Specific oral tolerance induction (SOTI) is a promising approach for severe food allergies. There are little data in the literature regarding the home-phase of SOTI, not only with regard to type and frequency of adverse reactions but also regarding the most suitable treatment and protocol.
AimsTo define the incidence and severity of adverse reactions, possible risk factors, and the safety and effectiveness of the home-phase of an original SOTI protocol in a large group of children with severe cow's milk (CM) allergy, after the hospital “rush” phase.
MethodsThe study was conducted by recording in-home phase adverse events, success and failure as reported by parents, and calling families. Adverse reactions were treated following the International Guidelines, arbitrarily modified by introducing nebulised epinephrine for respiratory reactions, oral beclomethasone for acute gastric pain and oral cromolyn for recurrent gastric pain.
ResultsOut of 140 patients, 132 were contacted; eight were inaccessible (follow-up 2–84 months). The number of adverse reactions was 1 in every 100 doses. The reactions were treated with nebulised epinephrine (221 reactions), IM epinephrine (6 reactions), and other drugs. Patients with high specific IgE levels (greater than 100kUA/L) and lower CM dose (less than 5ml) at the end of in-hospital phase showed a higher risk both for number of reactions and use of nebulised epinephrine.
ConclusionsThe home phase of SOTI was characterised by a significant number of adverse reactions, mostly managed with an acceptable rate of side effects. Nebulised epinephrine played a pivotal role in respiratory reactions.
Food allergy is the primary cause of anaphylaxis in children and cow milk's (CM) proteins are the main offender in Europe.1
Specific oral tolerance induction (SOTI) is a promising approach in the treatment of severe CM allergy. Recent reports have demonstrated the efficacy of different oral desensitisation protocols during the hospital phase with limited side effects.2–6 Nevertheless, the number of children who have undergone the treatment is still small and, as a result of this, SOTI is considered an experimental approach which is to be limited to highly defined settings. Furthermore, as the success of SOTI on the whole depends upon the outcome of the home-phase, further research is needed to document the safety and efficacy of this portion of the treatment. Before SOTI can be applied on a widespread scale, the most suitable protocol must be developed, in order to reduce the frequency of adverse reactions.
In fact, the available data on the follow-up of home phase SOTI refer to limited series, with low specific IgE levels and a short-term follow-up.7–9
The main aim of this study is to outline, after an hospital induction phase of SOTI,10 the adverse reactions and their treatment during the home phase of SOTI as experienced by a large series of patients diagnosed with severe CM allergy (high levels of specific IgE, recent systemic reactions, associated risk factors such as the presence of asthma) over a long follow-up period.
AimsTo define the incidence, severity and treatment of adverse reactions during the home phase of SOTI, the possible risk factors related to patient and protocol, and the safety and effectiveness of the home-phase SOTI protocol in a large group of children undergoing home treatment after the in-hospital rush phase.
Materials and methodsConsensus and ethics committee approvalInformed consent was obtained from all parents. The ethics committee of the I.R.C.C.S. Burlo Garofolo, Trieste, Italy approved the study.
Definition of casesThe rush phase was a hospital based SOTI which was applied to children with a clear history of CM allergy: allergy to CM with a positive oral food challenge test for less than a single dose of 4ml or with a history of recent severe reactions occurring within the previous year and requiring emergency room care, and all with positive specific IgE levels.
Children who had a positive (DBPCFC) or presented with an objective symptom during the course of hospital SOTI were included in the study. Children in this latter group were enrolled without DBPCFC for any or more of the following reasons: a positive oral challenge, a history of recent severe reactions requiring emergency room care within the previous year, specific IgE levels >70kUA/L or refusal of parents to allow the child to undergo a DBPCFC. All the parents of the children discharged after the in-hospital SOTI were instructed to report adverse events by phone or email, and were followed-up via email or a phone call by one of the doctors responsible for SOTI. Eventual emergency room admission or hospital re-admission at the Burlo Garofolo hospital was also recorded.
Home CM increaseEach patient was discharged with written instructions on how to gradually increase the dose of CM. The increase in CM was flexible and could be adapted to the patient's tolerance and symptoms by slowing down the rate of increase or keeping it a fixed dose for weeks or even months at a time, in the case of recurring symptoms.
This increase was adapted to each patient's outcome after the hospital phase. Patients discharged at the end of the in-hospital phase with a single dose of more than 15ml were instructed to increase by 2ml every 2 or 3 days until they reached 60ml; then 5ml every 2 or 3 days up to a single maximum dose of 250ml. Patients discharged with less than 15ml and more than 5ml were instructed to increase the dose by 1ml every 5–7 days up to 30ml; and to continue as above. Patients discharged with a dose of less than 5ml were advised to increase the dose by 0.5ml every 7–10 days. In general, the first CM dose at home was always 2ml less than the last in-hospital dose. This was done in order to provide a wider safety margin and to diminish the risk of reactions either due to the reduced frequency of CM administration (only once or twice daily versus multiple daily doses in the hospital) and/or to the possible delay between the last in-hospital CM dose and the first dose at home (almost all children came from different regions of the country and a few from abroad). Sublingual SOTI was recommended to children who were discharged with a tolerance of less than 2ml of CM. It consisted in keeping 2ml of CM under the tongue for 3min, after which the patient was required to spit out the CM.
From 2001 to 2004, the home phase of CM administration consisted of two daily doses. However, as of 2004, the daily double dose was reduced to one dose in order to simplify the protocol, thus improving the families’ quality of life. The daily amount of CM remained unchanged.
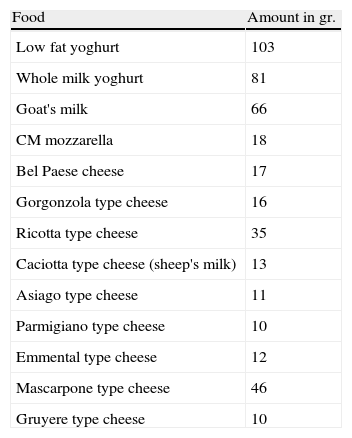
An equivalency table outlining the conversion of CM into cheese and yogurt was provided for the patients, in order to give them the possibility to vary their diet (see Table 1). Due to the significant amount of CM required to convert the CM dose into a small portion of cheese, the patients had to reach 80ml of CM before being able to use this option. Cheese could be used to replace a CM dose or could be added to a smaller CM dose. When the protocol consisted of two daily doses, a 4-h interval was required between them.
Equivalency table to be used in the conversion of CM doses into food doses. 100ml of CM corresponds to.
| Food | Amount in gr. |
| Low fat yoghurt | 103 |
| Whole milk yoghurt | 81 |
| Goat's milk | 66 |
| CM mozzarella | 18 |
| Bel Paese cheese | 17 |
| Gorgonzola type cheese | 16 |
| Ricotta type cheese | 35 |
| Caciotta type cheese (sheep's milk) | 13 |
| Asiago type cheese | 11 |
| Parmigiano type cheese | 10 |
| Emmental type cheese | 12 |
| Mascarpone type cheese | 46 |
| Gruyere type cheese | 10 |
Parents were told to keep the child under observation for 3h following the ingestion of CM and to avoid physical activity during this 3h period. In case of a respiratory infection, they were instructed to decrease the dose of CM by 30% and in the case of gastroenteritis or asthma by 50%, until a complete resolution of the symptoms was seen.
In the case of fever, parents were told to suspend the CM dose for that day. Once the symptoms resolved, they were free to slowly increase the daily dose over a seven-day period until reaching the previous maximum tolerated dose. Patients were instructed to avoid using straws (possible nebulisation effect), to skip a dose in the case of tooth extraction or cuts on the tongue, and to avoid hot showers in the 2h following CM administration. Patients who experienced significant repeated pharyngeal itching or gastric pain were advised to dilute the CM in a substantial amount of fruit juice or soy milk.
Emergency kitThe emergency kit consisted of an automatic epinephrine injector, nebulisation machine (Nebula®, Markos, Italy), spacer for use of beta-2 agonists, supply of drugs needed, written personalised instructions, 24h a day available phone number and e-mail address.
Instructions for parents in the treatment of adverse reactionsAt the time of discharge, parents received oral and written instructions on how to deal with the various reactions associated with the home phase of SOTI. They were trained in how to properly administer the automatic epinephrine injector, and how to use the nebuliser with epinephrine or beta-2 agonists. Parents were given a list of email (for non-urgent communications) and phone contacts (for urgent communications) and encouraged to call with questions or misgivings. All the contacts were doctors with SOTI experience.
The family physician was alerted to the fact that their patient was undergoing the home phase of SOTI and was also given a copy of the discharge report. Local hospitals were not systematically alerted, but each patient had a detailed discharge report to present in the case of reactions requiring hospital admission.
Adverse reactions were classified according to the Clark11 scale which was modified by introducing significant gastric pain as an additional reaction. This was defined as abdominal pain lasting more than 15min, interfering with the child's activities, forcing the child to remain in bed, and associated with an increase in heart rate or pallor. Adverse reactions were treated according to EAACI guidelines12 and the National Asthma and Prevention Program Guidelines13 adapted by means of some arbitrary changes. As a matter of fact, nebulised epinephrine was introduced as a first-line treatment for respiratory reactions and nebulised beclomethasone for mild rhinitis. Acute gastric pain was managed with oral beclomethasone (400mcg), while oral cromolyn (250mg) was administered 30min before each CM dose as prophylaxis for recurrent gastric pain episodes.
Indications for the treatment of home-phase reactions were as follows:
- -
Mild rhinitis and persistent pharyngeal itching: 400mcg of nebulised beclomethasone diluted in 2ml of standard saline solution.
- -
Mild urticaria: patients under 30kg to 5mg oral cetirizine, patients over 30kg to 10mg oral cetirizine.
- -
Coughing, tightness of the chest, rhinitis, wheezing and/or change in voice: 1mg per 10kg to a maximum of 3mg of nebulised epinephrine, followed by nebulised beta-2 agonists in patients with a history of wheezing or with persistent cough or wheezing after epinephrine nebulisation; oral steroids (0.15mg/kg of betamethasone) were also recommended in the case of associated severe urticaria, angio-oedema and persistent respiratory symptoms. A second epinephrine nebulisation after 15–20min was recommended in cases where only a partial response was seen after the initial nebulisation.
- -
Exacerbation of Departmental symptoms despite treatment, severe cyanosis, perception of a very severe crisis, loss of consciousness or collapse: IM epinephrine.
- -
Acute gastric pain: 400mcg oral beclomethasone.
- -
Recurrent gastric pain: 250mg oral cromolyn every day (30min before the ingestion of CM) for approximately one month, at which point the cromolyn was stopped. Diluting the CM in fruit juice or soy milk was also recommended in the cases of recurrent gastric pain.
Nebulised epinephrine was administered using a nebuliser (Nebula® Markos, Italy) at a dose of 0.1mg/kg (maximum dose 3mg) diluted either in 3ml of standard saline solution or 800mcg of beclomethasone. Parents were instructed to use epinephrine to manage any respiratory reaction (dysphonia, inspiratory and/or expiratory shortness of breath, wheezing and coughing). Beginning in 2006, parents were instructed to repeat the nebulised epinephrine dose, after 15–20min, a maximum of two times, in case of unsatisfactory response to the first administration. In 2008, the protocol was modified and nebulised epinephrine is always diluted in 800mcg of beclomethasone to enhance the local anti-inflammatory effect. Other drugs used in addition to epinephrine were: nebulised beta-2 agonists, oral antihistamines and nebulised or oral corticosteroids.
Documentation of in-home reactionsParents were asked to record the reactions occurring during the home phase of SOTI. In order to simplify the recording of the reactions, these were divided into three categories: cutaneous, gastrointestinal and respiratory. Mild and transient gastric pain (lasting less than 15min) and oral itching were considered to be insignificant reactions and parents were not asked to report them, unless they were repeated and disturbed the child.
Hospital readmissionPatients who, for whatever reason, stopped the normal CM increase and were still on fixed low doses after 6–9 months of home phase SOTI, were offered a three day readmission to the hospital in order to attempt a faster and safer CM dose increase in a controlled setting.
Interval between discharge and follow-upA follow-up call or visit was carried out a minimum of two months and a maximum of 84 months after discharge.
Specific IgE trendPatients were encouraged to have yearly blood tests in order to document the specific IgE trends. Specific IgG4 and cytokines were measured in a number of patients as part of a research protocol. Specific IgE and specific IgG4 were measured in all cases of readmission.
Collection of data and definition of resultsFrom the beginning of the study, all the data regarding the reactions reported by parents during the home phase were recorded. Many patients reported multiple reactions but to simplify parent's reporting, the focus was put mainly on treatment and on the main system involved. Starting in 2008, in order to complete the data, parents were contacted by either phone or email. The type and number of reactions, the quantity of CM, the possible triggers provoking the reactions, Emergency Department admissions and hospital readmissions in order to increase the CM dose were all reported. Continuous data were reported as mean and SD or minimum and maximum. Categorical data were reported numerically or as a percentage.
Statistical analysisFor categorical variables, data are presented as numbers and percentages; differences in Departmental outcomes were analysed with Chi square or Fisher exact test (if expected frequencies in contingency tables were less than 5). For continuous variables, data are presented as means and standard deviations; differences in Departmental outcomes were analysed with a non-parametric test (Mann–Whitney test or Wilcoxon test in the case of paired data), as a non-normal distribution of data were shown, both visually and with the Kolmogorov Smirnov test. All analysis was performed using SPSS 11 for Windows.
ResultsStudy populationThe study involved 209 patients with a history of severe CM allergy (high levels of specific IgE, recent systemic reactions, associated risk factors such as the presence of asthma), who were admitted to the Paediatric Department of the Burlo Garofolo hospital, between 2001 and 2008 for SOTI. Since 2008, 140 patients have been contacted; eight patients were inaccessible (due to changed address or phone number), while the others are still to be contacted. Patients were contacted at the beginning with those discharged in 2001.
Of the eight inaccessible patients, four had been discharged with a high dose (greater than 20ml); two with an intermediate dose (from 5 to 20ml); and two with a low dose (less than 5ml). Three out of eight had reported some kind of reaction, yet none required IM epinephrine use. Since, despite repeated attempts, none of them could be contacted to further verify the data, they were excluded from the analysis.
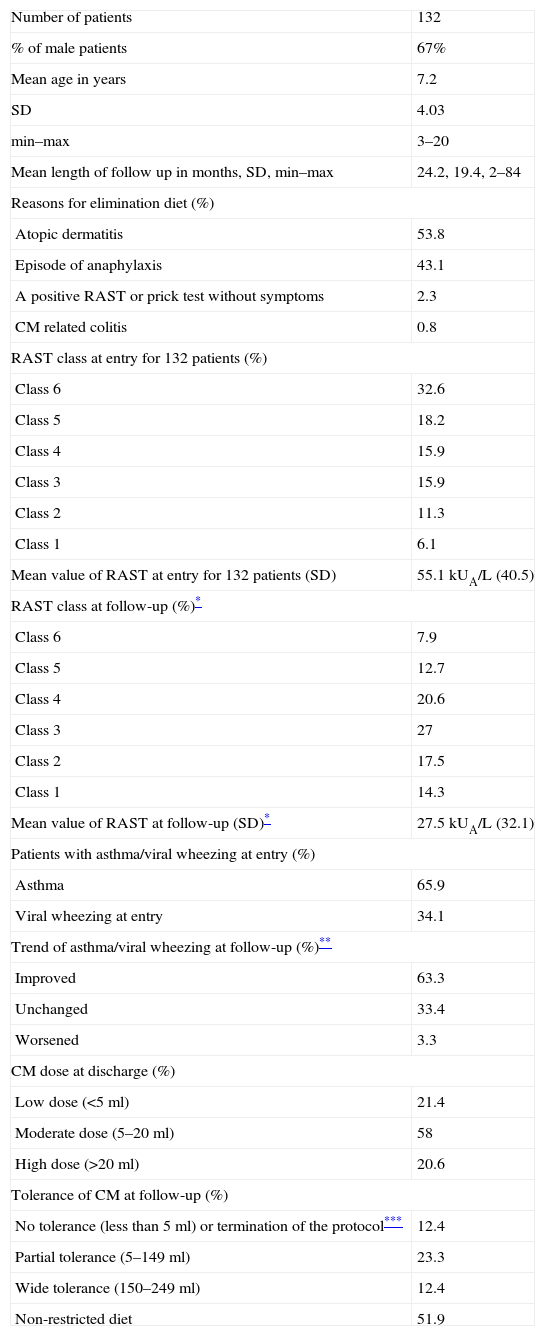
Table 2 outlines the mean age, male prevalence, mean length of follow up, cause of the initial elimination diet, CM RAST levels pre-SOTI and at follow-up, number of patients with asthma/viral wheezing pre-SOTI and at follow-up, and CM dose reached at discharge and at follow-up (see Table 2).
Baseline of patients’ characteristics.
| Number of patients | 132 |
| % of male patients | 67% |
| Mean age in years | 7.2 |
| SD | 4.03 |
| min–max | 3–20 |
| Mean length of follow up in months, SD, min–max | 24.2, 19.4, 2–84 |
| Reasons for elimination diet (%) | |
| Atopic dermatitis | 53.8 |
| Episode of anaphylaxis | 43.1 |
| A positive RAST or prick test without symptoms | 2.3 |
| CM related colitis | 0.8 |
| RAST class at entry for 132 patients (%) | |
| Class 6 | 32.6 |
| Class 5 | 18.2 |
| Class 4 | 15.9 |
| Class 3 | 15.9 |
| Class 2 | 11.3 |
| Class 1 | 6.1 |
| Mean value of RAST at entry for 132 patients (SD) | 55.1kUA/L (40.5) |
| RAST class at follow-up (%)* | |
| Class 6 | 7.9 |
| Class 5 | 12.7 |
| Class 4 | 20.6 |
| Class 3 | 27 |
| Class 2 | 17.5 |
| Class 1 | 14.3 |
| Mean value of RAST at follow-up (SD)* | 27.5kUA/L (32.1) |
| Patients with asthma/viral wheezing at entry (%) | |
| Asthma | 65.9 |
| Viral wheezing at entry | 34.1 |
| Trend of asthma/viral wheezing at follow-up (%)** | |
| Improved | 63.3 |
| Unchanged | 33.4 |
| Worsened | 3.3 |
| CM dose at discharge (%) | |
| Low dose (<5ml) | 21.4 |
| Moderate dose (5–20ml) | 58 |
| High dose (>20ml) | 20.6 |
| Tolerance of CM at follow-up (%) | |
| No tolerance (less than 5ml) or termination of the protocol*** | 12.4 |
| Partial tolerance (5–149ml) | 23.3 |
| Wide tolerance (150–249ml) | 12.4 |
| Non-restricted diet | 51.9 |
Since laboratory could not determine routinely specific IgE values above 100kUA/L RAST classes have been used. For the same reason in the determination of Mean RAST value 100kUA/L is the maximum possible value considered.
The follow-up data were only available for 63 patients. The CM RAST values decreased in 49 of the 63 patients (a reduction of 3 classes in four children, 2 classes in 20 children and 1 class in 25 children). More importantly, there were no increases in the RAST class. Only one child, remained stuck for six months on a single dose of 35ml, and reported an increase in the specific IgE values for whole milk, from 35 to 42kU/L in a one-year period.
The total number of doses (calculating the daily double dose in the first three years) was 89,853; the total number of reported reactions was 891.
Many patients had multiple reactions but to simplify parent's reporting the focus was put mainly on treatment and on the main system involved.
Number of reactions for each patientOf the 132 patients, 48 (36.3%) had no significant reactions (except for oral itching and mild, transient gastric pain that were not recorded), 39 (29.5%) had from one to five reactions, 31 (23.5%) from five to 15 reactions, and 14 (10.7%) had more than 15 reactions.
Percentage of reactionsOut of 891 reactions, 47.7% involved the respiratory tract, 28.8% the gastrointestinal tract, and 23.5% were cutaneous.
Factors triggering reactionsOf the 891 reactions, 28.5% were attributed to unknown causes, 27.4% were caused by an increase in the CM dose, 21.9% were due to physical activity, 17.8% were caused by simultaneous infection (especially upper respiratory tract infection), and 4.4% were due to various reasons (antihistamine treatment withdrawal, pollen season in allergic patients, continuous use of dairy products instead of CM, drinking with a straw (two patients), hot showers, cuts on the tongue (one patient), and vomiting due to car sickness resulting in nasal inhalation (one patient)).
Late onset reactionsOut of 132 patients, eight experienced allergic reactions after more than 3h from CM consumption. Six of these reactions occurred after eating cheese (delayed intestinal absorption could be the likely cause). All were characterised by diffuse urticaria with associated coughing and in two cases, mild wheezing. All the reactions were moderate and none required IM epinephrine.
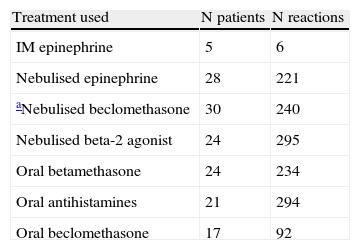
Treatments usedFive patients (with a total of six reactions) were given IM epinephrine, 28 (with a total of 221 reactions) were given nebulised epinephrine, 30 (with a total of 240 reactions) were given nebulised beclomethasone but in most cases this was associated with inhaled epinephrine, 24 (with a total of 295 reactions) were given nebulised beta-2 agonist, 24 (with a total of 234 reactions) were given oral betamethasone, 17 (with a total of 92 reactions) were given oral beclomethasone, and 21 (with a total of 294 reactions) were given oral antihistamines (see Table 3).
Treatment used for reactions. Reactions are given according to the treatments and numbers of reactions.
| Treatment used | N patients | N reactions |
| IM epinephrine | 5 | 6 |
| Nebulised epinephrine | 28 | 221 |
| aNebulised beclomethasone | 30 | 240 |
| Nebulised beta-2 agonist | 24 | 295 |
| Oral betamethasone | 24 | 234 |
| Oral antihistamines | 21 | 294 |
| Oral beclomethasone | 17 | 92 |
IM epinephrine was required on six occasions by five out of 132 patients. Of the five patients, four had a class 6 RAST (the other one had a class 4) and a history of asthma was present in three of the five. Four of the episodes were treated with IM epinephrine as a first-line treatment; two were treated after receiving treatment at home. In one of these episodes, a patient with a cat positive prick test, required IM epinephrine 3h after taking a dose of CM. In this case, the respiratory reaction, presenting with asthma and rhinoconjunctivitis, appeared after contacting with a cat and was treated by emergency response personnel. One patient received two IM epinephrine injections on two different occasions, due to physical activity.
Temporary stops in CM intakeFive patients had to suspend CM intake for few days (minimum three, maximum eight days) due to interfering illnesses (two had gastroenteritis with dehydration, one contracted Kawasaki disease, one developed appendicitis, and the last was involved in a car crash and sustained significant injuries). All restarted uneventfully with a lower dose (10–20% of the previously tolerated one).
Local Emergency Department admissionsFifteen out of 132 patients required 17 admissions to their local Emergency Department (one patient was admitted three times) due to the severity of the reactions. Twelve out of 15 patents were admitted despite receiving treatment at home, two were treated with IM epinephrine. None were admitted to the intensive care unit.
Readmissions to Burlo Garofolo hospitalOut of 132 patients, 22 (16.7%) required hospital readmission some months after discharge and two children (1.5%) required two separate readmissions. These patients remained on a small dose, ranging from 3 to 35ml of CM. They all demonstrated a significant specific IgE decrease with elevation of specific IgG 4 levels. Nineteen of the 22 patients were able to double their CM dose in just three days.
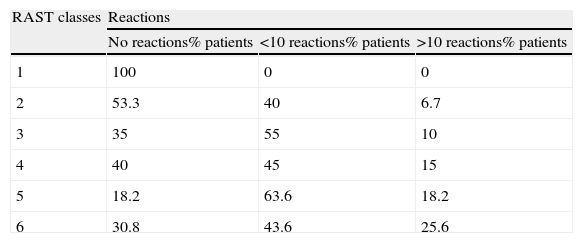
Correlation between IgE levels and home reactionsThere was a linear correlation between IgE levels and the number of home reactions. In fact, the higher the IgE class, the greater the risk of reactions is (see Table 4).
Correlation between IgE levels and number of home reactions.
| RAST classes | Reactions | ||
| No reactions% patients | <10 reactions% patients | >10 reactions% patients | |
| 1 | 100 | 0 | 0 |
| 2 | 53.3 | 40 | 6.7 |
| 3 | 35 | 55 | 10 |
| 4 | 40 | 45 | 15 |
| 5 | 18.2 | 63.6 | 18.2 |
| 6 | 30.8 | 43.6 | 25.6 |
p=0.003 difference between number of reactions in RAST classes 1–3 compared with 4–6.
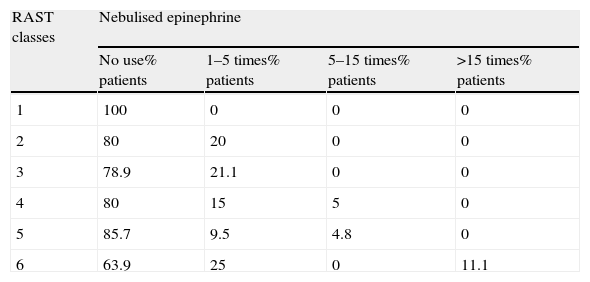
There was a linear correlation between IgE levels and the use of nebulised epinephrine for the treatment of respiratory reactions during the home phase. In fact, the higher the IgE class, the greater the risk is of using nebulised epinephrine in treatment of respiratory reactions (see Table 5).
Correlation between IgE levels and use of nebulised epinephrine.
| RAST classes | Nebulised epinephrine | |||
| No use% patients | 1–5 times% patients | 5–15 times% patients | >15 times% patients | |
| 1 | 100 | 0 | 0 | 0 |
| 2 | 80 | 20 | 0 | 0 |
| 3 | 78.9 | 21.1 | 0 | 0 |
| 4 | 80 | 15 | 5 | 0 |
| 5 | 85.7 | 9.5 | 4.8 | 0 |
| 6 | 63.9 | 25 | 0 | 11.1 |
p=0.014 difference between number of reactions treated with nebulised epinephrine in RAST classes 1–3 compared with 4–6.
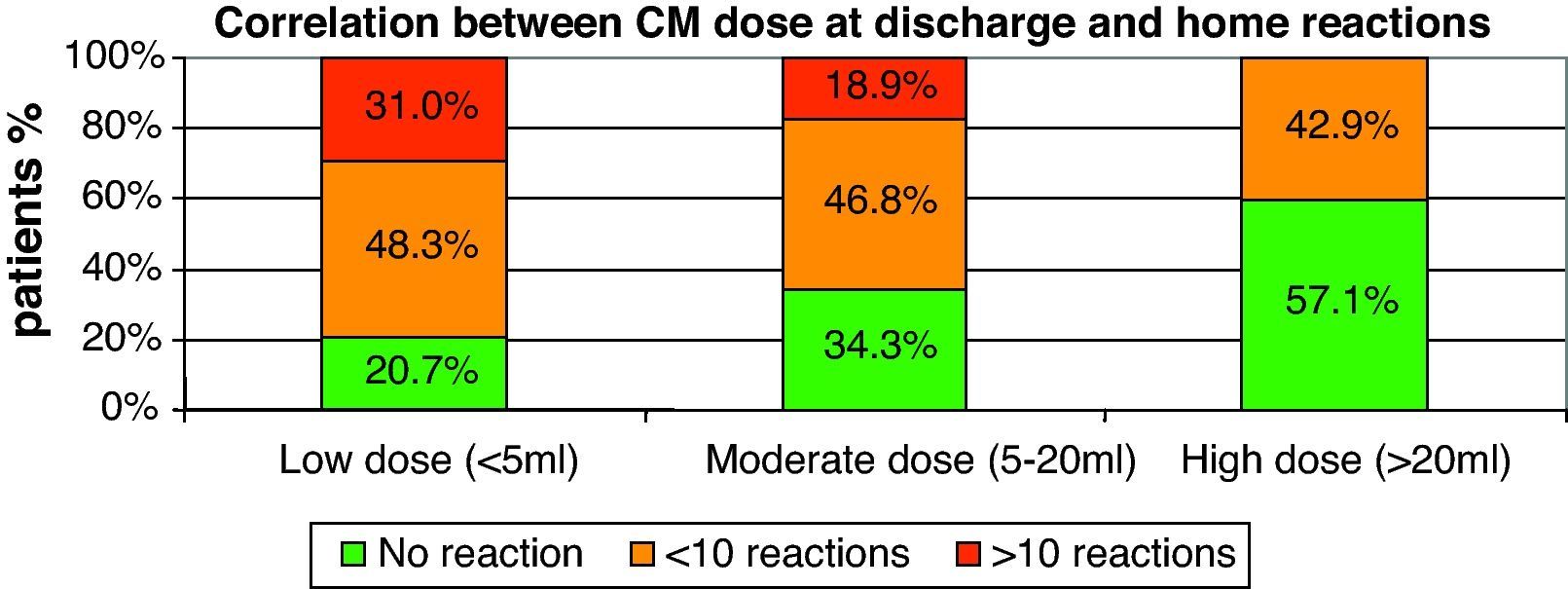
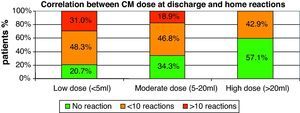
There was also a linear correlation between the amount of CM at discharge and the number of home reactions (p=0.003). The smaller the CM dose at discharge, the greater the possibility of a reaction is (see Fig. 1).
Correlation between CM amount at discharge and use of nebulised epinephrineThere was a linear correlation between the amount of CM reached at discharge and the use of nebulised epinephrine in the treatment of home-phase respiratory reactions. The lower the CM dose, the higher the risk is of requiring nebulised epinephrine for home use (p=0.036).
Correlation between presence of asthma and/or viral wheezing and home-phase reactionsThere was no significant correlation between the presence of asthma and/or viral wheezing, and the total number of home-phase reactions or the reactions requiring nebulised epinephrine.
Correlation between age and home-phase reactionsNo correlation was found between the patient's age and the number of home-phase reactions.
Timing of reactions in relation to SOTIOn average, the majority of reactions occurred in the first two years of SOTI and eventually decreased in frequency. Subsequent infrequent reactions after the two-year mark were usually triggered by gastrointestinal infections.
Sublingual SOTIFour children who could not tolerate CM ingestion were treated with sublingual CM administration. All had tolerated less than 2ml at the end of SOTI and were instructed to keep 2ml of CM under the tongue for 3min and then spit it out. All were evaluated with an open challenge after 9–12 months. Two of them experienced reactions at a dose lower than 2ml and stopped SOTI; two were able to tolerate higher amounts (6 and 8ml each) and subsequently moved on to the oral SOTI protocol.
DiscussionThe home phase of the Burlo Garofolo SOTI protocol was characterised by a significant number of adverse reactions. However, the great majority of these reactions were managed with an acceptable rate of side effects, even in children with very high levels of specific IgE and a history of recent severe reactions.
The literature regarding the adverse reactions during the home phase of SOTI is still limited and represented by studies with small sample sizes, and patients with relatively low RAST values.
Staden et al.14 used SOTI to treat 25 children with CM and egg allergies. All 25 patients (mean RAST of 10kUA/L) had mild reactions, and four had moderate reactions that were only treated with antihistamines and steroids. In this study the main triggers causing the reactions were: infection, physical activity, pollen allergies and irregular intake of CM. They found that a reduction in the CM dose always prevented side effects. Meglio et al.6 treated 21 children with CM allergy (mean RAST of 17.1kUA/L for whole milk). The treatment was successful in 71% of the patients and the reactions were mild requiring only antihistamines.
Skripak et al.15 treated with IM epinephrine during the home phase 2 out of 13 children (mean RAST of 34.8kUA/L).
Patriarca et al.2 treated 59 patients (mean RAST of 32kUA/L) with a success rate of 83%. None of the patients experienced any significant side effects, and did not require epinephrine administration or hospitalisation. Narisety et al.16 treated 25 children with a mean RAST of 29.9kUA/L, who were discharged after an initial introduction of CM in the hospital. Out of 2465 reported administrations of milk, there were 419 mild reactions, 90 gastrointestinal reactions and 21 respiratory reactions. In this study, five children received IM epinephrine injections. None of the aforementioned studies reported the use of nebulised epinephrine as a first-line treatment.
This protocol reveals that nebulised epinephrine can play a pivotal role in the management of these patients.
In the International Guidelines,12 nebulised epinephrine has only recently been introduced as a second line treatment, following IM injection for persistent respiratory symptoms. However, it is well known that respiratory symptoms during anaphylaxis are more prevalent than systemic symptoms in children, such as hypotension that is typical of adults.17 Therefore, it is reasonable to hypothesize that, even with a low level of epinephrine in the blood, the local anti-oedema action and the alfa 1-adrenergic effect provided by nebulisation play a major role in the control of symptoms and may arrest the negative chain of respiratory events. The positive effects of nebulised epinephrine were outlined by Hourihane and Warner18 who reported that in 20 years of practice the use of IM epinephrine was replaced by nebulised epinephrine. In their study Simons and Estelle19,20 have shown that most children are unable to inhale epinephrine from a pre-measured dosage nebuliser. However, a thorough search of the literature has not revealed evidence comparing continuous nebulisation to pre-measured dosages.
The data presented in this article require cautious interpretation and should not be transferred to any other study or taken out of context. Actually all the reactions occurred at home and were managed by trained parents. Nebulised epinephrine should only be used in cases of provoked anaphylaxis, as the event is expected and the epinephrine is ready to be used. Nebulised epinephrine should not be used to replace IM epinephrine in the case of spontaneous anaphylaxis.
This report outlines the progress of 132 children with a mean RAST of 55.1kUA/L, who were discharged after the 10-day in-hospital rush phase. During the home-phase, the patients received 89,853 administrations of CM resulting in: 256 gastrointestinal reactions, 425 respiratory reactions and 204 cutaneous reactions. Reactions were relatively frequent (one reaction per 100 CM doses), only five children required six IM epinephrine injections.
The most significant differences between this study and the others are the large number of children with RAST values greater than 100kUA/L (43 patients) and the length of time between the end of the hospital SOTI phase and the follow-up. This study involved the largest sample size documented in the literature, with the longest follow-up and the highest number of patients with high specific IgE levels (over 100kU/L). Children with high IgE levels are the patients that require SOTI the most, due to the low probability of spontaneous acquisition of tolerance and to the higher risk of severe reaction, as demonstrated by the data.
A large percentage of the reactions were trigged by specific factors, such as infections, physical activity and CM dose increase, but a significant part of the reactions was due to unknown causes. As a result, they were in essence unpredictable and unexplainable.
Late onset reactions occurring 3h or more after ingesting CM were rare but reported. Most of these reactions occurred late in the evening after eating cheese. In this case, reactions may be attributed to delayed intestinal absorption. More data are required in order to better understand this subset of reactions. This issue is alarming because parents following the SOTI protocol at home are instructed to keep their child under observation for only 3h after CM intake. The observation period may need to be extended when CM is being replaced by cheese or yogurt.
The use of the equivalency table in replacing CM with either yogurt or cheese turned out to be quite convenient and well accepted by patients, since CM aversion is a problem for some of these children. In few cases, there appeared to be an increased risk of reaction when the patient began ingesting CM again for the first time after a long period of eating cheese or yogurt. This is probably due to differences in antigenic composition between CM and dairy products and for this reason, it may help to alternate dairy products with CM every third day.
An important trend noted was the reduction in the frequency of reactions as the CM dose was increased. However, in a few cases, some significant reactions were experienced by patients who had arrived at a CM dose of 250ml and who had already been using the SOTI protocol for up to two years.
Our data require cautious interpretation and should not be transferred to other contexts. All the reactions occurred in a controlled environment (patient's home), with parents who kept the children under close observation for 3h and were able to administer the appropriate treatment for the reaction. It is extremely important to remember that nebulised epinephrine should not be used in the case of spontaneous anaphylaxis as a replacement for IM epinephrine.
Detailed treatment instructions, a list of emergency phone numbers and non-urgent email contacts were the key to the success of the home-phase protocol.
Children who experienced repeated reactions at a low dose of CM were maintained on a fixed dose for a few months. They were then readmitted to the hospital for two to three days in order to increase the CM dose in a safe and controlled setting. This strategy was successful with the majority of these patients and played a pivotal role in the success of SOTI in this subgroup of patients.
As expected, high IgE levels were the most significant risk factor predicting the adverse home-phase reactions. The other significant risk factor for home reactions was a low CM dose at discharge, which implied frequent and significant reactions during the in-hospital induction phase. In summary, patients with high IgE levels, frequent and significant reactions during the induction phase, lower CM dose at discharge and frequent infections (depending on age) are expected to be most at risk of home reactions.
SOTI failed to improve the condition in 12% of patients for a variety of reasons, including repeated significant reactions, unresponsive gastric pain, refusal by the patient to ingest CM, or severe parental anxiety related to CM intake. Unsurprisingly, four out of the five patients which without the continuous needed IM epinephrine stopped the protocol. The issue of quality of life is of particular relevance to these patients. Most of them are not eager to consume large amounts of dairy products and an unrestricted diet is a goal for a minority of patients only. However, everyone's main goal is to live with the fear of a reaction caused by accidental contact with the antigen. The stress related to CM intake, during the SOTI protocol, and its adverse effects can be a significant burden on the patients and their families. Even the simple fact of avoiding exercise every day for 3h after CM intake can be a significant limitation for any child or adolescent. For this reason it is important to bear in mind that the patient's quality of life should not be compromised to reach the maximum tolerated dose of CM at any cost. This study has some limitations: since slight reactions were not reported by parents, the number of significant reactions may have been underestimated due to incomplete or under-reporting. Further limitations are due to the fact that phone or email contacts were not scheduled at regular intervals. Lastly, the total number of SOTI doses was estimated based on the collected data. However, the possibility of missed doses was taken into account, as it was an occurrence that was systematically reported by the parents.
In conclusion the data presented in this article have been the result of a long-lasting and well-established experience and allow a reasonable definition of the risk related to SOTI both in hospital and at home.
Strict food avoidance is still the only current treatment of paediatric IgE-mediated food allergy. In achieving oral tolerance, SOTI appears to be the strategy that offers most chance of alternative cure, but it cannot be recommended for routine practice for the doubts concerning its effectiveness and safety.
This study adds new data about SOTI side effects and suggests that SOTI may be applied in the near future also outside research protocols by dedicated and well-trained staff.
Box 1 A recent case of very troublesome SOTI
The story of Nerea, the daughter of an Internal Medicine Specialist, begins at four months of age, when she was admitted to the ICU for laryngeal oedema and angio-oedema after the ingestion of milk formula. She had a class 6 RAST for CM so was put on an elimination diet. The most serious episode occurred at six years of age, when, after an accidental intake of bread contaminated with CM, she presented with cough, angio-oedema (swelling of the lips and body) and severe wheezing initially treated with IM epinephrine. Nerea was also allergic to egg, some types of meat and fish, all resulting in reactions before the age of 12 months. Nerea also had a history of asthma that started abruptly at the age of 18 months requiring ICU admission for severe dyspnoea. At the age of seven years, in 2008, she was admitted for SOTI at Burlo Garofolo Trieste, Italy. RAST class for CM was >100kUA/L. A RAST dilution for casein showed an absolute value of 403kUA/L. During the 10-day hospital phase Nerea presented with two episodes of gastric pain (treated with oral beclomethasone) and one episode of significant urticaria and mild wheezing (treated with nebulised epinephrine). At discharge Nerea tolerated 32ml of pure CM. Upon reaching a dose of 62ml she suffered from an upper respiratory infection resulting in a dramatic drop of her CM dose (down to 30ml). Over the next few months, attempts were made to return to the dose of 62ml. However, she continuously presented with bronchospasm and urticaria at every increase of CM dose. The high frequency of reactions, especially related to upper respiratory infections forced her back down to a dose of 5ml. Slowly she was able to tolerate 45ml before her scheduled hospitalisation at Burlo Garofolo. At that point her RAST had decreased to 88.4kUA/L (class 5) with a rise in specific IgG4 (50.1mgA/L, normal value <5.6mgA/L). During hospitalisation the CM dose was increased to 100ml. Once at home, Nerea quickly reached a dose of 250ml with only occasional episodes of gastric pain and urticaria. After two months, she suffered from a respiratory viral infection with gastrointestinal symptoms forcing her to stop CM ingestion for two days. She started again from 20ml on the third day and due to the persistence of gastrointestinal symptoms her CM dose was decreased to 5ml per day and she was taking 250mg of oral sodium cromoglycate daily, 30min before CM intake. She had a very difficult winter with repeated infectious episodes (including influenza A) and repeated gastrointestinal and respiratory reactions. From the late spring of the second year of treatment, she started to increase the dose reaching a 200ml CM dose during the summer. At present, after 28 months of treatment, she is eating ice cream and 200ml of CM daily without symptoms. Her asthma has been perfectly controlled during all this time, with no exacerbations taking place during viral respiratory infections. During the home-phase the total number of reactions was 70. This is the perception of the quality of life as reported by the family: “Our life has totally changed. Before SOTI, Nerea had frequent reactions, and she could not attend birthday parties or restaurants. All her reactions to inhaled CM disappeared. All the stress caused by SOTI-related reactions has been rewarded since she now faces a future with no limitations.”
This study was performed without any funding or grants. All the authors declare that they have no conflict of interest and do not have any financial relationships with any biotechnology and/or pharmaceutical manufacturers.
We thank Natalie Leone for revising the text.