Iodinated contrast media can cause pseudoallergic reactions associated with histamine release in significant numbers of patients. To clarify whether these adverse reactions may be aggravated by a compromised histamine catabolism we asked if radiographic contrast agents in vitro inhibit the histamine inactivating enzymes diamine oxidase (DAO) and histamine N-methyltransferase (HMT).
MethodsNine iodinated contrast agents were tested in vitro. Following pre-incubation of purified porcine kidney DAO and recombinant human HMT with 0.1–10mM of the respective contrast medium (H2O and specific inhibitors of DAO and HMT as controls) enzyme activities were determined by using radiometric micro assays.
ResultsNone of the contrast media irrespective of their structure showed significant inhibition of the activities of DAO and HMT. Pre-incubation of the enzymes with specific inhibitors led to complete inhibition of the respective enzymatic activity.
ConclusionsThe iodinated contrast media tested in vitro did not exhibit inhibition of histamine converting enzymes at physiologically relevant concentrations. However due to the in vitro character of this study these results do not directly reflect the in vivo situation.
Iodinated contrast media (CM) are widely used in computed tomography and angiography. Anaphylactoid reactions have been reported as a side effect after intravenous application of these contrast agents.1 These are usually characterised by cutaneous symptoms including erythema, pruritus, and urticaria, but gastrointestinal, respiratory, or cardiovascular complaints can occur as well.2 The incidence of these reactions has decreased after introduction of non-ionic substances and has been reported in various studies to be 0.6–3.1%.3 With an incidence of 0.01–0.04% severe anaphylactoid reactions are rare but unpredictable.3 Several pre-treatment schemes for their prevention have been discussed, but there is evidence that they are not effective in every case and the risk of adverse reactions can only be reduced rather than completely abolished.4,5
The pathophysiology of these anaphylactoid reactions has been discussed controversially and the biochemical pathways are still poorly understood.1,4 Serious reactions are similar to those of type 1 hypersensitivity according to Coombs and Gell.1 However, CM molecules are very small and would not likely form haptens that can bind to antibodies.6
The biogenic amine histamine acts as an inflammatory mediator, a neurotransmitter, and a regulator of hydrochloric acid secretion from gastric mucosa by binding to and activating four different histamine receptors.7,8 Histamine has been proposed to be the primary mediator of adverse reactions to contrast media, since symptoms of anaphylaxis can be reproduced by histamine infusions and antihistamines provide effective treatment.1 In addition, it has been shown that iodinated contrast media can lead to significant histamine release in vitro.9
In man, histamine can be inactivated by two alternative pathways catalysed by the enzymes diamine oxidase (DAO, EC 1.4.3.6) and histamine N-methyltransferase (HMT, EC 2.1.1.8), respectively.10 DAO oxidatively deaminates the primary amino group of histamine forming imidazole acetaldehyde. HMT catalyses the transfer of a methyl group from S-adenosylmethionine (SAM) to the imidazole ring of histamine forming Nτ-methylhistamine, which is then further oxidised by monoamine oxidase (MAO, EC 1.4.3.4).8,10 DAO is expressed in various mammalian tissues including intestine, kidney, and placenta.11 DAO is a secretory protein that is likely to be responsible for the inactivation of extracellular histamine.12 HMT is found in most of the human tissues including brain, lung, stomach, intestine, kidney, and liver.13 It is a cytosolic protein that can convert histamine only inside the cells.14,15
A large number of drugs including histamine antagonists, corticosteroids, antibiotics, antimalarials, tranquillisers, antidepressants, neuroleptics, local anaesthetics, dinatriumcromoglycate, and verapamil were reported to inhibit the enzymatic activities of DAO and HMT.16–18 The effect of iodinated contrast media on the activities of the histamine inactivating enzymes DAO and HMT has not been evaluated so far. Since inadequate histamine inactivation is associated with the development of histamine-related adverse reactions19 and could thus contribute to and aggravate the side effects reported for CM we analysed if these contrast agents inhibit DAO and HMT activities in vitro.
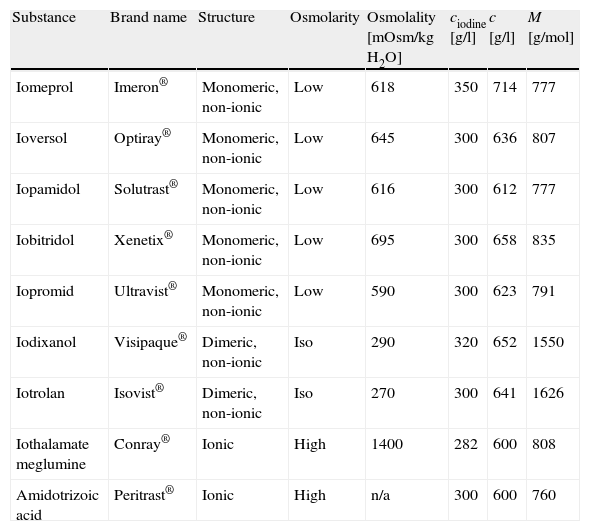
Materials and methodsContrast agentsThe non-ionic monomeric contrast media iomeprol (Imeron®, Bracco Altana, Konstanz, Germany), Ioversol (Optiray®, Guerbet, Roissy, France), iopamidol (Solutrast®, Bracco Altana, Konstanz, Germany), iobitridol (Xenetix®, Guerbet, Roissy, France), and iopromid (Ultravist®, Schering, Berlin, Germany), the non-ionic dimeric substances iodixanol (Visipaque®, GE Healthcare AS, Oslo, Norway) and iotrolan (Isovist®, Schering, Berlin, Germany), and the ionic contrast agents iothalamate meglumine (Conray®, Mallinckrodt, St. Louis, USA) and amidotrizoic acid (Peritrast®, Dr. Köhler Chemie, Alsbach-Hähnlein, Germany) were tested. The properties of these CM are summarised in Table 1.
Properties of radiographic contrast media used for inhibition studies. M=molecular weight of substance, ciodine=mass concentration of iodine in commercial preparation, c=mass concentration of substance in commercial preparation. n/a=data not available.
| Substance | Brand name | Structure | Osmolarity | Osmolality [mOsm/kg H2O] | ciodine [g/l] | c [g/l] | M [g/mol] |
| Iomeprol | Imeron® | Monomeric, non-ionic | Low | 618 | 350 | 714 | 777 |
| Ioversol | Optiray® | Monomeric, non-ionic | Low | 645 | 300 | 636 | 807 |
| Iopamidol | Solutrast® | Monomeric, non-ionic | Low | 616 | 300 | 612 | 777 |
| Iobitridol | Xenetix® | Monomeric, non-ionic | Low | 695 | 300 | 658 | 835 |
| Iopromid | Ultravist® | Monomeric, non-ionic | Low | 590 | 300 | 623 | 791 |
| Iodixanol | Visipaque® | Dimeric, non-ionic | Iso | 290 | 320 | 652 | 1550 |
| Iotrolan | Isovist® | Dimeric, non-ionic | Iso | 270 | 300 | 641 | 1626 |
| Iothalamate meglumine | Conray® | Ionic | High | 1400 | 282 | 600 | 808 |
| Amidotrizoic acid | Peritrast® | Ionic | High | n/a | 300 | 600 | 760 |
DAO activity was determined by using a radiometric micro assay based on the conversion of [14C]putrescine (1,4-diamino-[1,4-14C]butane) to 4-aminobutyraldehyde that is spontaneously converted to Δ1-pyrroline, which can be extracted into an organic solvent for quantitation by liquid scintillation counting.20,21 As a source of DAO activity we used homogenously purified porcine kidney DAO, which is highly homologous to human DAO and has almost identical enzymatic properties but is much easier to obtain in pure form in sufficient amounts.12,22 Briefly, in a total volume of 90μl, purified pig kidney DAO (81μU, 54ng) was pre-incubated in 100mM sodium phosphate pH 7.2 with 0.1–10mM of the respective CM for 30min at 37°C. Controls included pre-incubation without any additions or in the presence of 0.01–1mM of the specific DAO inhibitor aminoguanidine.23 The reaction was then started by addition of 10μl [14C]putrescine (0.222Ci/mol, 1nCi/μl, 4.5mM; GE Healthcare, Little Chalfront, UK), incubated for 30min at 37°C, and stopped by addition of 10μl 10% perchloric acid followed by alkalisation with 50μl sodium carbonate pH 12.2. The reaction product [14C]Δ1-pyrroline was extracted into 1600μl toluene containing 0.35% 2,5-diphenyloxazole (PPO) as a scintillator. Radioactivity was determined by liquid scintillation counting using a Packard Tri-Carb 2500TR Liquid Scintillation Analyzer. Mean enzymatic activity was determined from duplicate assays and inhibition of DAO activity was calculated relative to the uninhibited control that had a mean activity of 891dpm corresponding to 81μU (1μU=1nmol/min).
Radiometric histamine N-methyltransferase micro assayMeasurement of HMT activity is based on the transmethylation of histamine with [14C]SAM (S-adenosyl-l-[methyl-14C]methionine) forming radioactively labelled Nτ-methylhistamine that can be extracted at an alkaline pH into an organic solvent for quantitation by liquid scintillation counting.14,24 As a source of HMT activity we used homogenously purified recombinant human HMT that has identical enzymatic properties as the native enzyme purified from tissues.15,25 Briefly, in a total volume of 80μl, purified recombinant human HMT (28μU, 6ng) was pre-incubated in 100mM sodium phosphate pH 7.5 with 0.1–10mM of the respective CM for 30min at 37°C. Controls included pre-incubation without any additions or in the presence of 1–100μM of the specific HMT inhibitor amodiaquine.26 The reaction was then started by the addition of 10μl 500μM histamine and 10μl [14C]SAM (2Ci/mol, 1nCi/μl, 0.5mM; GE Healthcare, Little Chalfront, UK), incubated for 30min at 37°C, and stopped by addition of 60μl of a solution of 500mM boric acid and 1000mM sodium hydroxide causing a pH shift that facilitates the extraction of the product [14C]Nτ-methylhistamine into 1600μl of toluene/isoamylalcohol (1:1) containing 0.17% PPO as a scintillator. Radioactivity was determined by liquid scintillation counting using a Packard Tri-Carb 2500TR Liquid Scintillation Analyzer. Mean enzymatic activity was determined from duplicate assays and inhibition of HMT activity was calculated relative to the uninhibited control that had a mean activity of 2887dpm corresponding to 28μU (1μU=1nmol/min).
Statistical analysesStatistical analyses were performed by using the SPSS for Windows Version 15.0 software package (SPSS, Chicago, IL, USA). Differences in enzymatic activities of DAO and HMT between samples pre-incubated in the presence of CM and controls were assessed with repeated measures test. Correlation of enzymatic activities with CM concentrations was investigated by Spearman's correlation statistics. A two-tailed p value below 0.05 was considered statistically significant.
ResultsThe CM concentrations following bolus injection were calculated based on the recommended dosage. In a 70-kg person, a 100-mL injection of iodinated RCM (300mgI/ml) would yield an extracellular concentration of approximately 2mg iodine/ml corresponding to 3–8mmol/l in the equilibrium. Thus, the concentrations used in the assays (0.1–10mM) covered the whole physiological range.
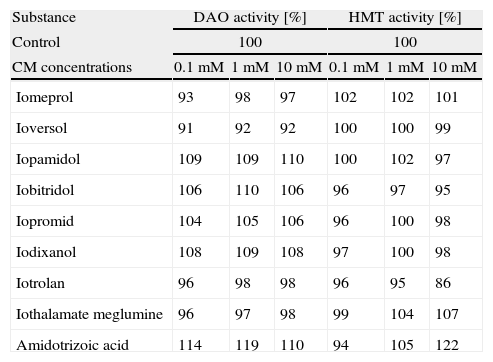
The results of the determination of the enzymatic activities of DAO and HMT following pre-incubation with physiologically relevant concentrations of the iodinated contrast media tested are shown in Table 2. As it is evident from these data and confirmed by statistical analyses using the repeated measures test, none of the radiographic CM exhibited significant concentration dependent inhibition of DAO (p=0.2145) and HMT (p=0.1911). In contrast, DAO and HMT were efficiently inhibited by the specific inhibitors aminoguanidine and amodiaquine, respectively, at concentrations of 10μM, showing that the inhibition assays worked as expected.
Enzymatic activities of DAO and HMT after incubation with 0.1–10mM of iodinated contrast media. Activities were calculated relative to uninhibited controls treated identically but incubated without any additions (100%), which were 891dpm corresponding to 81μU for DAO and 2887dpm corresponding to 28μU for HMT, respectively. For positive inhibition controls, DAO was incubated with 0.01–1mM aminoguanidine and HMT with 0.001–0.1mM amodiaquine, respectively. nd=not determined.
| Substance | DAO activity [%] | HMT activity [%] | ||||
| Control | 100 | 100 | ||||
| CM concentrations | 0.1mM | 1mM | 10mM | 0.1mM | 1mM | 10mM |
| Iomeprol | 93 | 98 | 97 | 102 | 102 | 101 |
| Ioversol | 91 | 92 | 92 | 100 | 100 | 99 |
| Iopamidol | 109 | 109 | 110 | 100 | 102 | 97 |
| Iobitridol | 106 | 110 | 106 | 96 | 97 | 95 |
| Iopromid | 104 | 105 | 106 | 96 | 100 | 98 |
| Iodixanol | 108 | 109 | 108 | 97 | 100 | 98 |
| Iotrolan | 96 | 98 | 98 | 96 | 95 | 86 |
| Iothalamate meglumine | 96 | 97 | 98 | 99 | 104 | 107 |
| Amidotrizoic acid | 114 | 119 | 110 | 94 | 105 | 122 |
| Substance | DAO activity [%] | HMT activity [%] | ||||
| Control | 100 | 100 | ||||
| Inhibitor concentrations | 0.01mM | 0.1mM | 1mM | 0.001mM | 0.01mM | 0.1mM |
| Aminoguanidine | 0 | 0 | 0 | nd | nd | nd |
| Amodiaquine | nd | nd | nd | 21 | 3 | 0 |
None of the substances irrespective of their monomeric, dimeric, ionic, or non-ionic structure, their osmolality, or their osmolarity inhibited the activities of DAO and HMT by more than 15% and no concentration dependence was observed for any of the inhibitory effects. Interestingly, pre-incubation with certain substances led to a slightly higher activity than that determined for the control. A slightly higher DAO activity was measured after pre-incubation with iopamidol, iobitridol, iopromid, iodixanol, and amidotrizoic acid whereas HMT activity was slightly higher after pre-incubation with iothalamate meglumine and amidotrizoic acid. However, these small stimulatory effects on DAO or HMT activity were not statistically significant and not significantly concentration dependent (Spearman correlation: DAO: r=0.1111, p=0.5812; HMT: r=0.0381, p=0.8503).
In summary, our results show that at concentrations up to 10mM the radiographic contrast media tested do not significantly inhibit the activities of the histamine inactivating enzymes DAO and HMT in vitro.
DiscussionAlthough iodinated contrast agents are routinely used in diagnostic imaging, relatively little is known about their biological effects in patients. Among the serious complications associated with the application of iodinated CM are the so-called anaphylactoid reactions that resemble type I hypersensitivity reactions but often without a clear involvement of specific IgE.1 It has been shown that iodinated CM can induce receptor-independent release of inflammatory mediators from basophiles and mast cells in vitro and in vivo, providing a possible explanation for their side effects in patients.1,27 However – although the pathophysiology of CM hypersensitivity reactions is still discussed controversially – actually its understanding seems to change. Recent studies described positive skin tests in at least 50% of the patients, and detected CM specific IgE antibodies in sera of immediate reactors. These observations suggest that severe immediate reactions may be IgE mediated and could therefore be really allergic. In contrast late adverse reactions appear to be T cell mediated.28–31
One of the most important of these inflammatory mediators is histamine whose plasma concentration was found to be elevated in patients with early adverse reactions to iodinated CM.32 Therefore, we asked if in addition to the possible release of histamine these substances might also inhibit histamine inactivation, thus aggravating the effect of this mediator in the observed anaphylactoid reactions.
We tested nine iodinated CM for their possible inhibitory effect on the enzymatic activities of the two histamine inactivating enzymes diamine oxidase and histamine N-methyltransferase in vitro. The radiometric assays used in this study are very sensitive, give highly reproducible results, and provide an accurate estimate of histamine degradation.
Our results clearly showed that at physiologically relevant concentrations, none of the substances tested exhibited significant inhibition of either DAO or HMT, the enzymes catalysing the first steps of histamine catabolism outside and inside cells, respectively. A few of the agents tested even slightly increased DAO and HMT enzymatic activities in vitro. Therefore, we conclude that the iodinated CM tested do not directly affect the inactivation of histamine. However, it is important to note that our observations are based on in vitro experiments which do not consider in vivo conditions of proteins, blood and endothelial cells, which may also play a role in CM hypersensitivity reactions. Therefore, our data do not directly reflect the in vivo situation since the study design only allows us to evaluate the direct effect of CM on histamine degradation.
As stated above, iodinated CM can release histamine from basophiles and mast cells in vitro and in vivo, leading to elevated plasma histamine concentration and severe pseudoallergic reactions.9,33–35 It is conceivable that this non-immunologic histamine release might overcharge the endogenous histamine degradation capacity, especially in patients with low enzyme levels of DAO and HMT. In fact, there is considerable individual variation in the expression and steady state tissue concentration of histamine inactivating enzymes and recently genetic polymorphisms were described for the DAO and HMT genes that appear to be associated with lower activities of the corresponding enzymes.36,37 Future studies will have to clarify whether patients with low basal DAO and HMT activities are more prone to develop adverse reactions upon administration of iodinated contrast media. This will help to minimise the risk for these patients and to develop efficient therapeutic strategies to counteract the side effects.
Ethical disclosuresProtection of human and animal subjects in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Patients’ data protectionConfidentiality of data. The authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestThe authors have no conflict of interest to declare.