To explore in the daily clinical practice setting that antimuscarinic, fesoterodine or solifenacin, provides a greater clinical benefit after changing the prior overactive bladder (OAB) therapy with tolterodine extended release (ER) to other novel antimuscarinic agents.
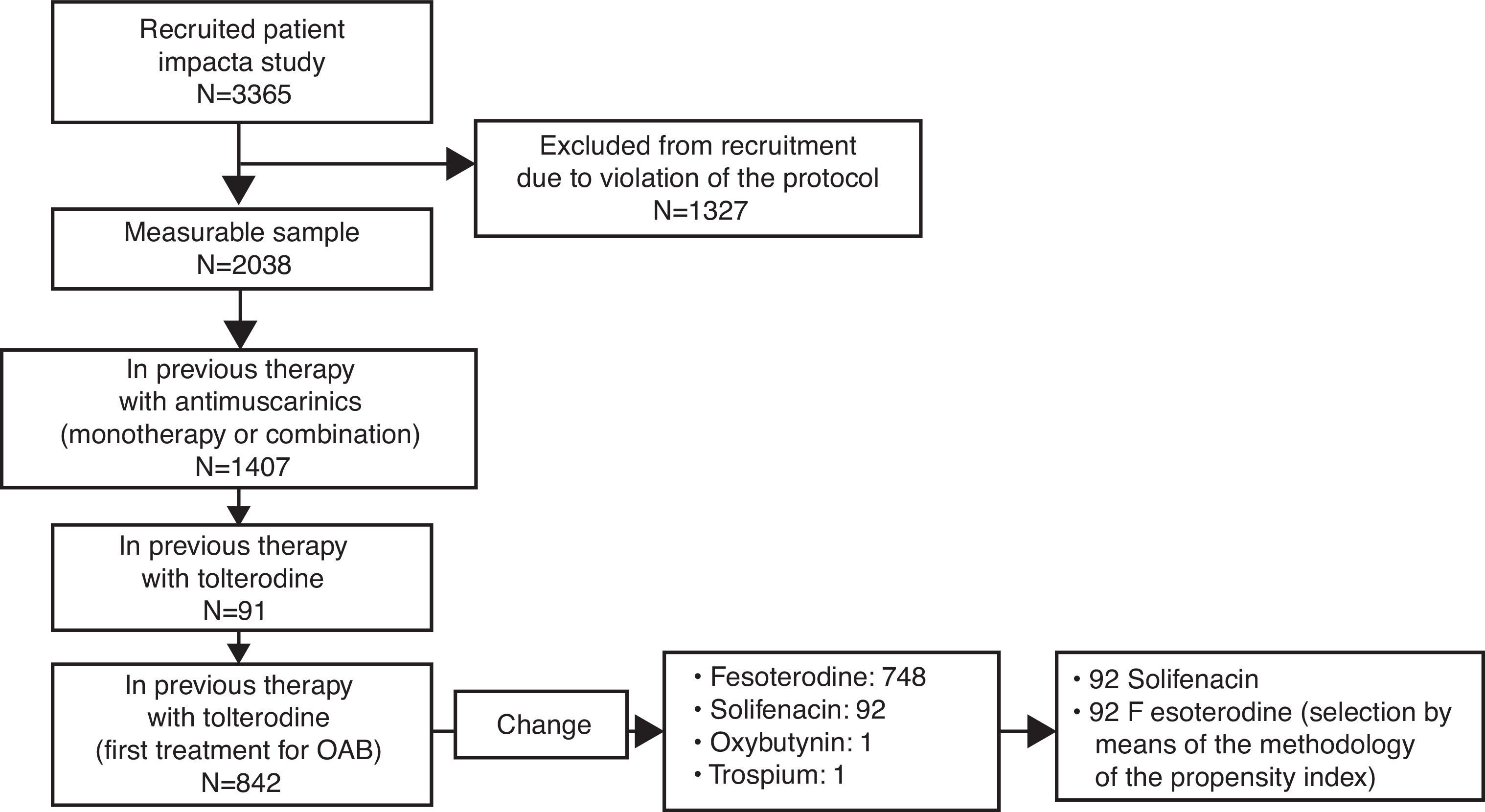
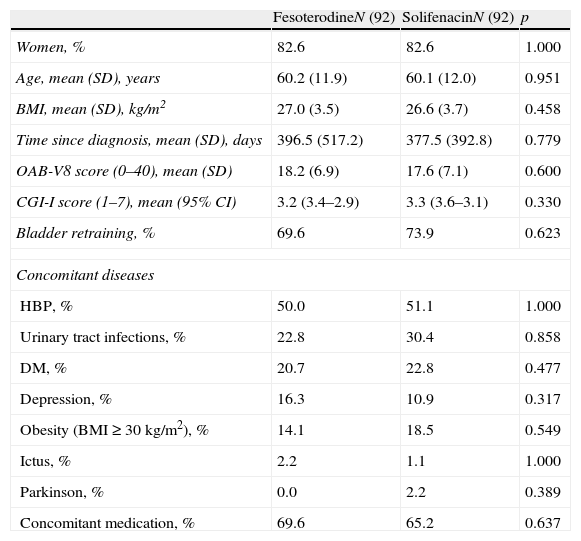
Material and methodsA post hoc analysis of data from an observational multicenter, cross-sectional, retrospective study. Adult patients of both sexes, with OAB and OAB-V8 score≥8, who switched to fesoterodine or solifenacin within the 3–4 months before study visit from their prior tolterodine-ER-based therapy due to poor response were included. 92 patients were selected for each treatment group, matched (1:1) according to conditioned probability using the propensity score. Benefit of treatment change perceived by the physician and patient was evaluated by means of the Clinical Global Impression of Improvement subscale (CGI-I) and Treatment Benefit Scale (TBS), respectively. Degree of worry, bother and interference with daily living activities due to urinary symptoms, level of satisfaction, and preference for current treatment were also assessed.
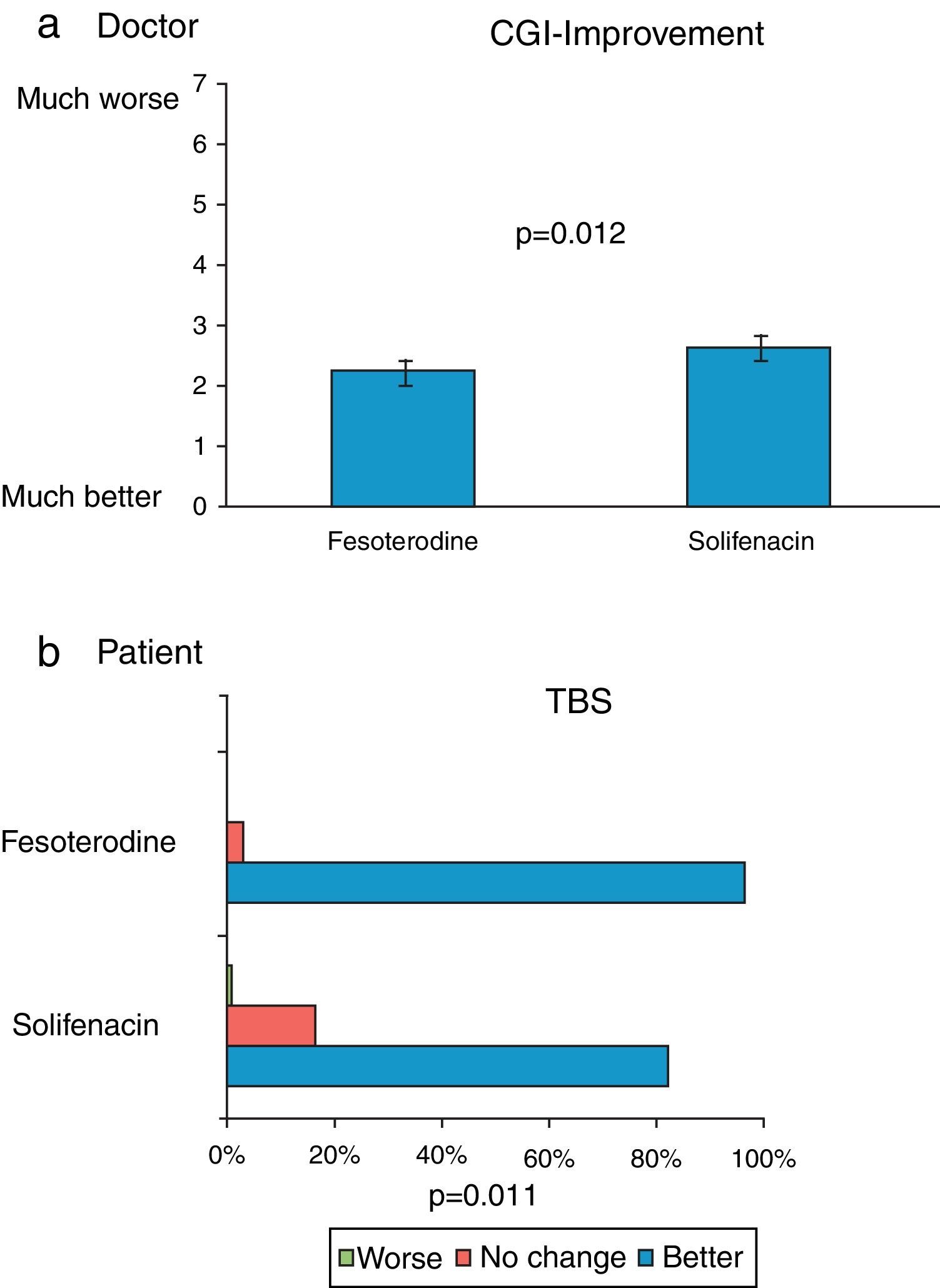
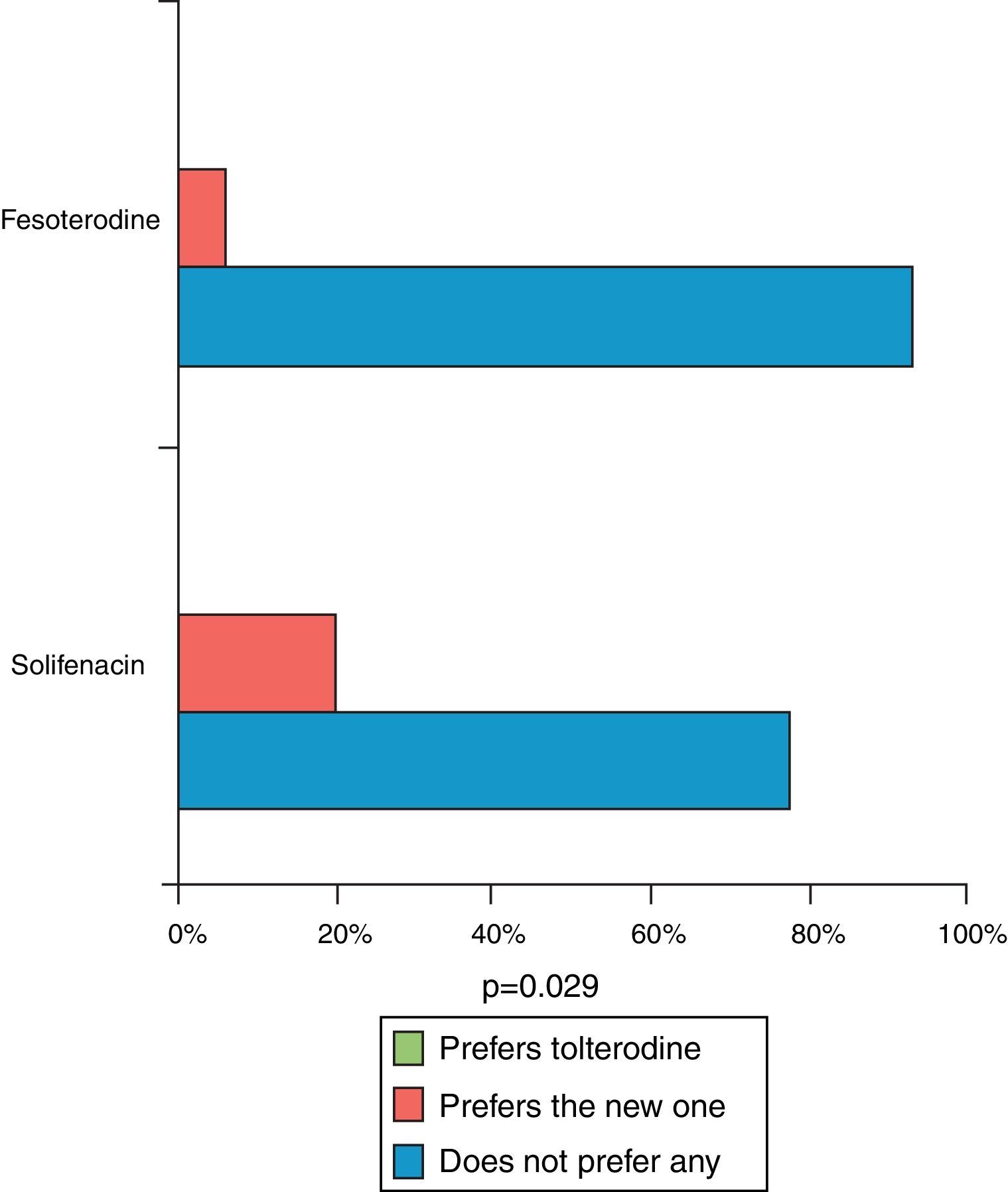
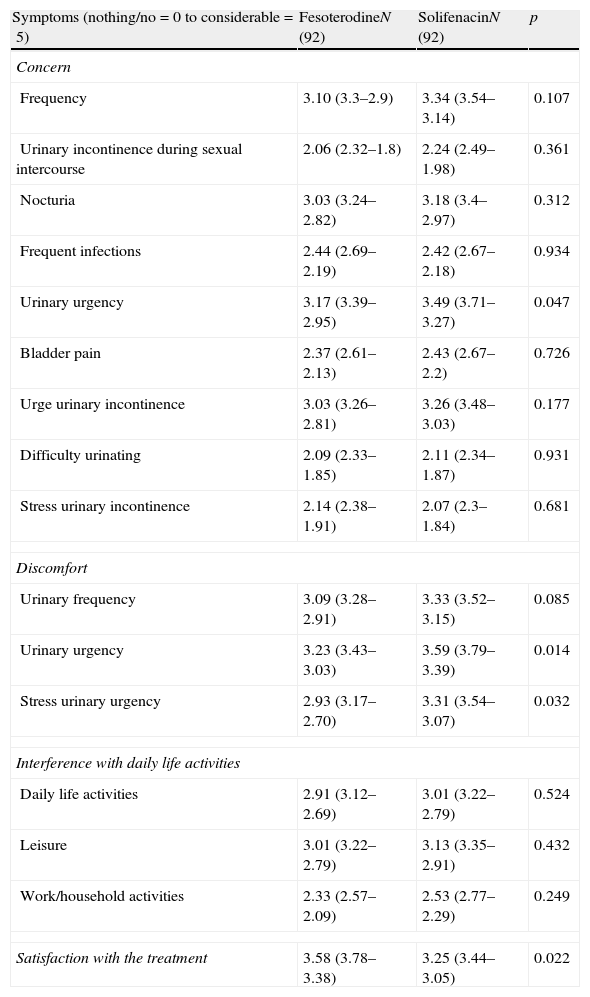
ResultsFesoterodine provided a significantly greater improvement than solifenacin in terms of therapeutic benefit perceived by the physician according to ICG-I. 96.7% of the patients on fesoterodine treatment vs. 81.6% of the solifenacin group showed a score of improvement in TBS (p<0.05). Fesoterodine was also better rated than solifenacin with regard to satisfaction and preference for the new treatment (93.4 vs. 78.2% p<0.05).
ConclusionsIn daily clinical practice the switch from tolterodine LP to fesoterodine seems to provide greater benefits both from the physician's and the patient's point of view compared with those provided by solifenacin.
Explorar en la práctica clínica diaria el beneficio clínico y del paciente conseguido tras cambiar su primer tratamiento para la vejiga hiperactiva (VH) con tolterodina de liberación prolongada (LP), por otro antimuscaríninico de última generación.
Materiales y métodosAnálisis post hoc de un estudio observacional, multicéntrico retrospectivo y transversal. Se incluyeron pacientes adultos de ambos sexos, con VH y puntuación OAB-V8≥8, con respuesta insuficiente al tratamiento previo con tolterodina LP sustituido por fesoterodina o solifenacina en los 3–4 meses previos. Se seleccionaron 92 pacientes para cada grupo de tratamiento, emparejados (1:1) según probabilidad condicionada utilizando el propensity score. Se valoraron el beneficio del cambio percibido por el médico y el paciente mediante las escalas de Impresión clínica global de mejoría (ICG-M) y del Beneficio del tratamiento (TBS) respectivamente. También se analizaron el grado de preocupación, la molestia y el impacto en la vida diaria de la VH, el grado de satisfacción y la preferencia por la medicación actual.
ResultadosFesoterodina proporcionó una mejora significativamente mayor que solifenacina en cuanto a beneficio terapéutico percibido por el médico según la ICG-M. El 96,7% de los pacientes tratados con fesoterodina vs. 81,6% con solifenacina mostraron una puntuación de mejoría en la TBS (p<0,05). La fesoterodina también resultó mejor valorada que la solifenacina en cuanto a la satisfacción y preferencia por el nuevo tratamiento (93,4 vs. 78,2%, p<0,05).
ConclusionesEn la práctica clínica diaria el cambio de tolterodina LP a fesoterodina parece proporcionar mayores beneficios tanto desde el punto de vista del médico como del paciente, comparado con el que aporta solifenacina.