Invasive fungal disease (IFD) treatment is challenging in hematologic patients due to drug interactions and toxicities that limit the use of the antifungal agents.
AimsTo analyze retrospectively in terms of safety and potential efficacy anidulafungin therapy, alone or in combination.
MethodsOur institutional guidelines recommended anidulafungin treatment in hematologic patients with suspected IFD and concomitant renal or liver impairment (to avoid drug interactions and preserve organ function).
ResultsFrom 2008 to 2013, 24 episodes of IFD occurring in 21 patients were classified as proven (4 cases), probable (15 cases) and possible (5 cases). Anidulafungin was administered alone (13%) or in combination (88%). Eight (33%) episodes were resolved, using monotherapy (1 out of 3, 33%) or a combined therapy (7 out of 21, 33%). Twelve cases (50%) were registered as failure (death due to IFD progression in 4 patients, and treatment change due to lack of efficacy in 8), and 4 cases (17%) were not evaluable (death unrelated to the IFD). Anidulafungin was not withdrawn in any case due to toxicity.
ConclusionsAnidulafungin therapy, alone or in combination, could be considered in hematologic patients with IFD and concomitant liver or renal impairment. Due to the low number of patients, we cannot draw any conclusion about efficacy.
El tratamiento de una infección fúngica invasiva (IFI) supone un importante desafío en los pacientes hematológicos debido a las interacciones farmacológicas y a la toxicidad de los agentes antifúngicos, que restringen su uso.
ObjetivosAnalizar de forma retrospectiva el tratamiento con anidulafungina, sola o combinada, en términos de su seguridad y posible eficacia.
MétodosEn los pacientes hematológicos con sospecha de IFI e insuficiencia renal o hepática concomitante, las guías clínicas de nuestro entorno recomendaban el tratamiento con anidulafungina (para evitar las interacciones farmacológicas y preservar la función orgánica).
ResultadosDe 2008 a 2013 se documentaron 24 episodios de IFI en 21 pacientes, que se clasificaron como IFI demostrada (4 casos), IFI probable (15 casos) e IFI posible (5 casos). Se administró anidulafungina como monoterapia (13%) y en combinación (88%). Se resolvieron 8 episodios (33%), 1 caso de 3 tratados con monoterapia (33%) y 7 casos de 21 tratados con terapia combinada, (33%). En 12 casos (50%), el tratamiento fracasó (muerte por progresión de la IFI en 4 pacientes y cambio de tratamiento por falta de eficacia en 8). Por último, 4 casos (17%) no se pudieron evaluar (muerte no relacionada con IFI). En ningún caso se retiró el tratamiento con anidulafungina por toxicidad.
ConclusionesEl tratamiento con anidulafungina, sola o combinada, podría considerarse apropiado para pacientes hematológicos con IFI e insuficiencia hepática o renal concomitante. Debido al reducido número de pacientes incluidos, no es posible extraer conclusiones respecto a la eficacia.
Invasive fungal disease (IFD) is a frequent and life-threatening complication in patients with hematologic diseases receiving immunosuppressive therapies like intensive chemotherapy or allogeneic stem cell transplantation (Allo-SCT).5,6 In spite of the availability of several efficacious antifungal agents, the management of IFD in this population remains challenging due to common drug interactions and toxicities that may limit the use of such agents.3,7
Anidulafungin is an echinochandin with in vitro activity against Candida (fungicidal) and Aspergillus (fungistatic) species and has not hepatic metabolism (it does not interact significantly with the cytochrome P450 isoenzymes) or renal elimination. Thus, this antifungal agent may be useful to treat IFD episodes in the setting of patients with hematologic malignancies and concomitant liver or renal dysfunction. However, anidulafungin has no indication to treat aspergillosis and has only demonstrated clinical benefits in non-neutropenic patients with invasive candidiasis.1,8 As far as we know, there is very limited information regarding the efficacy and safety of anidulafungin in the setting of hematologic patients developing IFD.4,9
The aim of this study is to analyze the safety profile of anidulafungin therapy in a series of 24 consecutive IFD episodes developed in patients with hematologic diseases and concomitant renal and/or liver impairment following chemotherapy regimens or Allo-SCT in a single institution. Although the study comprises a very limited number of patients with heterogeneous management and situations, we also aim to analyze, without drawing any conclusion, the efficacy of anidulafungin (alone or in combination with other agents) in this setting.
Material and methodsPatient selection and study designSince September 2008, the Hematology Service internal guidelines for the management of IFD episodes recommended, in an attempt to preserve liver and renal function in severely ill hematologic patients with IFD, anidulafungin therapy in adult patients with renal or hepatic impairment potentially limiting the administration of other antifungal agents. All IFD episodes were classified according to European Organization for Research and Treatment of Cancer and Mycoses Study Group (EORTC/MSG) revised definitions of 2008.2 Consecutive patients with hematologic diseases and IFD that received anidulafungin for at least two days were eligible. In addition, all eligible patients received chemotherapy or Allo-SCT. The clinical records of these patients were retrospectively revised and data collected in a specific form. This retrospective study was approved by the Research Ethics Board of the institution (study number 2013/0036).
Prophylaxis and management of IFD episodesAntifungal prophylaxis consisted of oral or intravenous fluconazole (100mg once a day) or itraconazole (200mg once a day) for patients receiving chemotherapy, and oral or intravenous voriconazole (100mg twice a day or 200mg once a day) for recipients of an Allo-SCT. Empirical antifungal therapy using caspofungin (70mg on the 1st day followed by 50mg once a day) was generally instituted in neutropenic patients with 5–7 days persistent fever. First line therapy for IFD episodes generally consisted of caspofungin or liposomal amphotericin (3mg/kg once a day). When the diagnosis of possible, probable or proven IFD was made, the combination of two antifungal agents (usually containing intravenous voriconazole 4mg/kg twice a day) was instituted at physician discretion. Start of anidulafungin (200mg 1st day followed by 100mg once a day), alone or in combination with other antifungal agent, was recommended in patients with hepatic and/or renal impairment limiting the use of other antifungal agents in opinion of the treating physician.
Data collection and definitionsClinical records, including serial serum Aspergillus galactomannan antigens, computerized tomography scans and other imaging tests, antifungal and immunosuppressive therapies, as well as other microbiological isolates, were retrospectively reviewed. Data were recorded at baseline (first day on anidulafungin therapy) and included age, gender, underlying disease, IFD characteristics, current chemotherapy or allo-SCT features, prophylaxis, prior and concurrent treatment (especially corticosteroids, antibiotics and antifungals), and laboratory tests (liver function and serum creatinine levels).
Renal and hepatic toxicity were classified according to World Health Organization (WHO) toxicity grades and were recorded at baseline, and at the end of anidulafungin treatment. Hepatic toxicity was recorded using the highest degree of toxicity of bilirubin, GOT, GPT, GGT, and alkaline phosphatase, whereas renal toxicity depended on serum creatinine levels. Improving or worsening outcome of hepatic and renal toxicity were based respectively on lowering or rising at baseline and end of anidulafungin treatment at least one grade of toxicity according to the WHO scale, otherwise it was recorded as stable. Additionally, we have assessed toxicity outcomes in hematologic patients with prolonged neutropenic fever needing empirical escalation to antifungal therapy that received anidulafungin due to hepatic or renal impairment at baseline.
Response was evaluated at the end of anidulafungin treatment. Resolved IFD was defined as clinical improvement and radiological recovery along with absence of positive microbiological results or progressive decline in mycological surrogate biomarkers. Treatment failure was divided into three categories: (1) death due to IFD; (2) change of anidulafungin treatment due to inefficacy: worsening of microbiological tests (e.g., raise of galactomannan levels) or site of infection (size of the lesions, local o systemic complications), or absence of improvement after prolonged therapy; and (3) change of treatment due to toxicity: anidulafungin withdrawal due to worsening of renal or hepatic laboratory parameters or other related toxicity. Patients still presenting signs or symptoms of IFD when dying by a cause other than IFD, were considered not evaluable.
Study endpoints and statistical analysisThe primary objective of the study was to evaluate the safety of anidulafungin therapy in patients with hematologic diseases, evaluating the need to stop the treatment due to toxicity and the outcome of renal and hepatic lab tests. Secondary objectives were response rate and death within the first 30 days. A descriptive analysis was performed; qualitative variables were analyzed by absolute and relative frequencies, whereas quantitative variables were evaluated with median and range intervals.
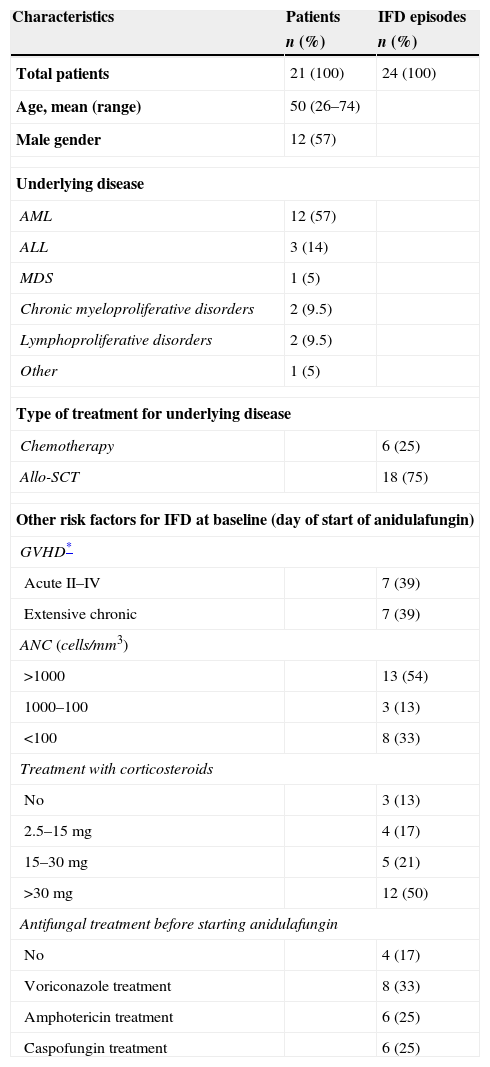
ResultsPatient characteristicsTwenty-one patients from September 2008 to June 2013 were included; three patients had two episodes of IFD, therefore a total of 24 episodes of IFD requiring administration of anidulafungin were recorded. Table 1 summarizes the main characteristics of the study cohort. Briefly, the median age was 50 years (26–74) and the most frequent underlying disease was acute myeloid leukemia (57%). In the course of 6 episodes (25%) chemotherapy was given; the remaining 18 episodes (75%) occurred in patients that received an allo-HSCT. Eight episodes (33%) occurred during neutropenic phase after chemotherapy or allo-SCT, and 21 (87%) were through corticosteroid therapy, mainly for the prophylaxis and treatment of concomitant graft-versus-host disease (GVHD) (Table 1). In 20 episodes (83%) anidulafungin therapy was preceded by antifungal therapy using agents with activity against Candida and Aspergillus (Table 1). Median time on previous antifungal therapy was 7 days (0–52). Three out of 4 proven IFD episodes corresponded to candidemia, while pulmonary aspergillosis was the main cause of probable IFD (14 out of 15). Five episodes of possible IFD were pulmonary nodules in the CT-scan. Nine episodes of IFD (38%) started anidulafungin therapy at intensive care unit (ICU). All patients had hepatic or renal impairment before starting anidulafungin.
Patients and episodes characteristics.
| Characteristics | Patients | IFD episodes |
|---|---|---|
| n (%) | n (%) | |
| Total patients | 21 (100) | 24 (100) |
| Age, mean (range) | 50 (26–74) | |
| Male gender | 12 (57) | |
| Underlying disease | ||
| AML | 12 (57) | |
| ALL | 3 (14) | |
| MDS | 1 (5) | |
| Chronic myeloproliferative disorders | 2 (9.5) | |
| Lymphoproliferative disorders | 2 (9.5) | |
| Other | 1 (5) | |
| Type of treatment for underlying disease | ||
| Chemotherapy | 6 (25) | |
| Allo-SCT | 18 (75) | |
| Other risk factors for IFD at baseline (day of start of anidulafungin) | ||
| GVHD* | ||
| Acute II–IV | 7 (39) | |
| Extensive chronic | 7 (39) | |
| ANC (cells/mm3) | ||
| >1000 | 13 (54) | |
| 1000–100 | 3 (13) | |
| <100 | 8 (33) | |
| Treatment with corticosteroids | ||
| No | 3 (13) | |
| 2.5–15mg | 4 (17) | |
| 15–30mg | 5 (21) | |
| >30mg | 12 (50) | |
| Antifungal treatment before starting anidulafungin | ||
| No | 4 (17) | |
| Voriconazole treatment | 8 (33) | |
| Amphotericin treatment | 6 (25) | |
| Caspofungin treatment | 6 (25) | |
Abbreviations: IFD: invasive fungal disease; AML: acute myeloid leukemia; ALL: acute lymphoblastic leukemia; MDS: myelodysplastic syndrome; GVHD: graft versus host disease; ANC: absolute neutrophil count.
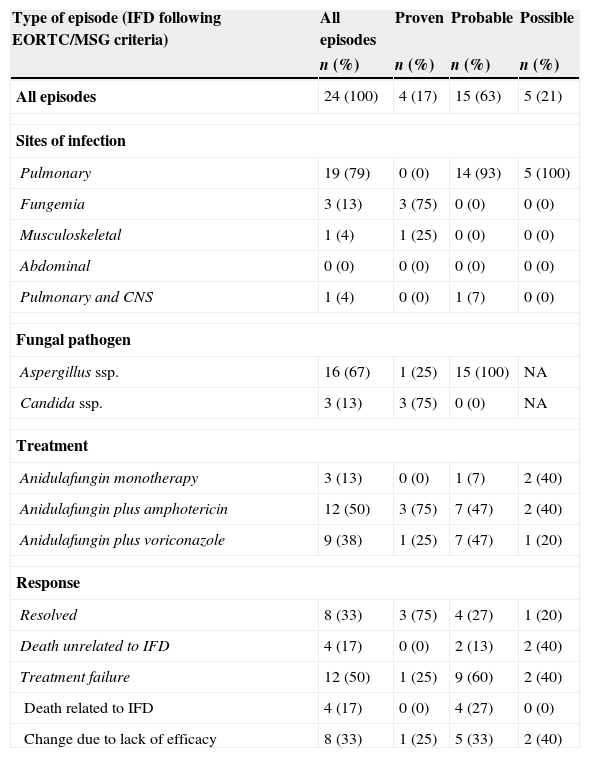
As it is shown in Table 2, anidulafungin monotherapy was administered in 3 (13%) episodes; one of them was a probable pulmonary aspergillosis that resolved in monotherapy. Combination regimens consisted of anidulafungin plus liposomal amphotericin or voriconazole (50% and 37% of all episodes, respectively). The median duration of anidulafungin therapy was 17 days (range, 3–97 days). Overall, 8 (33%) episodes were resolved (3/3 candidemias, 4/15 probable aspergillosis and 1/5 lung nodule, all but one in combination therapy). Twelve episodes (50%) were treatment failure as follows: 4 deaths related to IFD (4 aspergillosis) and 8 changes of anidulafungin due to lack of efficacy (in 6 proven/probable aspergillosis and 2 possible episodes). The remaining 4 episodes (17%) were not evaluable (death unrelated to IFD). Overall, 13 patients (62%) died in the first 30 days following start of anidulafungin. Eight of 9 (89%) patients with IFD that received anidulafungin in the ICU died within the first 30 days.
Treatment and response according to type of invasive fungal disease.
| Type of episode (IFD following EORTC/MSG criteria) | All episodes | Proven | Probable | Possible |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| All episodes | 24 (100) | 4 (17) | 15 (63) | 5 (21) |
| Sites of infection | ||||
| Pulmonary | 19 (79) | 0 (0) | 14 (93) | 5 (100) |
| Fungemia | 3 (13) | 3 (75) | 0 (0) | 0 (0) |
| Musculoskeletal | 1 (4) | 1 (25) | 0 (0) | 0 (0) |
| Abdominal | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pulmonary and CNS | 1 (4) | 0 (0) | 1 (7) | 0 (0) |
| Fungal pathogen | ||||
| Aspergillus ssp. | 16 (67) | 1 (25) | 15 (100) | NA |
| Candida ssp. | 3 (13) | 3 (75) | 0 (0) | NA |
| Treatment | ||||
| Anidulafungin monotherapy | 3 (13) | 0 (0) | 1 (7) | 2 (40) |
| Anidulafungin plus amphotericin | 12 (50) | 3 (75) | 7 (47) | 2 (40) |
| Anidulafungin plus voriconazole | 9 (38) | 1 (25) | 7 (47) | 1 (20) |
| Response | ||||
| Resolved | 8 (33) | 3 (75) | 4 (27) | 1 (20) |
| Death unrelated to IFD | 4 (17) | 0 (0) | 2 (13) | 2 (40) |
| Treatment failure | 12 (50) | 1 (25) | 9 (60) | 2 (40) |
| Death related to IFD | 4 (17) | 0 (0) | 4 (27) | 0 (0) |
| Change due to lack of efficacy | 8 (33) | 1 (25) | 5 (33) | 2 (40) |
Abbreviations: IFD: invasive fungal disease; NA: not applicable.
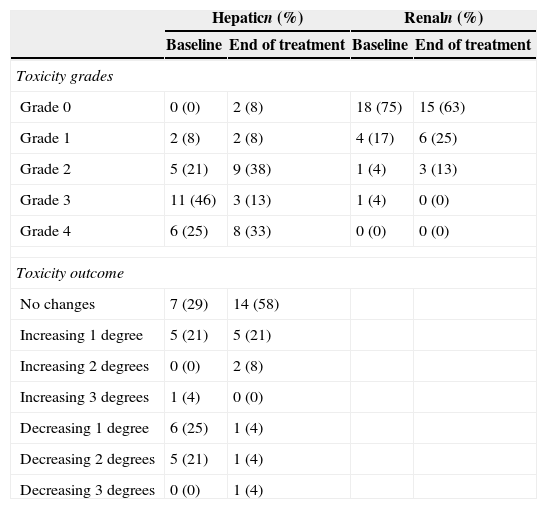
Liver and renal laboratory test and toxicity outcome are represented in Table 3. Anidulafungin was not withdrawn or exchanged by other antifungal agent in any case due to toxicity. No adverse events related to anidulafungin were observed.
Hepatic and renal toxicities in each episode (n=24), before and after anidulafungin treatment, according to WHO toxicity grades.
| Hepaticn (%) | Renaln (%) | |||
|---|---|---|---|---|
| Baseline | End of treatment | Baseline | End of treatment | |
| Toxicity grades | ||||
| Grade 0 | 0 (0) | 2 (8) | 18 (75) | 15 (63) |
| Grade 1 | 2 (8) | 2 (8) | 4 (17) | 6 (25) |
| Grade 2 | 5 (21) | 9 (38) | 1 (4) | 3 (13) |
| Grade 3 | 11 (46) | 3 (13) | 1 (4) | 0 (0) |
| Grade 4 | 6 (25) | 8 (33) | 0 (0) | 0 (0) |
| Toxicity outcome | ||||
| No changes | 7 (29) | 14 (58) | ||
| Increasing 1 degree | 5 (21) | 5 (21) | ||
| Increasing 2 degrees | 0 (0) | 2 (8) | ||
| Increasing 3 degrees | 1 (4) | 0 (0) | ||
| Decreasing 1 degree | 6 (25) | 1 (4) | ||
| Decreasing 2 degrees | 5 (21) | 1 (4) | ||
| Decreasing 3 degrees | 0 (0) | 1 (4) | ||
The first day of anidulafungin treatment all patients had abnormal liver tests (hyperbilirubinemia or hypertransaminasemia of at least one WHO toxicity grade). Hepatic toxicity improved or remained stable in 18 (75%) episodes, but worsened in 6 (25%) episodes (due to acute GVHD in 2 patients and sepsis with multiorgan failure in 4 patients). All patients with their hepatic function worsening died due to the aforementioned complications.
At baseline, renal function was abnormal in 6 (25%) episodes. Renal function improved or remained stable in 5/6 (83%) cases at the end of the anidulafungin treatment, but worsened in one case due to septic shock. In addition, in 5 episodes starting anidulafungin therapy with normal serum creatinine values, renal function worsened due to sepsis and multi-organ failure. In the studied cohort, all patients developing sepsis and secondary renal failure died within the first 30 days.
Empirical administration of anidulafunginIn addition to the 24 episodes of IFD, 29 patients with prolonged neutropenic fever and concomitant liver or renal impairment received empirical treatment with anidulafungin. The first day of anidulafungin treatment all but one patient (96%) had abnormal liver tests. Hepatic toxicity improved or remained stable in 21 (75%) episodes, but worsened in 7 (25%). At baseline, renal function was abnormal in 11 (38%) episodes. Renal function improved or remained stable in 9 (82%) cases at the end of anidulafungin treatment, but worsened in 2 cases due to septic shock. In addition, in 6 episodes starting anidulafungin therapy with normal serum creatinine values, renal function worsened due to sepsis and multi-organ failure. All patients with impairment of hepatic or renal function due to sepsis and multi-organ failure died within the first 30 days. Anidulafungin was not withdrawn or exchanged by other antifungal agent in any case due to toxicity. No adverse events related to anidulafungin were observed.
DiscussionThis study shows that anidulafungin is a safe drug for the management of IFD in patients with hematologic diseases and concomitant liver and/or renal dysfunction. As expected, in this series of severely ill patients with IFD the response and mortality rates were worse with respect to those reported in studies with other antifungal agents in patients without baseline renal or liver impairment.3,5–7
Although the major limitation of our study is that it is an observational and retrospective analysis, it should be noted that, in order to avoid selection bias, records of all patients hospitalized during the study period in the Hematology Service were reviewed to identify those receiving anidulafungin since the Service's policies recommended this drug in special situations. Another major limitation is that the number of patients is small, avoiding us to draw any conclusion about the efficacy of the drug. In addition, the prophylaxis and management of IFD before starting anidulafungin was heterogeneous, as well as the physician's decision to combine or not anidulafungin with amphotericin or voriconazole. Regarding the etiology of IFD episodes, most prevalent fungal pathogens belonged to Aspergillus and Candida genera, as in other studies performed in patients with hematologic malignancies and high-risk of IFD (i.e., chemotherapy-induced neutropenia and/or corticosteroids administration associated with allo-SCT and GVHD).5,8
Concerning anidulafungin safety and tolerability, our results are quite similar to those of previous studies performed in non-hematologic patients, reporting around 2% of adverse events leading to treatment discontinuation.1,8 In our series, treatment with anidulafungin as single agent or even in combination was feasible as the drug was not discontinued due to toxicity. In addition, the majority of patients showed stable or improved hepatic and renal function during anidulafungin therapy. This was also true for patients receiving empirical therapy with anidulafungin in the context of prolonged febrile neutropenia.
To our knowledge, there are only two studies reporting the use of anidulafungin in 2 and 5 hematologic patients, respectively.4,9 The first of those papers published, reported 2 patients with probable breakthrough aspergillosis while receiving anidulafungin monotherapy in hematologic patients.9 In fact, there is no indication for this drug in the setting of mold infections. In our series, the response rate of anidulafungin therapy (alone or in combination) in aspergillosis was 25%, which is lower than 52–63% reported with other antifungal agents as monotherapy or bi-therapy.3,5,7 Worthy of note is that one patient receiving anidulafungin monotherapy for probable aspergillosis showed resolution of IFD. Nevertheless, we cannot conclude from our data that anidulafungin was effective or not for aspergillosis because it was used in combination with amphotericin or voriconazole in most cases. Regarding the efficacy of anidulafungin monotherapy for invasive candidiasis, large studies performed in non-hematologic ICU patients reported around 70% of responses,1,8 and a small study showed successful treatment in 5 patients with hematologic diseases and candidemia.4 Although responses were observed in the 3 (100%) episodes of candidemia, our study, again, is clearly insufficient to draw any valid conclusion regarding efficacy of anidulafungin because of the very small number of cases and the concomitant use of amphotericin in this setting.
The early mortality rate observed in patients with IFD was 62%, higher than 42–47% reported in studies with other antifungal agents.3,5–7 These differences could be explained by the adverse baseline characteristics of patients included in our study.
In conclusion, our study suggests that anidulafungin is a safe and tolerable option to treat IFD in patients with hematologic diseases and concomitant liver or renal impairment. Due to the low number of patients, we cannot draw any conclusion about efficacy.
Conflict of interestThis study received financial support from Pfizer Spain in connection with the development of this manuscript. The authors declare no other conflicts of interest.
Author contributionsPau Montesinos, Rebeca Rodríguez-Veiga and Miguel A. Sanz conceived the study, analyzed, and interpreted the data; Pau Montesinos, Rebeca Rodríguez-Veiga and Miguel A. Sanz wrote the paper; Pau Montesinos and Rebeca Rodríguez-Veiga performed the statistical analyses; David Martínez-Cuadrón, Blanca Boluda, Inés Navarro, Carmen Alonso, Belen Vera, Jaime Sanz, Francisca López-Chulia, Guillermo Martín, Rosa Jannone, Guillermo Sanz, Aima Lancharro, Isabel Cano, Javier Palau, Ignacio Lorenzo, Isidro Jarque, Miguel Salavert, Paula Ramírez reviewed the manuscript and contributed to the final draft.
We thank Carlos Pastorini, David Pellicer, and Shirley Weiss for data collection and management.