To describe the results obtained in 25 men with metastatic castration-resistant prostate cancer (MCRPC) treated with abiraterone (AA). A comparative analysis of abiraterone effectiveness and safety between our results and data published in the literature was conducted.
Materials and methodBi-institutional prospective analysis of 25 consecutive patients with MCRPC undergoing treatment with abiraterone, with a mean follow-up 7.9 (3–15) months was carried out. Treatment effectiveness and safety analyses regarding baseline characteristics of patients (age, prior treatments, basal PSA, performance status, pain, and metastasis) were conducted.
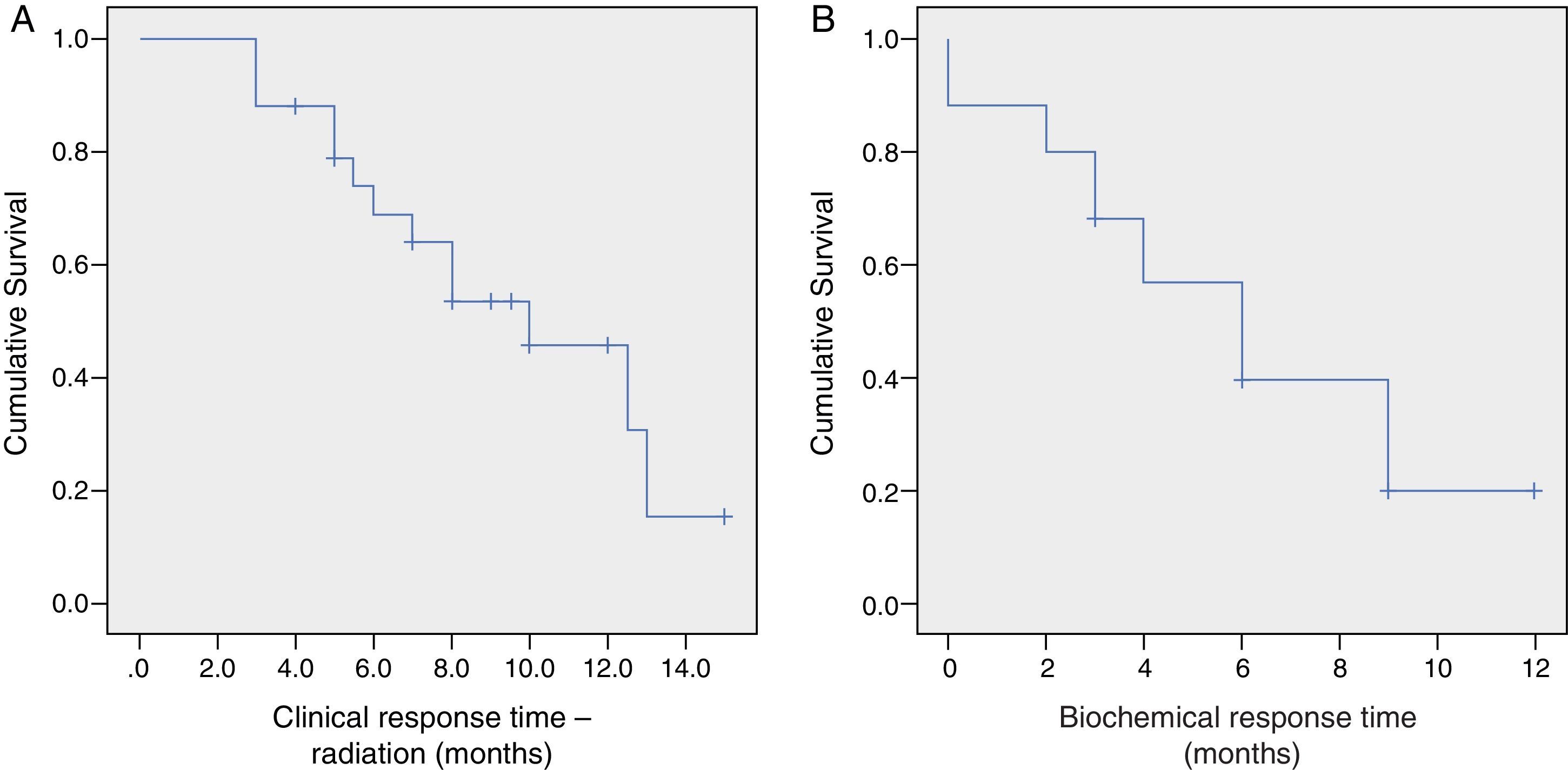
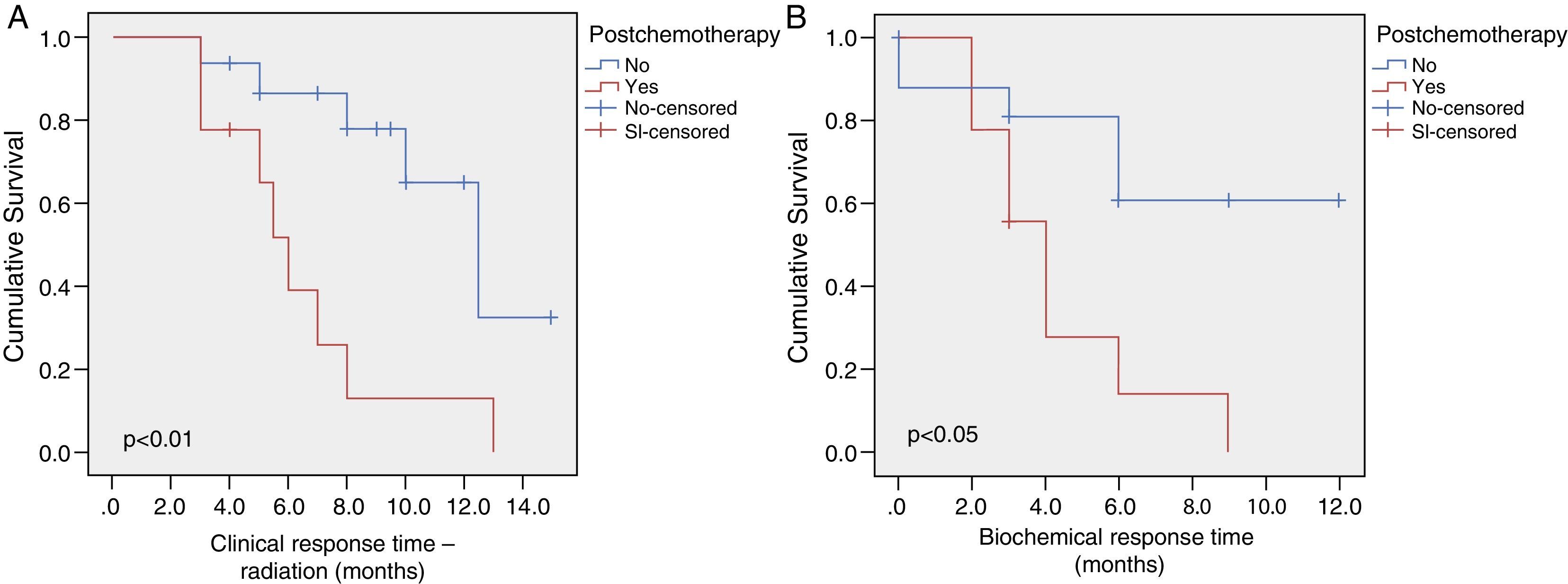
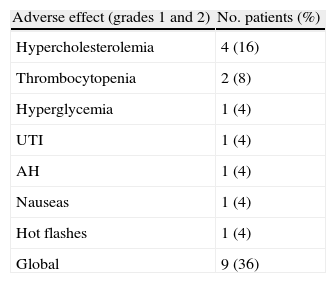
ResultsAt 13.6 months of follow-up, the overall survival is 80% (CI 95%: 11.8–15.4). Clinical and radiological-free progression survival is 9.5±1 months (CI 95%: 7.7–11.3) and biochemical response is 6.8±1 months (CI 95%: 5–8.7). Only the treatment with chemotherapy impaired significantly the response time to AA [6.4 months for radiological-free progression survival (CI 95%: 4.2–8.6) and 4.3 months for biochemical-free progression survival (CI 95%: 2.6–6)]. The incidence of adverse drug events was 36%; all of them were of grade 1–2/4 and, in no case, suspension or reduction of the dose of AA was needed.
ConclusionsThe treatment with AA has been effective in our series, with a tolerability considerably higher than what other studies published.
Describir los resultados obtenidos de la experiencia en el tratamiento con acetato de abiraterona (AA) en 25 hombres con cáncer de próstata metastásico resistente a la castración (CPMRC). Realizamos el análisis comparativo de la eficacia y seguridad de este fármaco en relación con la literatura existente.
Material y métodoEstudio biinstitucional prospectivo de una cohorte de 25 pacientes consecutivos que reciben tratamiento con AA por CPMRC, con un seguimiento medio 7,9 (3-15) meses. Análisis de la seguridad y eficacia del tratamiento en relación con las características basales de los pacientes (edad, tratamientos previos, PSA basal, performance status, dolor, metástasis).
ResultadosLa supervivencia global es del 80% a los 13,6 meses de seguimiento (IC 95%: 11,8-15,4). La supervivencia libre de progresión clínico-radiológica de la serie es de 9,5±1 meses (IC 95%: 7,7-11,3) y el de respuesta bioquímica de 6,8±1 meses (IC 95%: 5-8,7). Solo el tratamiento previo con quimioterapia empeora significativamente el tiempo de respuesta a AA (supervivencia libre de progresión radiológica 6,4 meses [IC 95%: 4,2-8,6] y bioquímica de 4,3 meses [IC 95%: 2,6-6]). La incidencia de efectos adversos fue del 36%, todos grado 1-2/4, y en ningún caso requiere suspender o disminuir la dosis de AA.
ConclusionesEl tratamiento con AA ha sido eficaz en nuestra serie, con una tolerabilidad considerablemente mayor a lo publicado en otros estudios.