Several studies to evaluate the accuracy of galactomannan (GM) in bronchoalveolar lavage fluid (BALF) as a diagnostic tool have been carried out; however, there are still controversies about the optimal cut-off point of BALF GM.
AimsThe objective of this study was to determine the diagnostic accuracy and the optimal cut-off point on BALF GM from patients with suspected invasive pulmonary aspergillosis (IPA) in a tertiary care hospital.
MethodsA cross-sectional study with 188 patients (≥18 years) that had undergone a bronchoscopy with BAL due to suspected IPA was carried out. IPA was diagnosed according to the EORTC/MSG guidelines.
ResultsThe optimal optical density cut-off point for BALF GM was 0.67, with sensitivity, specificity, positive predictive value, and negative predictive value of 100%, 70%, 32.3%, and 100%, respectively.
ConclusionsBALF GM detection proved to be a useful supplementary technique in the early diagnosis of IPA in both neutropenic and non-neutropenic patients.
Diversos estudios han evaluado la precisión del galactomanano (GM) como herramienta diagnóstica en el líquido de lavado broncoalveolar (LBA); sin embargo, todavía existen controversias sobre el punto de corte óptimo de LBA GM.
ObjetivosEl objetivo de este estudio fue determinar la precisión diagnóstica y el punto de corte óptimo en LBA GM de pacientes con sospecha de aspergilosis pulmonar invasora (API) en un hospital de tercer nivel.
MétodosSe realizó un estudio transversal en el que fueron incluidos 188 pacientes (≥18años) a los que se les había realizado una broncoscopia con LBA por sospecha de API. La API se diagnosticó de acuerdo con las guías EORTC/MSG.
ResultadosEl punto de corte óptimo para el valor densidad óptica de LBA GM fue de 0,67, con sensibilidad, especificidad, valor predictivo positivo y valor predictivo negativo del 100%, 70%, 32,3% y 100%, respectivamente.
ConclusionesLa detección de GM en LBA demostró ser un procedimiento útil en el diagnóstico precoz de la API, tanto en pacientes neutropénicos como en no neutropénicos.
Invasive pulmonary aspergillosis (IPA) is a potentially lethal infection caused by Aspergillus fumigatus, as well as other Aspergillus species, which are widely distributed in soil and other organic matter.31 It occurs almost exclusively in immunocompromised patients,10 with prolonged neutropenia being the main risk factor.4 Despite this, the incidence of cases is increasing in non-neutropenic patients, including pulmonary transplant recipients, critical patients and patients using corticosteroids.18 Globally, it is estimated that approximately 200,000 cases of IPA are diagnosed annually.25 The prevalence is around 1–15%25 and the overall mortality rate remains high, around 30–50% in the general population,8 and may exceed 90% in certain populations.12
The diagnosis of IPA depends on a constellation of clinical, radiological and microbiological criteria.13 The high mortality rate results, in part, from the difficulties of establishing an early diagnosis due to nonspecific clinical manifestations, delayed radiological findings and low culture yield.12 The gold standard for the diagnosis of invasive fungal diseases is histopathological analysis or culture of tissue samples, which is rarely possible due to the risks involved in performing biopsies in pancytopenic patients.25 Since diagnostic delay or misdiagnosis in these patients may lead to several problems, such as drug toxicity due to inappropriate treatments, high drug costs and mortality, early diagnosis is fundamental.29 Galactomannan (GM) is a polysaccharide component of the cell wall of Aspergillus species that is released during the growth of hyphae.14 The attribute of being detectable in body fluids and blood in the early stages of the disease makes it an ideal candidate for IPA diagnosis.13 Several studies10,13,29 have been carried out to evaluate the accuracy of GM in bronchoalveolar lavage fluid (BALF) in the diagnosis; however, there are still controversies about the optimal cut-off point of BALF GM. The objective of this study was to determine the diagnostic accuracy and the optimal cut-off point of BALF GM from patients with suspected IPA in a tertiary care hospital.
Material and methodsStudy design and locationWe conducted a cross-sectional study in a general, tertiary care, university-affiliated hospital with 750 beds. The ethics committee at the hospital approved the access to patients’ records in December 11th, 2017 (number 170657). Patients confidentiality has been kept.
Patients and data collectionAll adult patients (≥ 18 years) who underwent a bronchoscopy with BAL between January 2013 and January 2019 due to suspected IPA, and who had BALF tested for GM, were included in the study. The BALF sample was collected using a fiber-optic bronchoscope, under standard techniques. The Platelia Aspergillus GM Elisa immunoassay (Bio-Rad Laboratories, Marnes-laCoquette, France) was used to detect the presence of GM on BALF, according to the manufacturer's recommendations.
The diagnostic criteria applied for IPA, according to the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) guidelines,9 were: (1) the presence of host factors for IPA, including solid organ transplantation, connective tissue disorders or use of immunosuppressive agents, such as corticosteroids; (2) the presence of radiological features consistent with IPA on computed tomography, such as dense and well-circumscribed lesions with or without a halo sign, air crescent sign or cavity; (3) mycological evidence of Aspergillus, such as a positive Aspergillus culture from qualified specimens (including sputum and BAL) or a positive serum GM (at a cutoff value of 0.5); and (4) histological evidence of Aspergillus hyphae on lung biopsy specimens or a positive Aspergillus culture from pulmonary biopsy specimens. A proven diagnosis is made when patients meet all four criteria, a probable diagnosis is made when patients meet the first three criteria and a possible diagnosis when patients meet the first two criteria. In order to assess the accuracy of GM in BALF, the studies performed,10,13,29 as well as the present study, excluded BALF GM results from the EORTC/MSG criteria. All assessments of the EORTC/MSG criteria were done by two independent investigators who were blinded to the BALF GM results.
A standardized form for each patient was completed with the following information: demographic, clinical, laboratory and radiological data in addition to the EORTC/MSG criteria.
Statistical analysisData analysis was performed using SPSS 18.0 (Statistical Package for the Social Sciences, Chicago, Illinois) and MedCalc 16.4.3 software package (MedCalc Software, Mariakerke, Belgium). Data were presented as number of cases, mean±standard deviation, or median with interquartile range. Sensitivity, specificity, positive predictive value and negative predictive value were calculated for two of the most used optical density (OD) cut-off points for BALF GM (≥0.5 and ≥1.0). The gold standard was a proven or probable IPA diagnosis by the EORTC/MSG criteria. The absence of disease (IPA) was considered in cases with possible diagnosis or without IPA according to the EORTC/MSG criteria. We also constructed receiver operating characteristic (ROC) curves to analyze the best cut-off point for BALF GM OD index. The best cut-off point was chosen based on the Youden index and on the area under the ROC curve closer to 1. We excluded from the analysis of sensitivity, specificity, positive predictive value and negative predictive value those patients who had previously used antifungal agents active against Aspergillus in order to avoid false-negative results, and piperacillin-tazobactam and amoxicillin-clavulanate due to the possibility of false-positives. Considering that the sensitivity of BALF GM varies from 70% to 87.5% in previous studies,1,5,26 with a 95% confidence interval and a power of 80%, 61 patients, at least, had to be included in the study.
ResultsDuring the study period, 188 patients met the inclusion criteria and were included in the study. According to EORTC/MSG criteria, 35 (18.6%) patients were classified as IPA and 153 (81.4%) patients as non-IPA. The characteristics of the study population are shown in Table 1. The age, gender, underlying diseases/conditions, and a previous use of antifungal drugs and antibiotics did not significantly differ between the two groups (p>0.05). Fungal isolation in culture, and direct microscopy observation of fungal structures, were more frequent in IPA group. A higher percentage of patients with dense, well-circumscribed lesion(s) with or without a halo sign on chest CT was found in the IPA group when compared with the non-IPA group (71.4% vs 38.6%, p=0.001). The presence of GM in both serum and BALF was also higher in the IPA group (p<0.0001). The mortality rate was significantly higher in the IPA group (54.3% vs. 30.1% in the non-IPA group, p=0.024).
Data collected of IPA and non-IPA patients.
| Characteristics | IPA (n=35) | Non-IPA (n=153) | p value |
|---|---|---|---|
| Age (years) | 46.9±17.1 | 46.4±17.7 | 0.872 |
| Gender male | 16 (45.7%) | 76 (49.7%) | 0.814 |
| Underlying disease/condition | |||
| Hematologic malignancy | 14 (40%) | 71 (46.4%) | 0.618 |
| Solid organ transplantation | 27 (77.1%) | 131 (85.6%) | 0.327 |
| HIV | 33 (94.3%) | 121 (79.1%) | 0.062 |
| Solid organ malignancy | 33 (94.3%) | 141 (92.2%) | 0.497 |
| Other | 29 (82.9%) | 137 (89.5%) | 0.202 |
| Previous use of antifungal drugs | 19 (54.3%) | 61 (39.9%) | 0.172 |
| Previous use of antibioticsa | 10 (28.6%) | 40 (26.1%) | 0.935 |
| Fungal structures in BALF (microscopy observation) | 9 (25.7%) | 5 (3.3%) | <0.0001 |
| Fungal isolation in culture | 14 (40%) | 3 (2%) | <0.0001 |
| Neutropenia | 13 (37.1%) | 32 (20.9%) | 0.07 |
| Corticosteroid therapy | 11 (31.4%) | 26 (17%) | 0.089 |
| Immunosuppressive therapy | 26 (74.3%) | 93 (60.8%) | 0.193 |
| Allogenic stem cell transplant | 9 (25.7%) | 26 (17%) | 0.34 |
| Chest CT | |||
| Dense, well-circumscribed lesion(s) with or without a halo sign | 25 (71.4%) | 59 (38.6%) | 0.001 |
| Air-crescent sign | 1 (2.9%) | 3 (2%) | 0.565 |
| Cavity | 4 (11.4%) | 11 (7.2%) | 0.296 |
| Serum GM | 0.45 (0.26–0.96) | 0.21 (0.17–0.3) | <0.0001 |
| BALF GM | 1.75 (0.68–5.31) | 0.40 (0.27–0.77) | <0.0001 |
| Mortality | 19 (54.3%) | 46 (30.1%) | 0.024 |
Data are presented as mean±SD, or median (interquartile range).
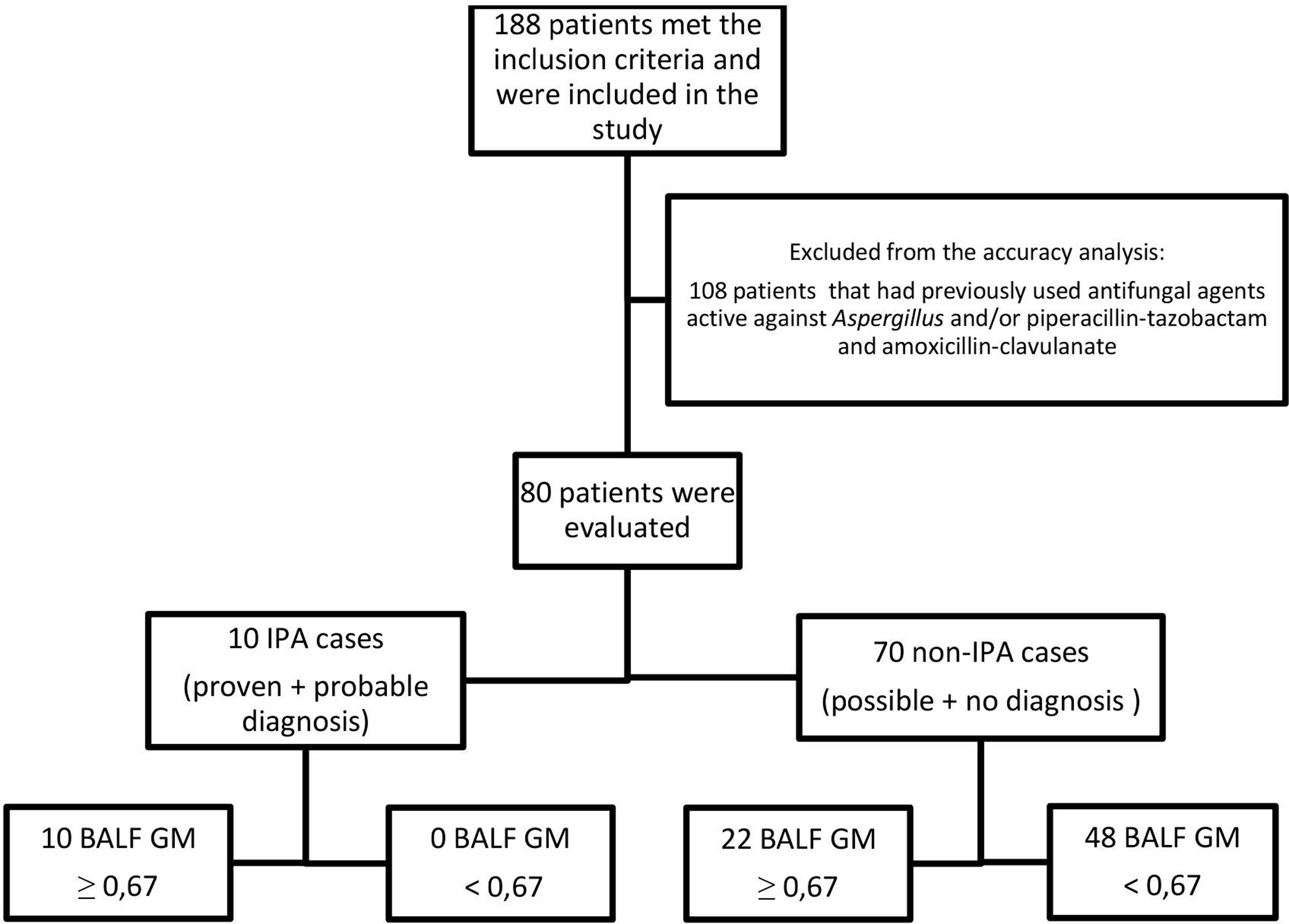
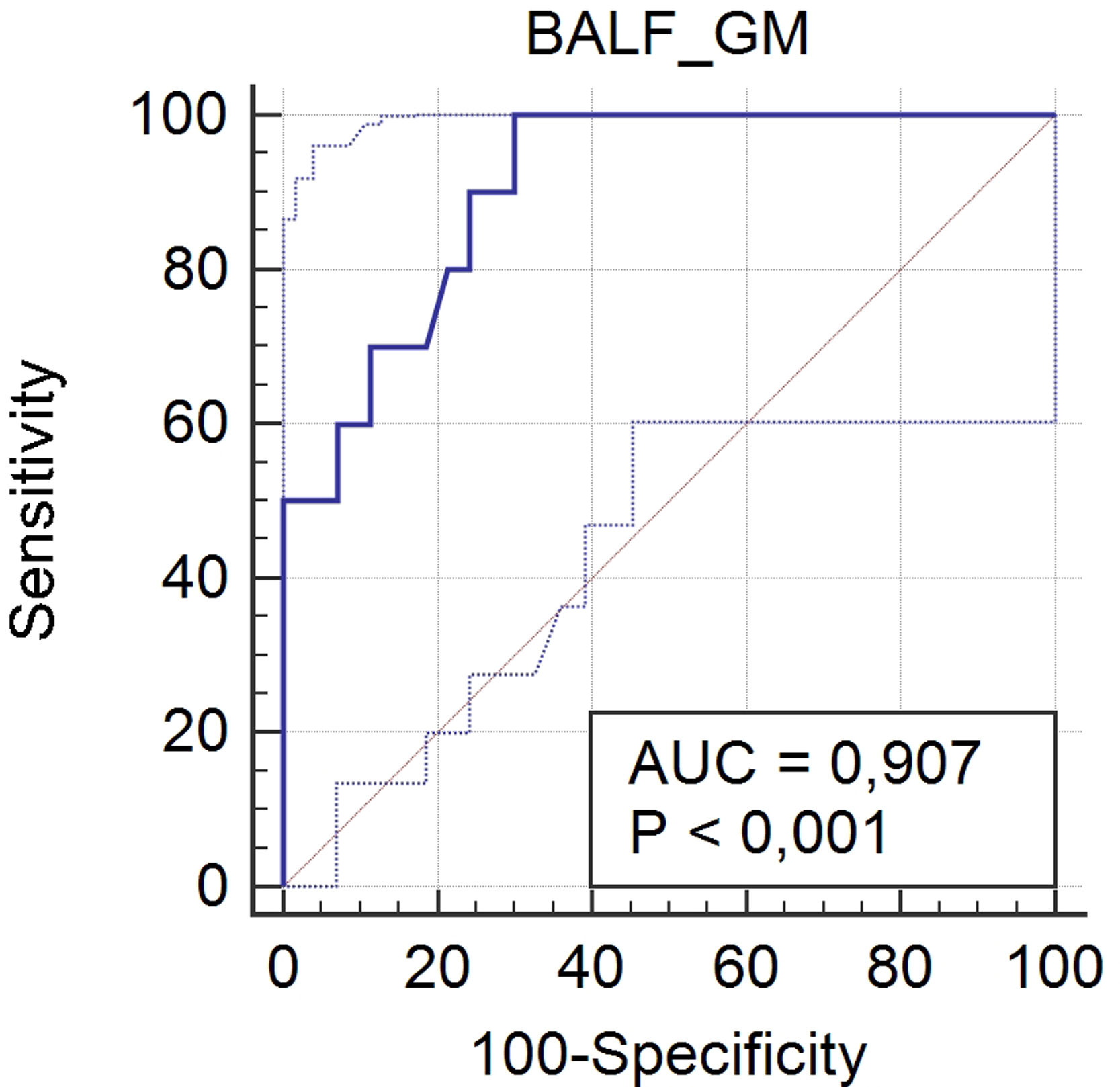
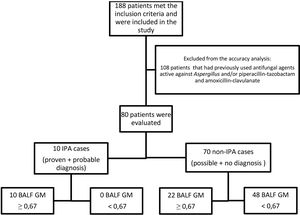
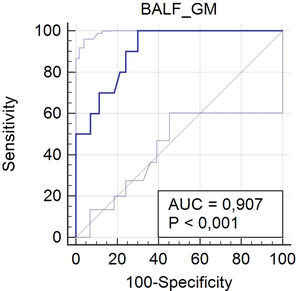
One hundred and eight patients have had previously antifungal agents active against Aspergillus, and/or piperacillin-tazobactam and amoxicillin-clavulanate, and were excluded from the analysis. Ten patients (12.5%) out of the 80 patients included in this analysis were defined as IPA cases and 70 (87.5%) as non-IPA cases (Fig. 1), according to EORTC/MSG criteria (gold standard). The optimal cut-off point for BALF GM OD index was 0.67, with sensitivity, specificity, positive predictive value, and negative predictive value of 100% (95%, CI 69.2–100), 70% (95%, CI 57.9–80.4), 32.3% (95% CI 25–40.5), and 100% (95%, CI 90.9–100), respectively. The area under the ROC curve was 0.907 (95%, CI 0.82–0.96; p<0.0001) (Fig. 2).
Using a threshold OD index≥0.5, the sensitivity, specificity, positive predictive value, and negative predictive value were 100% (95% CI 69.2–100), 55.7% (95% CI 43.3–67.6), 24.4% (95% CI 19.9–29.6), and 100% (95% CI 90.9–100), respectively, whereas an OD index≥1 resulted in a sensitivity, specificity, positive predictive value, and negative predictive value of 70% (95% CI 34.8–93.3), 81.4% (95% CI 70.3–89.7), 35% (95% CI 22.2–50.4), and 95% (95% CI 88–98), respectively (Table 2). The diagnostic odds ratio for the cutoff values 0.5, 0.67, and 1 were 24.9, 48.3, and 14.7, respectively.
Performance of BALF GM for diagnosing IPA at different cutoff values.
| Cutoff value | Sensitivity% (95%CI) | Specificity% (95%CI) | PPV% (95%CI) | NPV% (95%CI) | Overall accuracy% (95% CI) |
|---|---|---|---|---|---|
| ≥0.5 | 100 (69.2–100) | 55.7 (43.3–67.6) | 24.4 (19.9–29.6) | 100 (90.9–100) | 60 (48.4–70.8) |
| ≥0.67 | 100 (69.2–100) | 70 (57.9–80.4) | 32.3 (25.0–40.5) | 100 (90.9–100) | 73.8 (62.7–82.9) |
| ≥1 | 70 (34.8–93.3) | 81.4 (70.3–89.7) | 35 (22.2–50.4) | 95 (88–98) | 80 (69.6–88.1) |
In the present study we found that the best cutoff point for BALF GM OD index was ≥0.67, with sensitivity, specificity, positive predictive value, and negative predictive value of 100%, 70%, 32.3%, and 100%, respectively. Using a threshold OD index≥0.5, the sensitivity and negative predictive value were the same, but specificity and positive predictive value were lower (55.7% and 24.4%, respectively). When using an OD index≥1 sensitivity and negative predictive value decreased (70% and 95%, respectively), and specificity and positive predictive value increased (81.4% and 35%, respectively).
GM is a polysaccharide component of the cell wall of Aspergillus that is released into body fluids during the early stages of fungal invasion.15,22 BALF GM concentration may be higher than serum GM, being useful for the early diagnosis of IPA.24 It provides additional sensitivity compared to culture, exceeding 70% in most studies.5,16,21,23 However, the best cutoff value for BALF GM OD index is still under debate. The most common used cutoffs are ≥0.5 and ≥1, although the results vary widely across studies.7 In the present study, the optimal cutoff point for BALF GM was ≥0.67. Our results stand in accordance with at least two previous studies.29,30 Zhuang et al.30 evaluated the usefulness of BALF GM in 183 non-neutropenic patients and found an optimal cutoff value of 0.76, with a sensitivity of 100% and a specificity of 76.2%. In another study29 with 128 non-neutropenic patients, the authors identified the best cutoff as 0.7, with lower sensitivity (72.97%) and higher specificity (89.16%). Notably, both studies were conducted on a non-neutropenic population. In our study, 62.9% of the patients were also non-neutropenic. In recent years, studies have reported several IPA cases among patients without neutropenia.2,6 As in other studies,3,19,27 we found that increasing the cutoff point for galactomannan increases specificity to the detriment of sensitivity. Wu et al.27 set the optimal cutoff value of GM in BALF in 0.87, with sensitivity and specificity of 91.7% and 92.5%, respectively. Other authors19 found that a BALF GM cutoff value of 0.88 showed the highest diagnostic efficacy for pulmonary aspergillosis, with sensitivity and specificity of 77.2% and 93%, respectively. In a recent metanalysis3 it was demonstrated that when the cutoff is greater than 1, the sensitivity is higher.
Dense, well-circumscribed lesion(s) with or without the halo sign were statistically more frequent in IPA cases when compared with non-IPA cases in the present study. The air-crescent sign and the presence of cavities were less common in both groups, and there is not statistically difference between patients classified as IPA and those non-IPA. In a previous study with non-neutropenic patients, Zhuang et al. showed that radiological findings had no significant difference between proven or probable IPA and non-IPA groups.30 In fact, suggestive lesions of IPA on CT scans, such as the classic halo sign and the air-crescent sign, were less specific in non-neutropenic patients.11,17 We included both neutropenic and non-neutropenic patients in our sample, which justifies the different results.
We found a higher mortality rate in the IPA group (54.3%) when compared to the non-IPA group (30.1%). IPA is well known to cause high mortality. Yu et al.,28 investigating non-neutropenic patients, observed that the 30-day mortality rate was higher in IPA patients, almost 4-fold than that of non-IPA patients (24.4% vs. 6.6%). In another study,20 the mortality rate of patients treated for IPA was significantly higher than that of only colonized patients (27.3% vs. 9.5%).
This study has some limitations that we have to take into account. First, we recruited patients from a single center. However, we believe the results may apply to other settings. Second, this was a retrospective study, but the information we obtained retrospectively from chart review was as complete as if it was collected prospectively. Third, the prevalence of IPA in our study is high compared to the usual prevalence found in other clinical settings, and this fact could be reflected in the predictive values. Lastly, we included both neutropenic and non-neutropenic patients and maybe some results would be different between these groups; nevertheless, a mixed population reflects the real clinical practice. In spite of these concerns, the definition of the best cutoff value for BALF GM is important to define the best approach for IPA diagnosis, once it depends on the local incidence.7
In summary, BALF GM detection proved to be a useful technique in the early diagnosis of IPA. In our retrospective study, the best cutoff point for BALF GM OD index was ≥0.67, including neutropenic and non-neutropenic patients. Further studies on homogeneous population are needed to confirm these results.
Conflict of interestThe authors declare that they have no conflict of interest.