The aim of the study was to evaluate the efficacy and safety of high-dose mite sublingual immunotherapy (SLIT) administered in children with allergic rhinitis in real-life clinical practice. Moreover, we analysed the clinical course of asthma severity.
MethodsRetrospective, observational, monocentre study. Medical records of patients treated between 2001 and 2008 were reviewed. Patients received a standardised Dermatophagoides pteronyssinus+Dermatophagoides farinae extract (300IR/ml) manufactured by Stallergenes (Staloral® 300). Patients were evaluated before SLIT initiation and at 6, 12, 24, 36 and 48 months. Global assessment of SLIT efficacy was measured using a visual analogue scale (VAS) and a rhinitis medication consumption score (RMCS). A global asthma score was used to estimate the clinical course of asthma severity.
ResultsWe obtained data from 78 patients, 43.6% male. The mean (±SD) age was 11.0±3.0 years. Most patients (69.2%) suffered from allergic rhinitis plus asthma. Patient evaluation of allergy severity (VAS) revealed a highly significant improvement between baseline and six months (p<0.001, Wilcoxon test): 4.0±1.7cm vs. 7.3±4.6cm. This improvement was maintained throughout the four-year follow-up period. The use of medications (RMCS) was significantly reduced in the first six months (4.6±2.5 points at baseline vs. 0.8±1.6 points at six months visit, p<0.001, Wilcoxon test) and remained very low until the end of follow-up. We did not find a temporal improvement in asthma severity.
ConclusionsThis retrospective study indicates that high-dose SLIT in children with rhinitis caused by house dust mites is well-tolerated and could be an effective treatment.
Immunotherapy is a wide term for the available treatments for immunological diseases. Among them, allergen-specific immunotherapy is a well-documented aetiological therapy for allergic rhinitis; IgE-mediated asthma; and venom hypersensitivity,1–3 and it is considered to be the only treatment strategy able to alter the natural course of the disease.4 Allergen-specific immunotherapy consists of administering gradually increasing quantities of an allergen product to an individual with IgE-mediated allergic disease to reduce the symptoms associated with subsequent exposure to the allergen involved.5 This therapy induces clinical and immunological tolerance, has long term efficacy and may prevent the progression of the allergic disease.5 Such characteristics are especially important in children because they can prevent new sensitisations,6 and progression from rhinitis to asthma.7
Subcutaneous immunotherapy has been widely applied and has been shown to be effective in reducing allergic airway disease symptoms.2 However, uncommon but severe systemic reactions have caused concern among physicians,8,9 and repeated injections have led to serious complications, especially among children. Therefore, alternative routes of immunotherapy have been proposed. Among them, sublingual immunotherapy (SLIT) has been suggested to be an attractive treatment option for children, where safety is of extreme importance and outpatient, home-based therapy is preferred. SLIT has been gaining the confidence of physicians because of its good safety profile and its effectiveness in the context of allergic airway disease. The efficacy and safety of high-dose sublingual immunotherapy (SLIT) has been demonstrated in several double-blind, placebo-controlled studies.10 However, there are still few data available concerning paediatric patients in clinical practice, although recently the efficacy of SLIT in children and adolescents with grass pollen-related allergic rhinoconjunctivitis has been demonstrated in two double-blind placebo studies.11,12
The aim of the present study was to evaluate the efficacy and safety of high-dose SLIT administered in children with allergic rhinitis to house dust mites in real-life clinical practice. Moreover, we analysed the clinical course of the severity of asthma in children treated with SLIT who presented rhinitis plus asthma due to house dust mites.
Material and methodsThis was a retrospective, observational study conducted in a local hospital, at the Paediatric Department Asthma Unit of Sant Pau Hospital, Barcelona, Spain. Medical records of patients treated between 2001 and 2008 were reviewed. The study was adherent to the Declaration of Helsinki. We used medical record files in which the patients’ personal identification data were kept separately from the clinical data, thereby ensuring anonymity. Spanish legislation does not require the approval of an institutional review board for observational, post-authorisation studies conducted using entirely medical record files in which the patients’ personal identification data are kept separately from the clinical data.
In Spain, SLIT is indicated in allergic rhinitis when there is proven IgE-mediated sensitisation to a single antigen or very small group of antigens and in patients not controlled with pharmacotherapy. The diagnosis of house dust mite allergy (Dermatophagoides pteronyssinus and/or D. farinae) was based on medical history, positive skin test using standardised extracts, and the presence of specific IgE as shown by RAST (radioallergosorbent test). Patients mono-sensitised to house mites, with or without asthma, received SLIT with IR (index of reactivity)-standardised D. pteronyssinus+D. farinae extract (300IR/mL) manufactured by Stallergenes (Staloral® 300). The major allergen content (average of different batches) of final extract corresponding to 100IR/mL was 20μg/mL of Der p 1, 4μg/mL of Der p 2 and 50μg/mL of Der f 1. After an 11-day or 1-day (ultra-rush procedure) build-up phase, patients received maintenance treatment consisting of five applications (equivalent to 0.5mL) of 300IR/mL five times a week. The mean duration of the treatment was 29.4 months (range 2–52 months). All the patients were asked to follow some standard recommendations in order to avoid house-dust-mite exposure.
We collected demographic and clinical data on baseline patient variables before SLIT initiation: gender, age, diagnosis, duration of allergic disease, total IgE, specific IgE, RAST classification, proportion of muconasal eosinophils and the results of the prick test to D. pteronyssinus and D. farinae.
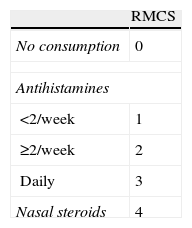
Patients were evaluated, according to clinical practice, before SLIT initiation (baseline visit) and at 6, 12, 24, 36 and 48 months. A physician asked the patient or caregiver to evaluate nasal symptoms related to allergy (itching, sneezing, rhinorrhoea and obstruction). Patients subjective assessments regarding their allergic symptoms were recorded using a 10cm visual analogue scale (VAS, 0: very bad, 10: very good). Allergy severity was assessed according to the VAS and classified as mild (VAS≥5) or moderate-severe (VAS<5). Additionally, patients were asked to describe medication consumed during the previous months. A rhinitis medication consumption score (RMCS) summarising treatments for rhinitis in the previous month was also used as an efficacy endpoint.13 RMCS (0–7 points) was calculated by adding specific scores based on the class of drug: 0 points for no consumption, 1–3 points for antihistamines and 4 points for nasal steroids (Table 1). The frequency of antihistamine and/or nasal steroid consumption during the inter-visit period was also gathered.
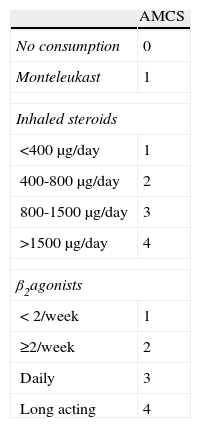
Clinical course of asthma severity was estimated through a global asthma score, commonly used in clinical practice. Severity of asthma was summarised in a total score resulting from the addition of bronchodilator use, nocturnal symptoms, effects on lifestyle and exacerbation symptoms scores from a questionnaire completed at each clinical visit. The lower the score was, the better for the patient (0–27 points). An asthmamedication consumption score (AMCS) summarising treatments for asthma in the previous month was used to estimate changes in asthma medication during SLIT treatment.13 AMCS (0–9 points) was calculated by adding specific scores based on the class of drug: 0 points for no consumption, 1 for Montelukast, 1–4 points for inhaled steroids and 1–4 points for β2-agonists (Table 2). Additionally, respiratory function tests were conducted to determine Forced Expiratory Volume in 1 Second (FEV1) and Peak Expiratory Flow (PEF). Adverse reactions (ARs) occurring during the treatment period were recorded in order to determine tolerance to SLIT.
Asthma-medication consumption score (AMCS, 0-9 points). Calculations take into account all drugs consumed for asthma in the previous month
| AMCS | |
| No consumption | 0 |
| Monteleukast | 1 |
| Inhaled steroids | |
| <400μg/day | 1 |
| 400-800μg/day | 2 |
| 800-1500μg/day | 3 |
| >1500μg/day | 4 |
| β2agonists | |
| <2/week | 1 |
| ≥2/week | 2 |
| Daily | 3 |
| Long acting | 4 |
The Mann-Whitney U-test was used to compare VAS and RMCS at baseline between the subgroup of patients with rhinitis plus asthma and the subgroup of patients with rhinitis alone. Wilcoxon’s nonparametric test for paired comparisons was used to compare VAS, RMCS, GAS and AMCS at the various visits. The comparison of proportions was performed using the Chi-square test. Paired Student’s t-test was used to compare respiratory function tests at the various follow-up time points. All the analyses were performed using SPSS version 12.0 (SPSS Inc., Chicago, Illinois, USA).
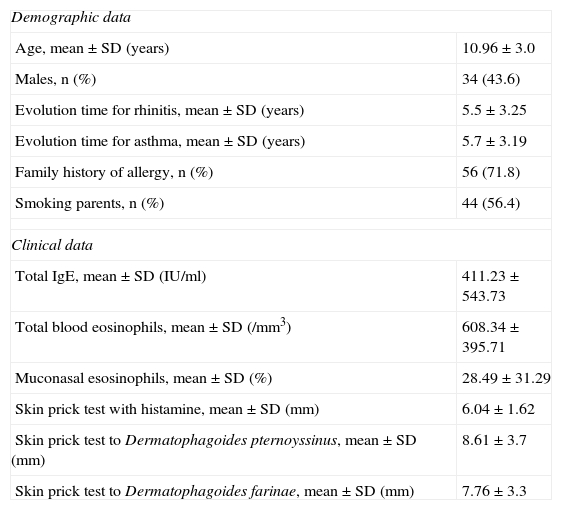
ResultsWe obtained data from 78 patients, 34 male and 44 female. The mean (±SD) age was 11.0±3.0 years (range, 6–18 years). Most patients (n=54; 69.2%) suffered from allergic rhinitis plus asthma and 24 (30.8%) were diagnosed with rhinitis but not asthma. Mean duration of rhinitis and asthma were 5.5±3.3 years and 5.7±3.2, respectively. According to the Global Initiative for Asthma (GINA) classification, 51% of the patients presented intermittent asthma and 49% mild to moderate persistent asthma. Table 3 summarises demographic and clinical data for the paediatric patients at the initiation of treatment with SLIT.
Demographic and clinical data at baseline N=78
| Demographic data | |
| Age, mean±SD (years) | 10.96±3.0 |
| Males, n (%) | 34 (43.6) |
| Evolution time for rhinitis, mean±SD (years) | 5.5±3.25 |
| Evolution time for asthma, mean±SD (years) | 5.7±3.19 |
| Family history of allergy, n (%) | 56 (71.8) |
| Smoking parents, n (%) | 44 (56.4) |
| Clinical data | |
| Total IgE, mean±SD (IU/ml) | 411.23±543.73 |
| Total blood eosinophils, mean±SD (/mm3) | 608.34±395.71 |
| Muconasal esosinophils, mean±SD (%) | 28.49±31.29 |
| Skin prick test with histamine, mean±SD (mm) | 6.04±1.62 |
| Skin prick test to Dermatophagoides pternoyssinus, mean±SD (mm) | 8.61±3.7 |
| Skin prick test to Dermatophagoides farinae, mean±SD (mm) | 7.76±3.3 |
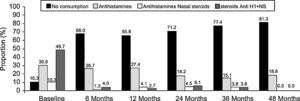
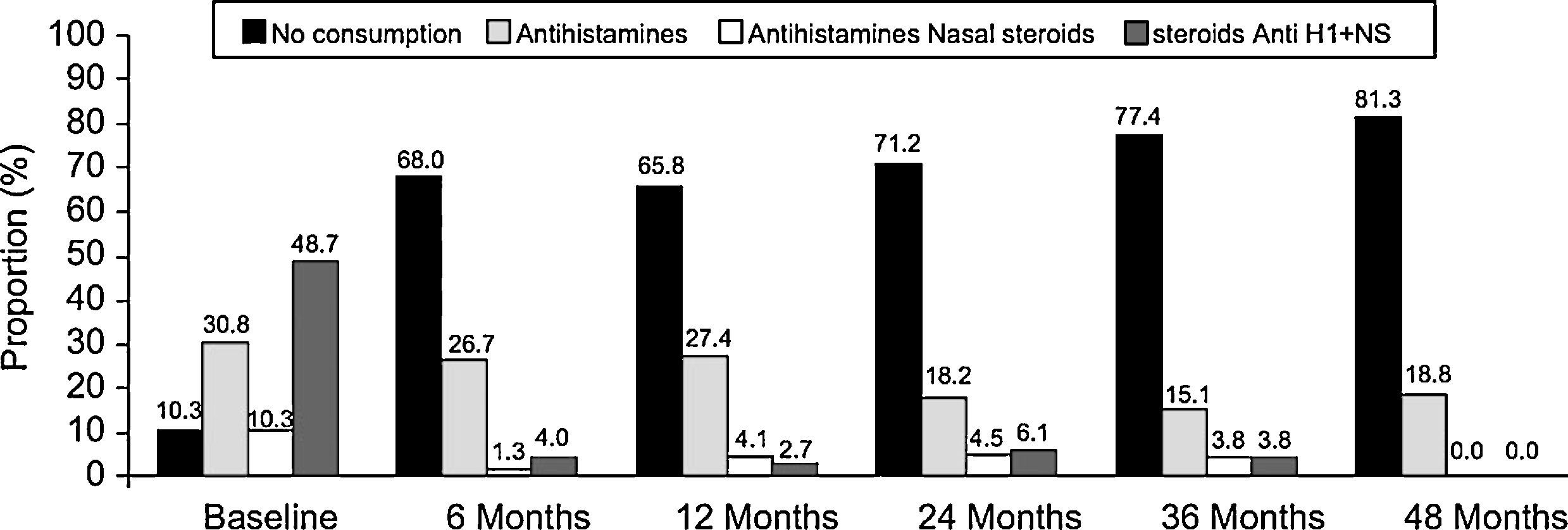
During the month prior to SLIT initiation, the most common treatment for allergic rhinitis was the combination of antihistamines and nasal steroids (n=38; 48.7%). Of the remainder, 24 (30.8%) subjects were being treated with antihistamines, 8 (10.3%) with nasal steroids, and 8 (10.3%) were not taking any rhinitis treatment. No statistically significant differences were found in VAS or RMCS at baseline visit between the subgroup of patients with rhinitis plus asthma and the subgroup of patients with rhinitis alone (p>0.05, Mann-Whitney U-test).
Patient evaluation of allergy severity (VAS) revealed a highly significant improvement between baseline and six months (p<0.001, Wilcoxon test): 4.0±1.7cm vs. 7.3±1.6cm, respectively. This significant improvement was maintained throughout the four-year follow-up period, the VAS at four years being 8.1±1.6 (p<0.001, Wilcoxon test) (Figure 1). Similar results were obtained in the subgroup of patients with rhinitis plus asthma and in the subgroup of patients with rhinitis alone (results not shown). The severity classification of allergic disease according to VAS changed dramatically between baseline and six months; the proportion of moderate-severe (VAS<5) rhinitis decreased from 78.2% at baseline to 9.3% at six months (p<0.05, Chi-square test). A gradual reduction continued over the course of the study period.
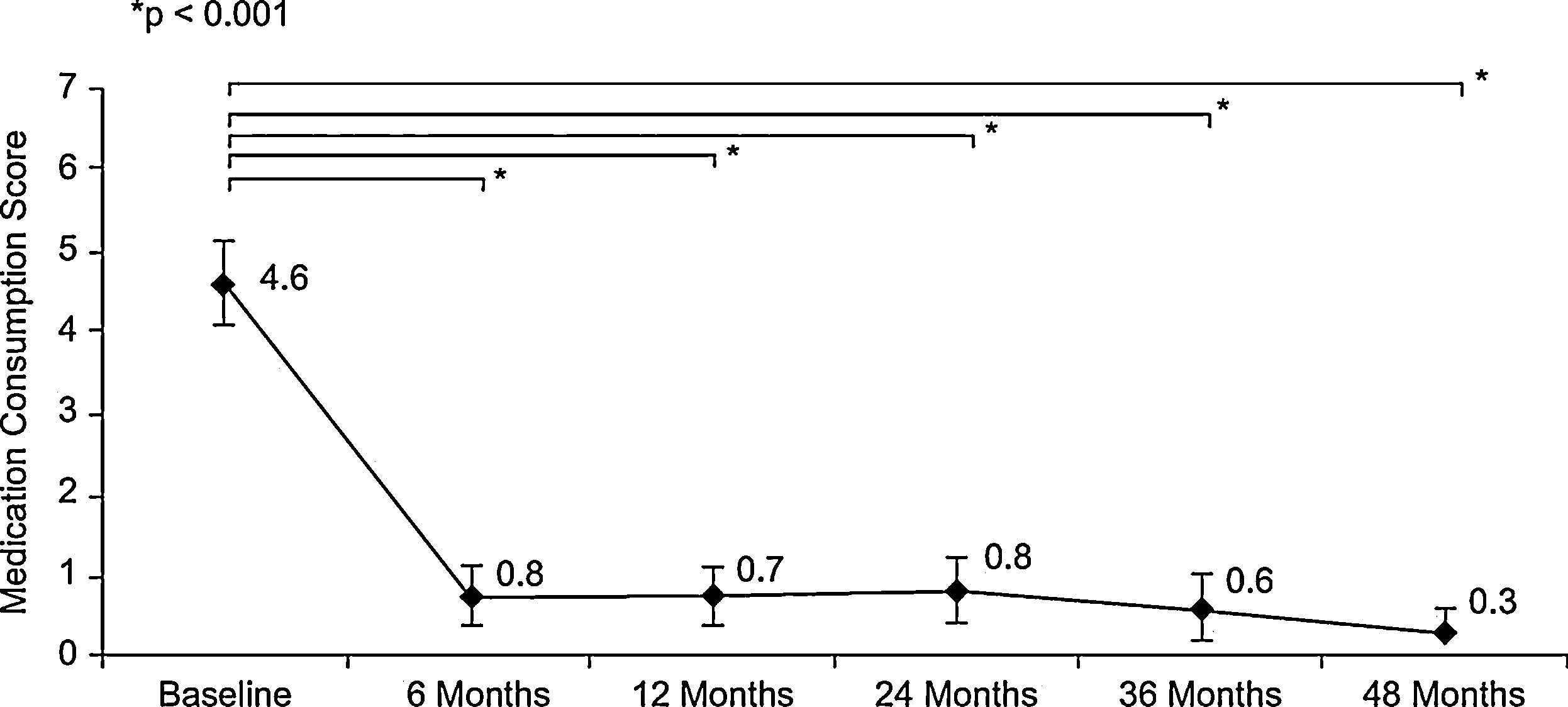
The use of medications during the month before each visit, assessed by the mean of the RMCS, was significantly reduced in the first six months (4.6±2.5 points at baseline vs. 0.8±1.6 points at six-months visit (p<0.001, Wilcoxon test)), and remained very low until the end of follow-up (0.3±0.8 points at 48-months visit) (Figure 2). Similar results in temporal changes in RMCS were obtained in both the subgroup of patients with rhinitis plus asthma and the subgroup of patients with rhinitis alone (results not shown).
There was a reduction in the consumption of antihistamines and nasal steroids in the month before each visit throughout the study period (Figure 3). The proportion of patients who had not consumed any treatment for rhinitis in the month before each visit increased over time, especially between baseline and the six-month visit (10.3% versus 68%). At the end of the study period (48 months) the proportion of patients with no consumption was 81.3%. Moreover, we observed a temporal reduction in the frequency of consumption of antihistamines and/or nasal steroids during the intervisit period (p<0.001, Chi-square test). At baseline, 47.4% patients reported a regular consumption of antihistamines and/or nasal steroids, 5.3% at the six-month visit, 1.4% at the 12-month visit, 0% at 24- and 36-month visits, and 5.9% at the end of the study period. At the same time, the proportion of patients who declared no consumption of antihistamines and/or nasal steroids increased over time: 9.0% at baseline and 73.5% at 48-month visit.
With regard to asthma medication, we did not find statistically significant differences between baseline, six-month visit and subsequent visits in AMCS (p>0.05, Wilcoxon test). The GAS was significantly reduced in the first six months (p<0.05, Wilcoxon test): 4.1±3.5 points at baseline vs. 2.9±3.2 points at six-month visit). However, in the subsequent visits, no such significant reduction in GAS was found. Additionally, no significant differences in FEV1 and Peak Flow were found over time (p>0.05, Student’s t-test).
Eighteen patients presented an adverse reaction (AR) (23.1%), most of them being local reactions (tongue oedema, tongue irritation and small ulcers on the tongue): 10 of these patients had received conventional regimen in the build-up phase (10 out of 60: 16.7%) and 8 the ultra-rush regimen (8 out of 18: 44.4%). Five patients (6.4%) experienced seven gastrointestinal reactions (nausea and/or abdominal pain) and only one patient (1.3%) showed one systemic reaction (SR): headache. All ARs were mild or moderate. There were no cases of anaphylactic shock or life-threatening events. The statistical analysis showed a significantly higher proportion of ARs in patients who received an ultra-rush regimen during the build-up phase compared to those receiving conventional regimen (p<0.05, Chi-square test).
Of the 78 patients included in the study, 12 patients discontinued immunotherapy prematurely: one due to lack of efficacy; two due to an AR (stomach pain and tongue oedema and irritation); two due to lack of efficacy and AR (tongue oedema); five due to loss to follow-up; and another two for reasons not related to the study treatment. A dose increase was made in seven patients based on investigator criteria. In these patients the dose was increased from five applications of 300IR/mL five times a week to eight applications of 300IR/mL five times a week due to a bad response to the treatment and/or an increased use of concomitant allergy medication. Good control of allergy was achieved in one or two months, so the dose was maintained.
DiscussionThe efficacy and safety of high-dose sublingual immunotherapy (SLIT) has been demonstrated in several double-blind, placebo-controlled studies.10 However, there are still few data available concerning paediatric patients in clinical practice. In the present study, we aimed to evaluate the efficacy and safety of high-dose SLIT administered in children with allergic rhinitis to house dust mites in real-life clinical practice. This retrospective study indicates that high-dose SLIT is well-tolerated and could be an effective treatment. It considerably increased the clinical global assessment of efficacy during SLIT and reduced the use of concomitant medication. These results are consistent with evidence that SLIT is effective in the treatment of allergic rhinitis in paediatric patients.14–16
There are few controlled trials which have evaluated SLIT efficacy in the treatment of allergic rhinitis caused by house dust mites carried out exclusively in paediatric patients.13,17–19 Recently, Marogna et al.19 published an open randomised controlled study in children with allergic rhinitis, with or without intermittent asthma. In this study, SLIT significantly reduced nasal and bronchial symptoms in comparison with the control group from the first year of treatment. Conversely, previous studies have reported negative results of SLIT in the treatment of paediatric allergic disease.17,18 Thus, in the study conducted by Hirsch et al.,17 SLIT did not show consistent clinical or immunological benefits compared to placebo. Some authors have pointed out that negative results might be related to a low amount of allergen administered during treatment.17,20 Variability in treatment effects could be explained also by variable responses to treatment according to the type of allergen used, the age of patients, or the dose and duration of treatment.10,14 Thus, Penagos et al.14 in a meta-analysis indicated that SLIT courses shorter than 18 months were not effective. However, in our study SLIT demonstrated a good efficacy in children six months after treatment initiation.
As for the long-term effect of SLIT on asthma symptoms, we did not find any reduction in the consumption of treatments for asthma between baseline and the sixmonth visit. Moreover, we did not find any temporal improvement in asthma severity, assessed using the GAS, or in respiratory function. Our results do not support the results of an open-label, controlled, observational study which included 60 mite-sensitive asthmatic children aged from 3 to 17 years.21 In that study, SLIT was given for 4 to 5 years and the children were followed up for 10 years, at which time there was a significant reduction in the prevalence of asthma, use of asthma medication and a significant increase in peak expiratory flow rate in the SLIT group compared with the control group. Other controlled studies had revealed clinical efficacy of SLIT in asthmatic children.19,22–24 The clinical condition of the patients selected in our study might explain the lack of a significant improvement in asthma severity: peak flow and FEV1 at baseline were markedly high, indicating that asthma was well controlled in these patients when they initiated the treatment with SLIT. Similar results were obtained in a double-blind, placebo controlled trial performed in 111 children with house dust mite-induced mild-to-moderate asthma.25 This study indicated that SLIT does not provide significant additional benefits when patients are well controlled with pharmacological treatment and dust mite-avoidance measures.
The safety of SLIT in children was confirmed in many trials, and no severe ARs had been reported. In our study, SLIT at high doses was well tolerated by children. Mild or moderate local ARs were the most frequently reported, mainly related to the direct contact of the vaccine with mucosal surfaces. There were no cases of anaphylactic shock or life-threatening events. In spite of the higher proportion of ARs among patients with an ultra-rush build-up phase, all ARs recorded were mild or moderate, which indicates that such a regimen could be an alternative to classic regimens if administered under the strict control of health care professionals.
The present study was carried out in real-life clinical practice. Indeed, a double-blind, placebo-controlled study would have enhanced the power of our results and demonstrated the efficacy of SLIT. However, since patients were not selected according to restrictive criteria, our results may be better extrapolated to real conditions. Moreover, the magnitude of the effects of SLIT in rhinitis patients found in our study cannot be easily explained only by a placebo effect.
During recent decades, allergic airway disease has been effectively controlled in paediatric patients, mainly with inhaled and nasal corticosteroids which seek to relieve symptoms. However, disease activity usually returns after discontinuation of treatment. As stated earlier, allergen-specific immunotherapy is believed to be the only treatment capable of changing the natural history of the disease. A recent study has demonstrated the preventive effect of SLIT on the onset of mild persistent asthma and new skin sensitisation in a group of allergic children with allergic rhinitis, with or without intermittent asthma. These results confirms that SLIT as well as SCIT act as a biological response modifier.19 The benefits of immunotherapy, together with the fact that dust mites are the leading cause of allergies, especially asthma, have to encourage us to perform more well-designed and powered clinical studies in children with dust mites-induced rhinoconjunctivitis and asthma, in a similar way as was done in pollen allergy.11,12
In conclusion, this retrospective study might indicate that high-dose SLIT has a good efficacy and safety profile in children with mites-induced allergic rhinitis in real-life clinical practice. Further studies are needed to confirm short and long-term efficacy and preventive effects of SLIT in rhinitis as well as asthma in paediatric patients.
Conflict of InterestJosé-Luis Justicia and Víctor Alvà are employees of Stallergenes Ibérica. The corresponding author declares no other potential conflict of interest.




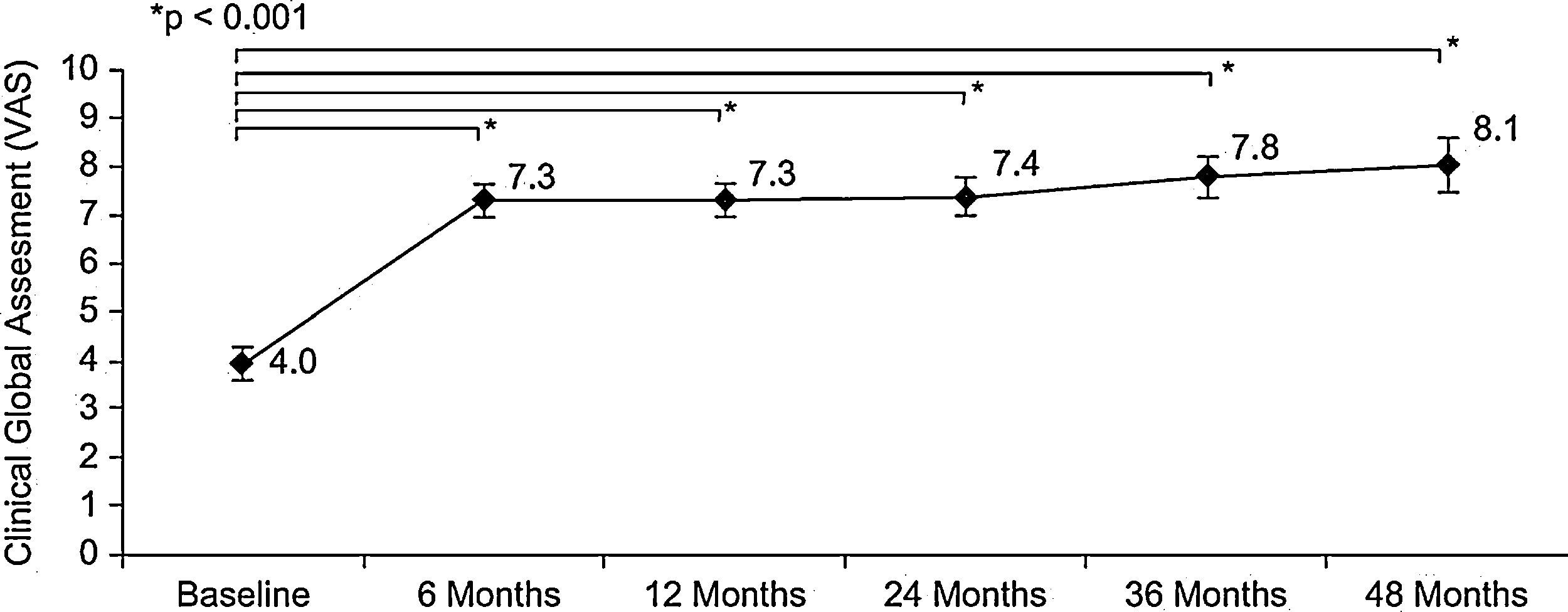
![Evolution of clinical global assessment of efficacy during sublingual immunotherapy according to visual analogue scale (mean [95%CI]); baseline (n=78), 6 months (n=75), 12 months (n=73), 24 months (n=66), 36 months (n=53) and 48 months (n=32). Evolution of clinical global assessment of efficacy during sublingual immunotherapy according to visual analogue scale (mean [95%CI]); baseline (n=78), 6 months (n=75), 12 months (n=73), 24 months (n=66), 36 months (n=53) and 48 months (n=32).](https://static.elsevier.es/multimedia/03010546/0000003900000003/v1_201304101047/S0301054610001217/v1_201304101047/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Evolution of consumption of medications for rhinitis during the month before each visit according to Rhinitis Medication Consumption Score (mean [95%CI]); baseline (n=78), 6 months (n=75), 12 months (n=73), 24 months (n=66), 36 months (n=53) and 48 months (n=32). Evolution of consumption of medications for rhinitis during the month before each visit according to Rhinitis Medication Consumption Score (mean [95%CI]); baseline (n=78), 6 months (n=75), 12 months (n=73), 24 months (n=66), 36 months (n=53) and 48 months (n=32).](https://static.elsevier.es/multimedia/03010546/0000003900000003/v1_201304101047/S0301054610001217/v1_201304101047/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)