INTRODUCTION
Specific immunotherapy is traditionally performed by several subcutaneous injections of the sensitizing allergen. During the first phase, called induction or build-up phase, progressively increasing dosages of the allergen extract are administered at variable intervals until the individual maximum tolerated dose is reached.
This dose usually corresponds to 0.8-1.0 mL of the top concentration vial of the commercial allergenic preparations. Ideally, this dose should represent the administration of 5-20 mg of the major allergen (1). During the following phase, usually called maintenance phase, the maximum tolerated dose is administered at intervals in the range 2-8 weeks for 3-5 years. The dose the immunotherapy can be started with, and the progression of the intermediate doses, has been adjusted and established on an empyrical basis supported by a long and consolidated clinical experience, paying special attention to the frequency of adverse events.
However, it must be admitted that careful and systematic studies aimed at establishing the best combination of starting and final dosages, their progression during the build-up phase, and the frequency of administration during the build-up and the maintenance phase, are lacking.
The history of specific immunotherapy starts with aqueous extracts, later on almost completely substituted with the important exception of hymenoptera venoms by depot extracts. Depot extracts have a better tolerability profile, i.e. they are linked to a lower frequency of local and systemic side effects, and allow for a longer interval between administrations during the maintenance phase.
The experience accumulated up to now has led mainly for the build-up phase to different schedules characterized by different intervals between administrations and, therefore, by different lengths of the build-up phase.
Each schedule can be useful for different purposes and may involve the need for special precautions to be taken or experience to be owned by the allergologist for its performance.
The schedules reported in the literature or really used in the common practice can be summarized as follows.
1. Standard schedule. This is the schedule routinely followed in allergology. It is performed with depot extracts, with single increasing dosages administered on average weekly during the build-up phase. Maintenance dosages are administered routinely every 3-4 weeks, the possible range being from 2 to 8 weeks. The starting dosage is usually 1.000 to 10.000-fold lower than the maintenance dosage.
2. Rush schedule. This schedule is usually performed almost exclusively for hymenoptera venom immunotherapy. Rush schedules can be also used for inhalant allergens, but mainly in experimental protocols. These schedules are performed with aqueous extracts, administering a few increasing dosages during the same day and for a few consecutive days, until the maximum tolerated dose is reached. Intervals between administrations during the same day are 20-30 minutes. The maintenance follows at 2-6 weeks intervals, often with aqueous extracts but also, sometimes, with depot extracts. The starting dosage is usually 10.000 to 100.000-fold lower than the maintenance dosage.
4. Ultrarush schedule. This is a special kind of rush schedule allowing to reach the maintenance dosage in a few hours by means of several administrations at 20-30 minutes intervals during the same day.
5. Cluster (or clustered) schedule. The basic schedule resembles the rush schedule, because there are a few administrations during the same day at 20-30 minutes intervals, but administrations are not performed in consecutive days. The most common interval between days is one week although 15-day intervals have also been reported. This schedule is usually performed with both aqueous and depot extracts or combinations of aqueous (for the beginning of the build-up phase) and depot extracts.
The standard schedule normally involves 12-16 weekly administrations made by the allergologist. Patients have therefore to accept to undergo a visit every week for 3-4 months, and this means economical and time wasting drawbacks leading in some cases to a non-compliance of patients with the therapy.
The use of non-standard schedules in the common practice may have different reasons and explanations. The rush or ultrarush schedule is used for hymenoptera venoms when there is the need for the induction of a quick tolerance toward the venom. In this way the patient is protected against the real risk of severe reactions when exposed to a field sting, and/or it is protected from the psychological fear of the consequence of a sting, with a quick improvement of his/her quality of life. Rush schedules have also been used in the past in experimental protocols with inhalant allergens. This choice was made because in this way both the maintenance dose and the induction of detectable changes in immunological (IgE, IgG, ICAM-1, etc.) as well as clinical parameters (specific and aspecific challenge tests, skin reactivity, etc.) are quickly reached. Rush and ultrarush schedules involve nonetheless higher risks for adverse events for patients. Their use is therefore limited to hospitalized patients under careful monitoring with the supervision of adequately trained personnel.
More recently, a further impulse towards schedules different from the standard one so as to reach more quickly the maintenance dose has been identified in the patient's compliance. This aspect, combined with the overestimation of the frequency and relevance of local and systemic adverse events related to the injective immunotherapy, has become the rational basis to develop and use, mainly during the last decade, non-injective forms of immunotherapy to be used as self-administered treatments. From the economical, safety and compliance points of view this is obviously a very interesting chance, but it can lead to a loss of contact between specialists and patients. The need for a regular attendance to the allergologist's required by the injective therapy can be seen as a good opportunity for a general check of the pathology and for a revision/adjustment of the drug treatment protocol. This means of course a better follow up of the patient and could turn out in a better clinical outcome. For this reason, also for self-administered non injective immunotherapy (namely SLIT), a regular attendance to the allergologist's should be recommended.
According to the points above, we can identify two different kinds of cluster schedules, developed in different times and designed for different purposes. A first group of schedules includes cluster schedules developed within an experimental design not aimed at evaluating the cluster schedule itself, and therefore not intended to be used in the common practice. In most cases, the build-up phase has been performed with aqueous extracts, whereas the maintenance phase has been conducted with aqueous or depot extracts. A premedication with antihistamines has been used in some of these studies.
A second group of cluster schedules designed for a possible use in the common practice has been generated more recently. They are based for both the build-up and the maintenance phase on depot extracts, with no use of premedication.
Obviously, the frequency and relevance of local and systemic side effects is the main key element for the development and usefulness of this second group of cluster schedules, whereas it is only a secondary problem or outcome for the first group of schedules.
Other key points in favor of cluster schedules, such as minor cost, better compliance for both clinicians and patients if combined with a safety profile comparable to the standard schedule, should nonetheless be considered.
DOCUMENTATION ON CLUSTER SCHEDULES (FIRST GROUP)
There are several papers dealing with the use of cluster schedules within an experimental design devoted to the evaluation of parameteres different from the tolerability of the schedule itself. For this reason, in some of these papers frequency and relevance of adverse events related to the administration of the treatment are incompletely reported or are not reported at all.
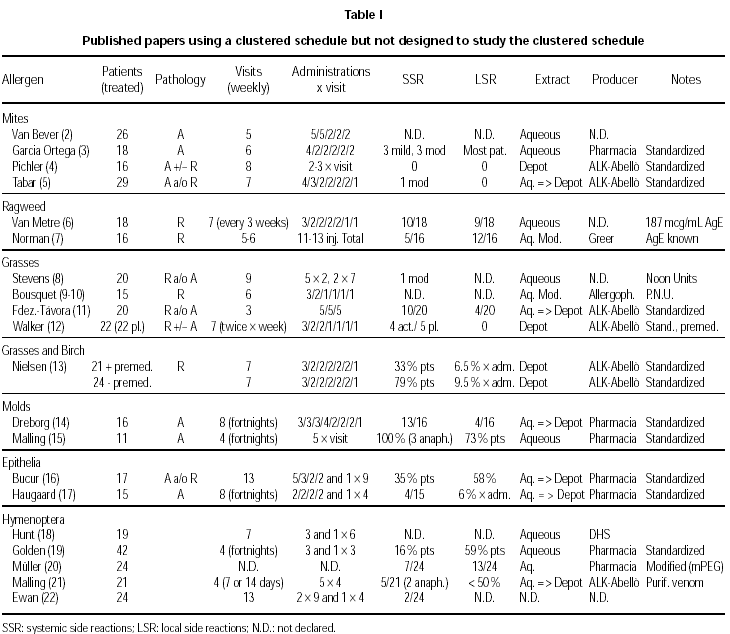
Available studies, organized according to the allergen involved, are summarized in table I (2-22).
The comparison among different allergens, different extracts (aqueous, depot, modified) and schedules used is obviously difficult. Moreover, the use in some studies of a premedication does not allow for a correct interpretation of the outcomes.
DOCUMENTATION ON CLUSTER SCHEDULES (SECOND GROUP)
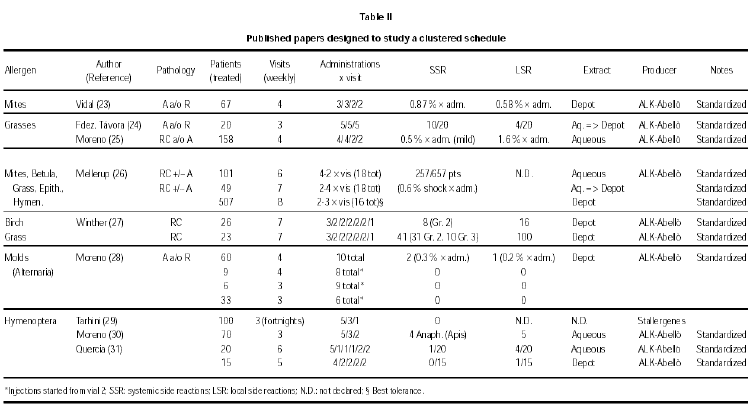
These papers have been produced in the last years and, because they have a very practical aim, in some cases they have been published in journals of lower scientific profile and diffusion. These papers are summarized in table II (23-31), organized according to the allergen involved.
EFFECT OF DIFFERENT VARIABLES ON TOLERANCE PROFILE
Many different variables can obviously affect the tolerance of the administration of an allergenic preparation. The following ones are the most relevant, with the most common possible different choices for each of them:
1. Use of premedication (kind of drug, dosage, time of administration before allergen administration).
2. Kind of allergen (mite, pollen, epithelium, mould, hymenoptera venom).
3. Purification, standardization, consistency and stability of the allergenic extract.
4. Pharmaceutical presentation of the allergen (native or chemically modified allergens; aqueous or depot extracts; use of aluminum hydroxide, or calcium phosphate, or tyrosine to obtain the slow release of the allergen).
5. Clinical manifestations before treatment (conjunctivitis, rhinitis, asthma, and their combinations).
6. Actual clinical situation of the patient when the allergen is administered.
7. Schedule followed (number of clusters, number of injections per cluster, time interval between clusters, starting dose, progression of doses, top dose reached).
PREMEDICATION
Premedication was used in one study with depot grass extract (12), and in one with birch or grass depot extract (13). The results are quite different, in spite of the common origin of the extracts used (ALK-ABELLÓ). In the trial by Nielsen (13), reaching the maintenance dose in seven visits for a total of 14 injections, a high rate of total systemic reactions in patients not premedicated (79 %) as compared to premedicated patients (33 %) was observed. However, in no case a life-threatening reaction Grade 4 (grading of reactions according to ref. 32) was detected. In the trial by Walker (12), reaching the maintenance dose in seven visits for a total of 11 injections, the same low rate of mild, delayed systemic side effects was observed during the build-up phase, i.e. 4/22 and 5/22 in the active and placebo group, respectively. The premedication was in both trials 1 tablet of loratadine. There are anyway two differences between these two studies, that may help to explain the difference in the rate and relevance of side effects. The premedication was given 120 minutes before the allergen administration in one case (13), whereas it was given at least 15 minutes before in the other case (12). Moreover, the schedules seem to be very similar, but in one case each cluster was given at weekly intervals (13), whereas in the other case (12) two clusters per week were administered.
PHARMACEUTICAL PRESENTATION (AQUEOUS-DEPOT)
Aqueous and depot preparations of several allergens (Mites, Birch, Grass, Epithelia, Hymenoptera) from the same producer (ALK-ABELLÓ) have been compared in one study (26). The cluster regimen for the build-up phase was performed only with the aqueous preparation, or with the depot preparation, or with a mixed regimen, administering 5, 7, and 6 clusters respectively with 2-4 injections per visit. For the mixed regimen, the first 3 clusters were performed with the aqueous preparation.
Considering all patients presenting at least one immediate systemic side effect, there was no difference among pure aqueous (53/101), pure depot (223/507) or mixed aqueous/depot regimen (24/49). A lower rate of immediate systemic reactions Grade 3 or 4 could be seen for the pure depot treatment, but the regimen used in this case was perhaps less aggressive. The top dose was reached after 7 weeks, instead of 5 (pure aqueous regimen) or 6 (mixed regimen), and the number of administrations was 3 for the first cluster and 2 for the following ones, instead of 4 for the first 3 (pure aqueous regimen) or 2 (mixed regimen) clusters. Patients treated only with the depot treatment showed nonetheless a slightly higher rate of delayed systemic reactions.
Because of these differences, it is hard to conclude that depot preparations are better tolerated for cluster schedules than aqueous preparations, used alone for the whole build up phase or used only for the first part of it.
Adrenaline was used in 4 (4/101) patients treated with the aqueous preparation, in 2 patients (2/507) treated only with the depot treatment, and in 1 patient (1/49) treated according to the mixed regimen but during the depot treatment.
ALLERGEN
A direct comparison among allergens can be attempted only in a few cases. In one paper (26) a similar rate of systemic side reactions for birch, grass, cat and mites, and a significantly lower rate for hymenoptera venom was found. Considering only grade 2-4 reactions, a lower rate was registered for birch and hymenoptera venom as compared to cat, mites and grass. Winther et al (27) used a similar treatment schedule but with an administration less and a depot extract for birch (26 patients) and grass (26 patients) from the same producer. They found again a higher rate of systemic reactions grade 2-4 for grass (41 in total, 31 grade 2 and 10 grade 3) as compared to birch (8 in total, all Grade 2).
Most of the SRs (> 80 %) occurred with accumulated daily doses below 30000 SQ-U (top dose according to the treatment schedule, 100 000 SQ-U). Also the local late reaction rate for the same dose expressed as SQ-U was higher for grass (100 > 8 cm) as compared to birch (16 > 8 cm). According to the authors of this paper, an unbalanced potency between birch and grass extract is the most likely explanation for the clinically assessed difference in tolerance. However, in one study (13) as much as 79 % of patients treated with birch or grass depot extract without premedication showed SR, whereas only 42 % of birch allergic patients and 53 % of grass allergic patients treated with aqueous and/or depot birch or grass extract showed SR without premedication in another study (26). These differences are difficult to explain, because both studies used a very similar treatment schedule and allergen preparations from the same producer (ALK-ABELLÓ).
Mellerup et al (26) reported on average a 41 % of SRs in patients treated with depot extracts, cat and mite being the allergens more often involved in SRs. This finding was not confirmed by Pichler et al (4) who reported no SRs and no local side reactions administering a depot mite extract from the same producer and according to the same schedule. Using a different schedule with an aqueous mite extract, Tabar et al (5) reported only 1 case of moderate SR, corresponding to a rate as low as 3.5 %. It is interesting to note again that the extracts used by Mellerup, Pichler and Tabar were from the same producer (ALK-ABELLÓ).
Very high rates of SRs (from 80 to 100 %) have been reported in two papers where mold-allergic patients were treated with aqueous extracts, at least during the build-up phase (14,15). However, in another study (28) only 2/108 patients showed SRs during treatment with a depot preparation of Alternaria extract.
The schedules used in these 3 trials were different and so was the allergen producer and perhaps the allergenic potency of the treatment, as detailed in table II.
CHEMICALLY MODIFIED ALLERGENS
Allergens can be chemically modified, for instance with formaldehyde, glutaraldehyde, methoxypoliethylenglycol, or alginic acid, to reduce their capacity to link IgE molecules. Because modified allergens are expected to be better tolerated than native allergens, their use seems to fit well with cluster schedules. However, only four trials studying modified allergens in cluster schedules have been published.
Three studies have been run with inhalant allergens (7,9,10) and one with hymenoptera venom (20). No data about tolerance are available for one study (9), whereas patients showed SRs rates of 46.6 %, 15.3 %, and 29.2 %, respectively, in the other three studies. The lowest rate for SRs was reported for a special high-molecular-weight allergoid preparation, not commercially available. Local adverse reactions rates per patient as high as 66.6 %, 41.6 %, and 54.2 %, respectively, were reported (7,10,20).
Apart from one study with aqueous mold extract (15), these rates are not different from the rates reported in cluster studies with native allergens.
CLINICAL MANIFESTATIONS BEFORE TREATMENT
Asthmatics as compared to patients suffering only from rhinitis are more prone to develop SRs (33). Apart from the considerations already given for the schedules and the treatments used, this was especially true in asthmatic patients submitted to immunotherapy with molds extracts who showed very high rates of SRs (from 80 to 100 %). However, in another study with an Alternaria depot extract only 2/108 patients suffering from asthma and/or rhinits showed SRs Grade 2 (28). A relatively low rate of SRs is reported in general in patients treated with Hymenoptera venom as compared to inhalant allergens, mainly because these patients do not normally have respiratory symptoms when submitted to immunotherapy (tables I and II). These considerations are supported by the results from others (26) who reported a 79 % rate of SRs Grade 2-4 in asthmatic patients, a 42 % rate for patients with both asthma and rhinitis, and a 19 % rate for patients in the Hymenoptera venom group.
On the other hand, also patients suffering only from rhinitis can present a rate of SRs as high as 79 %, as shown in the study by Nielsen et al (13) with grass and birch in patients not premedicated and in spite of the administration of depot extracts. Moreover, in the study by Van Metre et al (2) with aqueous ragweed 10/18 rhinitic patients showed SRs, whereas in another study (27) 23 patients suffering from rhinoconjunctivitis under treatment with depot grass extract showed a total of 41 SRs.
TREATMENT SCHEDULE
Many different schedules have been used in different trials. The number of visits ranged from 3 to 8, with 1 to 5 shots per visit. In general, a higher number of administrations have been given at the beginning of the treatment with the most diluted vial, whereas 2 to 1 injections have been administered with the most concentrated allergen vial, with no clear-cut differences between aqueous and depot preparations. Visits took place in one trial twice per week (12), in 6 trials at fortnigth intervals, but in the majority of trials every week. The best results for tolerance were obtained in the study with administrations twice a week of a depot grass extract in patients protected by a premedication (12), and in the study with weekly clusters of 2-3 administrations of a depot Alternaria treatment (28). In the first case the rate of SRs was similar in the placebo and in the active group (5/22 and 4/22, respectively), whereas in the second case only 2/108 patients under active treatment showed a SR. However, with a very similar schedule but with weekly administrations of a depot grass extract from the same producer in premedicated patients, Nielsen et al (13) reported a 79 % rate of SRs. Moreover, Walker et al (12) treated patients suffering from rhinitis and/or asthma, whereas Nielsen et al treated patients suffering form rhinitis only. In this situation, a better tolerance would have been expected in the latter study, but the opposite took place.
FINAL COMMENTS AND CONCLUSIONS
Many important improvements have been reached in the purification, characterization, and standardization of inhalant allergens, that have led to the official recognition of the efficacy of immunotherapy performed with these allergens (1). However, the schedule for the build-up phase of the injective immunotherapy for inhalant allergens has experienced no development or progress and it is still normally performed according to long and time-consuming schedules already in use some decades ago. On the other hand, cluster, rush and ultrarush schedules are now commonly used for the build-up phase of Hymenoptera venom immunotherapy but very seldom followed for inhalant allergens. The research, development and use of these schedules have been obviously pushed and justified by the need of a very quick protection of sensitized patients towards the life-threatening field stings by the culprit insect.
Patient's compliance is a point of increasing importance for every therapeutic approach. For sure, the build-up phase of the immunotherapy for inhalant allergens as it is currently performed in the common practice is a crucial point for both acceptance of and compliance with this form of therapy. The development and the increasing use of non-injective, self-administered preparations for immunotherapy, are clearly related to this situation. As for Hymenoptera venoms, but for different reasons, there seems to be the opportunity to develop and validate new schedules for immunotherapy with inhalant allergens able to meet the combined needs of tolerance, safety, efficacy, and patient's compliance.
A cluster schedule for the build-up phase, allowing to reach the maintenance dose in half or less the time normally needed with the standard schedule, seems to be a first step in the right direction. Apart from the effects on patient's and clinicians' compliance, this has a relevant economical impact because of savings in time and personnel for the administration of the therapy.
Many studies dealing with a cluster schedule have been published but, because several variables can or must be considered and combined, no clear conclusion can be drawn from the available evidence.
However, according to the published papers, the following seem to be the best basic conditions to be further studied and properly combined for an optimal schedule:
1. Use of a premedication to be administered between 15 and 60 minutes before the first administration of each cluster, especially in asthmatic patients.
2. Use of depot preparations.
3. Not more than 4 administrations per cluster.
4. Between 4 and 6 clusters.
5. Administration of one-two clusters per week.
We hope that in the near future the interesting and promising results already available for the cluster schedule with inhalant allergens will be further confirmed and developed by adequately designed and properly conducted trials.