Fungi of the genus Fusarium are primarily plant pathogens and saprobes that produce disseminated infections in immunologically deficient humans. After aspergillosis, disseminated fusariosis is the second most common cause of invasive infection by filamentous fungi in patients with hematologic malignancies or those undergoing transplants of hematopoietic progenitors.
AimsDisseminated fusariosis (DF) is considered an extremely rare infection and has reached a stable incidence rate, but its high mortality rate and the lack of an optimal management protocol have raised increasing interest in this mycosis.
MethodsWe present three cases of DF produced by Fusarium oxysporum species complex, Fusarium solani species complex and the highly unusual Fusarium dimerum in patients with advanced hematological malignancies diagnosed in our hospital between 2007 and 2011. The species level identification of the Fusarium isolates was established by sequencing their TEF1 gene.
ResultsThe isolates showed low susceptibility to most of the antifungal agents analyzed, except that observed for F. dimerum to amphotericin B (AmB) and terbinafine, and F. oxysporum species complex to AmB. Interestingly, the strain of F. solani species complex exhibited high MIC values for AmB and voriconazole, notwithstanding these drugs were used for treatment with good results. Other relevant aspects to be considered in the treatment of DF are surgically cleaning foci of infection, withdrawing presumably contaminated catheters and recovery from neutropenia.
ConclusionsThe prevention of infection in colonized patients, the maintenance of a high level of diagnostic suspicion for early diagnosis, and the combined, vigorous and prolonged use of L-AmB and voriconazole are essential to decrease the mortality rate of this devastating infection.
Los hongos del género Fusarium son principalmente patógenos vegetales que producen infecciones diseminadas en personas con deficiencias inmunológicas. Tras la aspergilosis, la fusariosis diseminada es la segunda causa de infección invasora por hongos filamentosos en pacientes con enfermedades hematológicas malignas o en receptores de trasplantes de progenitores hematopoyéticos.
ObjetivosLa fusariosis diseminada es muy infrecuente y ha alcanzado una tasa de incidencia estable. Sin embargo, el interés por estas micosis se ha incrementado debido a su alta tasa de mortalidad y a la falta de un tratamiento óptimo.
MétodosSe presentan tres casos de fusariosis diseminada por Fusarium oxysporum species complex (SC), Fusarium solani SC y Fusarium dimerum en pacientes de nuestro hospital con enfermedades hematológicas avanzadas, diagnosticados entre 2007 y 2011. Los aislamientos de Fusarium se identificaron mediante secuenciación del gen TEF1.
ResultadosLa sensibilidad a los antifúngicos ensayados fue baja salvo a la anfotericina B (AmB) y la terbinafina en F. dimerum, y a la AmB en F. oxysporum SC. Aunque F. solani SC mostró valores altos de CMI para la AmB y el voriconazol, su uso para el tratamiento del paciente dio buenos resultados. Otros aspectos relevantes para el tratamiento de la fusariosis diseminada son la limpieza quirúrgica de los focos de infección, la retirada de catéteres presumiblemente contaminados y la recuperación de la neutropenia.
ConclusionesLa prevención de la infección en pacientes colonizados, el mantenimiento de un alto grado de sospecha para un diagnóstico temprano y el uso combinado, vigoroso y prolongado de L-AmB y voriconazol son esenciales para disminuir la mortalidad de esta infección devastadora.
Fungi of the genus Fusarium are primarily recognized as plant pathogens since they are responsible for a wide variety of diseases in the plant kingdom, sometimes with devastating socioeconomic impact.29 Under favorable environmental conditions, they can also produce mycotoxins, with remarkable implications for animal and human health.29 Regarding human beings, Fusarium spp. can produce syndromes ranging from allergic sinusitis, food poisoning, or surface infections such as onychomycosis or keratitis, to locally invasive or disseminated infections depending on the immunological competence of the host.15,29,32 Like other invasive fungal infections (IFIs), disseminated fusariosis (DF) is observed most frequently in patients with hematologic malignancies or those undergoing hematopoietic stem cell transplant (HSCT).14,25,33,34 According to some authors, DF is, after aspergillosis, the second most prevalent cause of IFIs by filamentous fungi in this group of patients.8,13 Despite being considered an extremely rare infection and having reached a stable incidence rate,6,9,30,38–41 this IFI has attracted growing interest in recent years due to its high mortality rate and the lack of an optimal treatment protocol.9,22,32 Here we present three cases of DF produced by Fusarium oxysporum species complex, Fusarium solani species complex and the extremely rare Fusarium dimerum in three patients with advanced hematological malignancies observed in our hospital during the period between 2007 and 2011. We also review the current state of the art for the clinical diagnosis and treatment of this devastating infection.
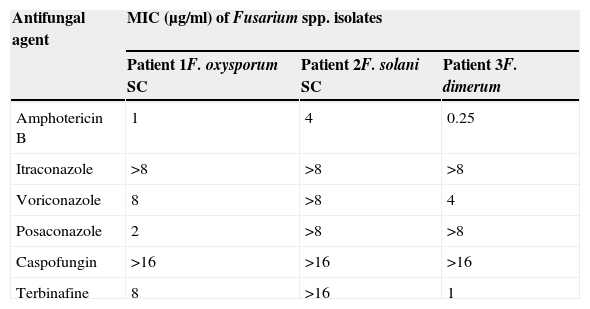
Patients and methodsPatient 1A 55-year-old man diagnosed with advanced cutaneous lymphoma, mycosis fungoides type, with fever and painful oral ulcers was admitted to our unit in April 2007. He had received the third cycle of treatment with denileukin diftitox (Ontak, anti-CD25) (day 0) for rescue therapy due to resistance to bexarotene, psoralen+ultraviolet A light, interferon, and polychemotherapy (nitrogen mustard, gemcitabine, fludarabine, methotrexate, cisplatin, etoposide and cytarabine). He presented an extensive skin involvement due to lymphoma and a large ulceration with necrotic background on the left area of his calf. Cutaneous lesions and blood cultures rendered mixed cultures of Stenotrophomonas maltophilia and Enterobacter cloacae, and a high viral load of cytomegalovirus was also detected in the blood. Fusarium sp. was identified in the wound on his left leg on day 18 since hospital admission. The use of imipenem, vancomycin, metronidazole, ganciclovir and surgical debridement of the ulceration temporarily restored the clinical stability of the patient. However, on days 26 and 29 he presented a new febrile ascent and Fusarium sp. was identified in blood cultures (Bactec 9240, Becton Dickinson). The isolate was later identified as F. oxysporum species complex. Despite adding liposomal amphotericin B (L-AmB) (5mg/kg of body weight, intravenously every 24h) to the antimicrobial regimen the clinical picture evolved to bilateral pneumonia that ended with the death of the patient on day 53 of follow-up. The halo sign was not detected in chest X-ray images and necropsy was not performed. Retrospective determinations of β(1–3)-d-glucan (Fungitell; Associates of Cape Cod, East Falmouth, MA, USA) in sera on days 45, 49 and 51 gave positive results with the following values: 212, 421 and 460pg/ml, respectively. The observed sensitivity of the F. oxysporum isolate to various antifungal agents is shown in Table 1.
In vitro antifungal susceptibility of Fusarium spp. isolated from patients. Minimal inhibitory concentration (MIC) values were estimated following the EUCAST-AFST broth microdilution method.11
| Antifungal agent | MIC (μg/ml) of Fusarium spp. isolates | ||
|---|---|---|---|
| Patient 1F. oxysporum SC | Patient 2F. solani SC | Patient 3F. dimerum | |
| Amphotericin B | 1 | 4 | 0.25 |
| Itraconazole | >8 | >8 | >8 |
| Voriconazole | 8 | >8 | 4 |
| Posaconazole | 2 | >8 | >8 |
| Caspofungin | >16 | >16 | >16 |
| Terbinafine | 8 | >16 | 1 |
SC: species complex.
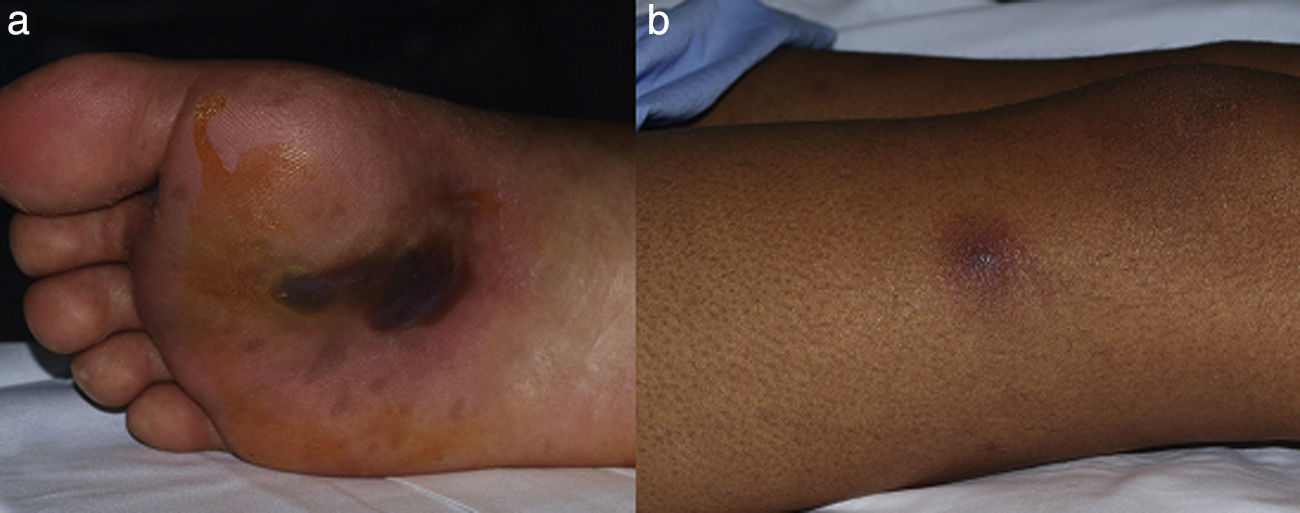
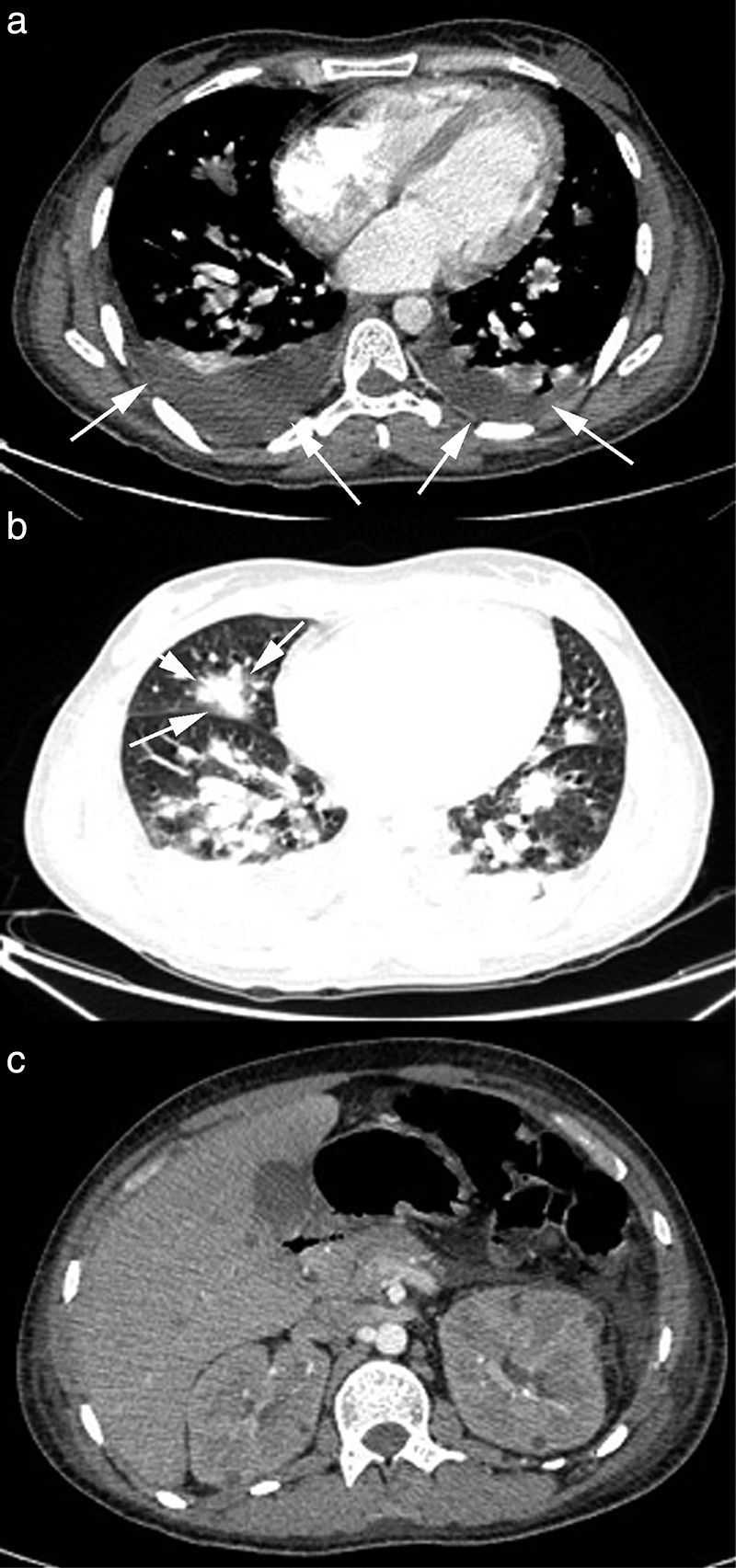
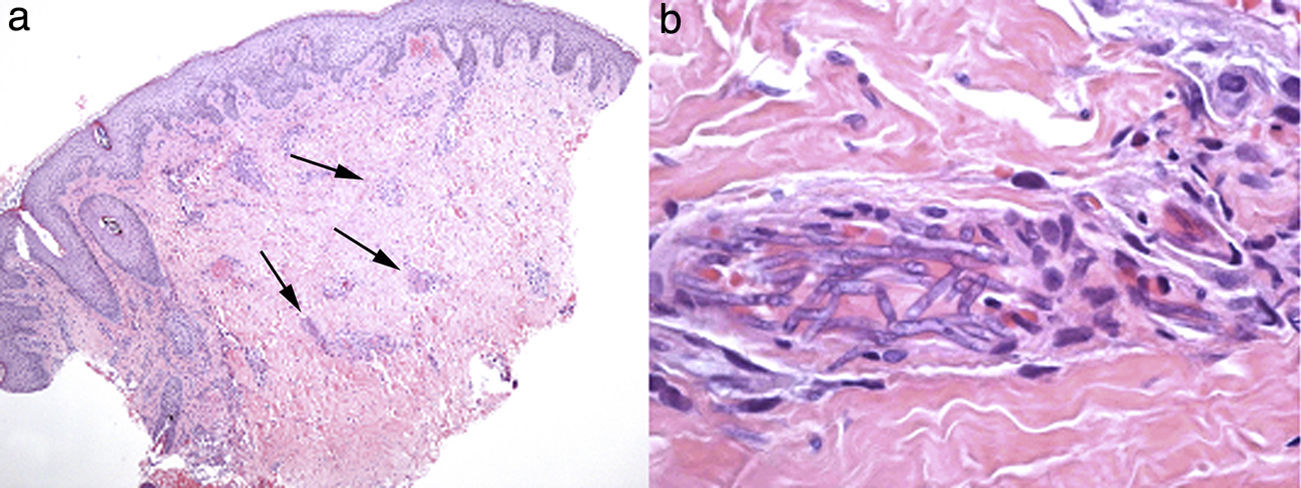
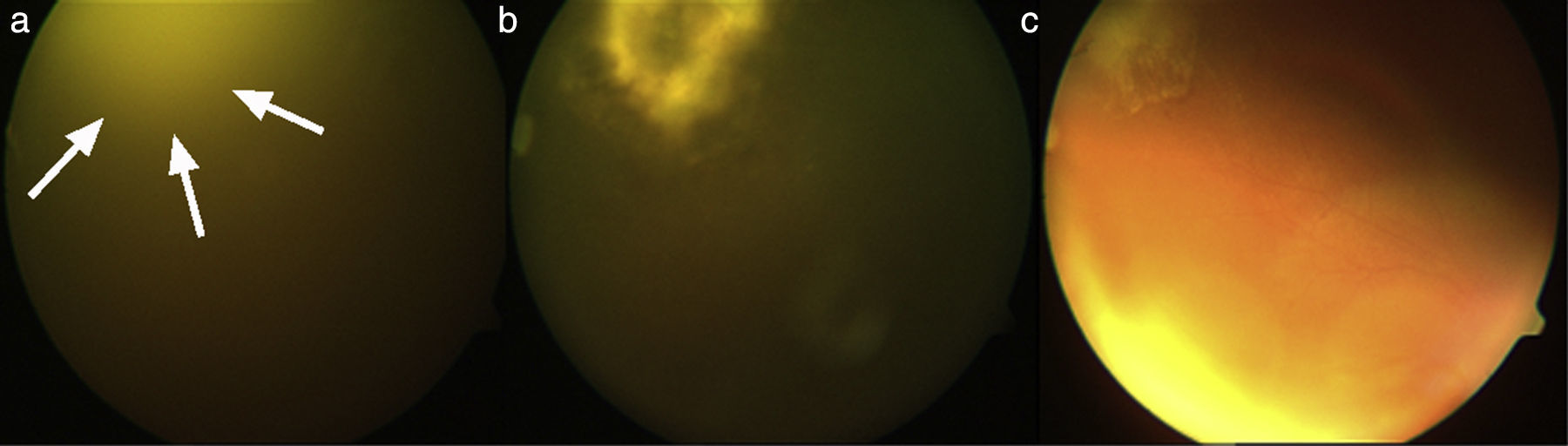
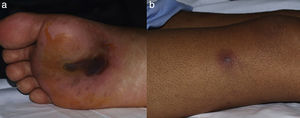
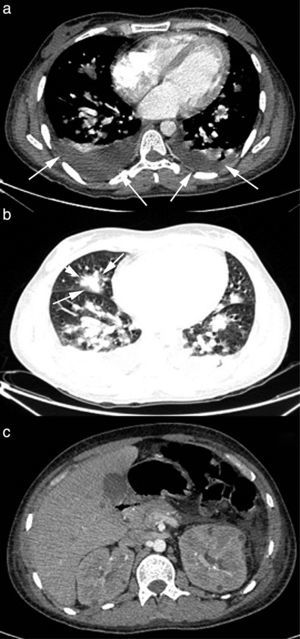
A 29-year-old male patient, recently arrived from Brazil, was diagnosed with an acute myelogenous leukemia (AML) in April 2010. Referred from another center, he was subjected to re-induction treatment in June 2010 (day 0) with cytarabine, idarubicin and etoposide due to lack of response to an earlier administered treatment. To prevent cerebral relapse, intrathecal chemotherapy with methotrexate, cytarabine and hydrocortisone was administrated on day 1. He received oral prophylactic fluconazole (200mg/day). On day 12 postchemotherapy, with profound neutropenia (<0.1 neutrophils×109/l), he presented an erythematous and indurated lesion on the sole of his foot (Fig. 1a) and treatment with vancomycin and meropenem was started. Fever appeared on day 14, and on day 16 subcutaneous nodules and lesions with central necrosis surrounded by spreading erythema, reminiscent of ecthyma gangrenosum, erupted on his limbs and scalp (Fig. 1b). L-AmB (5mg/kg every 24h) was started and biopsies of skin lesions were taken. A computer tomography (CT) of the chest, abdomen and pelvis showed bilateral pleural effusions, pulmonary nodules without lobar predominance, and hypodense lesions in renal, hepatic and splenic parenchyma suggesting microabscesses (Fig. 2). There was an incipient sign of halo surrounding a nodule in the right lung (Fig. 2b). Skin biopsy revealed dermis infiltration by septated, non-pigmented fungal hyphae with vascular invasion (Fig. 3). On day 18, after 52h of cultivation, Fusarium sp. was detected in skin sample cultures. The strains isolated in three blood cultures (Bactec 9240, Becton Dickinson) were identified as F. solani species complex. Granulocyte colony-stimulating factor (G-CSF) was administered every other day (300μg/48h subcutaneously) to prevent immune reconstitution syndrome, together with voriconazole (VRZ; two doses 6mg/kg i.v. every 12h, followed by 4mg/kg every 12h) despite the antifungigram results that indicated high MIC values for VRZ (>8μg/ml) and AmB (4μg/ml) (Table 1). On day 33, coincident with recovery from neutropenia, he presented a sudden loss of visual acuity in his right eye being subsequently diagnosed as endogenous endophthalmitis (Fig. 4). After vitrectomy and intravitreal instillation with VRZ (0.01% w/v), the patient experienced a complete recovery of visual acuity and clinical restitution. Galactomannan determinations (Platelia Aspergillus EIA test, Bio-Rad) in the sera on days 20, 27, 34, 55, 59, 64 and 70 were negative (index <0.5). After a prolonged treatment with L-AmB and VRZ (80 and 93 days, respectively) the patient consulted for headache and fever and died a few hours later, after hospital admission (day 103). The necropsy study revealed multiple foci of leukemic meningitis in the cerebral parenchyma. Fungal hyphae were observed in the pulmonary parenchyma but cultures were negative. No fungus was found in the skin, brain tissue, cerebrospinal fluid or any other parenchyma studied.
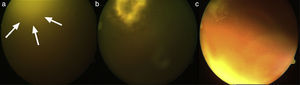
Lesions of patient 2 due to Fusarium solani species complex infection. (a) Erythematous lesion on a hemorrhagic background on the sole of his right foot, the probable gateway of the invasion, and (b) subcutaneous nodule with central necrosis surrounded by spreading erythema, reminiscent of ecthyma gangrenosum, on his right limb.
Evolution of endogenous fungal endophthalmitis corresponding to patient 2. (a) Severe vitritis with “headlight in the fog” sign due to underlying chorioretinitis caused by Fusarium solani species complex; (b) chorioretinitis area in the temporo-superior quadrant of the right eye after vitrectomy, and (c) resolving chorioretinitis one month after therapy with liposomal amphotericin B and voriconazole.
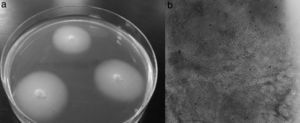
A 15-year-old male patient diagnosed with acute myeloid leukemia (AML) in September 2010 suffered an early relapse of his disease after autologous peripheral blood stem cell transplantation. A second salvage chemotherapy course was started with cytarabine and clofarabine in June 2011 (day 0) due to persistent AML after a previous treatment with fludarabine and cytarabine at high doses with G-CSF priming. He received antifungal prophylaxis with oral fluconazole (200mg/day), and intrathecal therapy with methotrexate, cytarabine and hydrocortisone was given during intensive treatment to prevent CNS leukemia. During deep neutropenia he presented with fever, and coagulase-negative Staphylococcus was isolated in blood cultures. The lack of response to imipenem, gentamicin and vancomycin advised the addition of L-AmB (5mg/kg/day) to the antimicrobial regimen. On day 14 postchemotherapy, erythematous and painful nodular lesions were observed on both legs. Hyphal structures were detected in the skin biopsy treated with 10% KOH and a filamentous fungus was isolated four days later. The appearance of the colonies was filamentous with a smooth and elevated central area, and the isolate was identified as F. dimerum (Fig. 5). Serum Aspergillus galactomannan determination was negative on day 14. VRZ was added to the antimicrobial regimen (two doses of 6mg/kg i.v. at 12h intervals, followed by 4mg/kg every 12h). Chest, abdominal and pelvic CT showed hypodense nodular lesions suggestive of microabscesses in lungs, kidneys, paraspinal muscles and spleen. The halo sign was not seen. Fundus examination was normal. The use of G-CSF (300μg/24h subcutaneously) and recovery from neutropenia were accompanied by the disappearance of fever on day 28 and clinical restitution of the patient. As a consequence of persistent leukemia (25% blast cells in bone marrow), the patient received a third course of reinduction treatment similar to the previous one on day 41. A new CT study, carried out prior to chemotherapy, revealed no new lesions while the previously observed pulmonary, renal and splenic nodular lesions were reduced in size. Nevertheless, on day 78, the emergence of new and painful skin nodules was observed and histopathological study and cultivation led to F. dimerum isolation. Unfortunately, our patient died a few days later (day 98). The antifungal treatment consisted of L-AmB for two days followed by combination with VRZ for 33 days, and finally VRZ alone the following 39 days. The antifungal sensitivity of the F. dimerum isolate is shown in Table 1.
Identification of fungal isolates and antifungal susceptibility testsThe fungal isolates were identified as Fusarium spp. based on the characteristics of colonies grown on potato-dextrose agar (PDA) at 35°C, and microscopic observation of fungal structures stained with lactophenol blue.
Species level identification was accomplished by partial sequencing of the TEF1 gene. DNA extraction from a seven-day old culture on PDA was accomplished with the UltraClean Microbial DNA isolation kit (MoBio Laboratories, USA) following the manufacturer's instructions with some modifications; after adding the MD1 solution, cells were maintained in liquid nitrogen for 15min followed by another 15-min period in a water bath at 56°C. The extracted DNA was quantified (BioPhotometer plus; Eppendorf AG, Germany) and stored at −70°C. The primers for DNA amplification were EF1 (5′-ATGGGTAAGGA(A/G)GACAAGAC-3′) and EF2 (5′-GGA(G/A)GTACCAGT(G/C)ATCATGTT-3′).23 The 50-μl final volume of the PCR mix contained 250μM each of deoxynucleotide triphosphates (Applied Biosystems, USA), 0.25μM of each primer EF1 and EF2, 1.5 units of AmpliTaq®DNA Polymerase (Applied Biosystems), 5μl of GeneAmp®10X PCR Buffer (Applied Biosystems) and 10μl of DNA extract. Amplification was performed in a Perkin Elmer GeneAmp PCR System 2400 (Roche Diagnostics Systems, USA), and the cycling conditions were 94°C for 5min, followed by 30 cycles of 94°C for 30s, 52°C for 30s and 72°C for 90s, and a final extension step at 72°C for 10min.23 Amplicons were checked by electrophoresis and PCR products were purified with the commercial NucleoSpin® Extract II kit (Macherey-Nagel, Germany) and stored at −20°C until processing. PCR amplicons were sequenced using the BigDye Terminators v3.1 (Applied Biosystems) and the following conditions: 94°C for 3min, followed by 25 cycles of 96°C for 10s, 50°C for 5s, and 60°C for 4min. DNA samples were processed in an ABI 3130XL sequencer (Applied Biosystems), analyzed with the software program Sequence Scanner v1.0 (Applied Biosystems), and the consensus sequence calculated with the Multiple Sequence Alignment ClustalW2 (UCB, Ireland) program. These sequences were then compared with the BLAST program in GenBank to establish the isolates’ identity. The sequences were incorporated into the GenBank database with reference numbers KJ534573 for F. solani species complex, KJ534574 for F. oxysporum species complex, and KJ534575 for F. dimerum. The amplified TEF gene sequence of F. solani species complex isolate matched that of the recently described species Fusarium keratoplasticum45 included in the Fusarium MLST database (http://www.cbs.knaw.nl/fusarium/).37
In vitro antifungal susceptibility tests for Fusarium isolates were performed according to the EUCAST broth microdilution technique11,50 at the Unidad de Micología del Centro Nacional de Microbiología (Instituto de Salud Carlos III, Madrid, Spain).
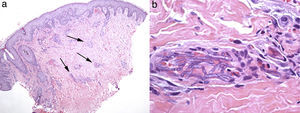
DiscussionFusarium species are ubiquitous fungi in soils rich in organic substrates all over the world, from tropical or temperate regions to the desert, or even Arctic areas, as a consequence of their high adaptability.19,29 They travel great distances using wind or rain and, like Aspergillus, their main portal of entry to the human body is the inhalation of fungal conidia. Wounds in the skin, catheter insertion and cellulite adjacent to paronychia or onychomycosis are also common ways of acquiring a spreading infection,59 which may apply to cases of patients 1 and 2. Fusarium has been identified in the water supplies of hospitals, where showers may contribute as a very effective aerosol system facilitating the inhalation of conidia.2 Under permissive local conditions, Fusarium shows a high capacity to invade blood vessels causing hemorrhagic infarction and tissue necrosis (Fig. 3), and to spread further from there. This characteristic of Fusarium could be related to their recognized capacity for adventitious sporulation, resulting in production of blastoconidia in tissues.21 In addition, it is relatively easy to retrieve it from biological samples since it grows well in culture media, including necropsy material.51
Unlike immunocompetent hosts where onychomycosis and keratitis are the most common Fusarium infections,32 disseminated forms are the most frequent clinical presentation of fusariosis in patients with hematologic neoplasms or subjected to hematopoietic stem cell transplant (HSCT), representing 79% and 90% of the observed Fusarium infections, respectively.33,34 The most common DF pattern shows cutaneous papules or nodules with central necrosis, giving the lesions an ecthyma gangrenosum-like appearance, combined with the isolation of the organism from blood cultures,32 as observed in patients 2 and 3. In contrast to Aspergillus spp. or zygomycetes infections, the presence of Fusarium in blood is commonly observed in 40–60% of DF patients.9,22,32,59 In addition, DF may be accompanied by sinusitis, necrotizing pneumonia or endophthalmitis, clinically indistinguishable from other IFIs,32 as observed in patients 1 and 2. The cultivation and histopathological analysis of cutaneous lesions are cost-efficient diagnostic tools of DF, as evidenced in Nucci and Anaissie's series that showed that in 76 of 148 patients (51%) with disseminated skin lesions, analysis of this unique location allowed the diagnosis of the infection.31 In our patients, analysis of cutaneous lesions was the only diagnostic source in patient 3. In contrast, although patients 1 and 2 had cutaneous lesions as well, the Fusarium isolates were only identified in blood cultures.
An early diagnosis is critical for infection control. Thoracic CT dramatically improves the sensitivity of simple X-ray to diagnose pulmonary involvement of DF, and consequently it is highly recommended for suspected fungal pneumonia. Similar to aspergillosis, the presence of nodules or masses is the most frequent finding but, in contrast, according to Marom et al.24 “the halo sign” is not usually found. This situation was observed in patients 1 and 3 of this study, but patient 2 showed an incipient sign of halo in the thoracic CT scan that may reflect a different stage of the infection (Fig. 2b). Serum determination of fungal antigens such as galactomannan and/or β(1-3)-d-glucan has limited value in the diagnosis of DF. However, some authors believe it may be useful in a highly suggestive clinical context of IFI, where positive β(1-3)-d-glucan and negative galactomannan would force the possibility of DF to be considered.32 Nevertheless, the usefulness of the Platelia Aspergillus EIA assay for discriminating against fusariosis has been recently called into question, since positive results have been reported for DF patients as well.55 In the present work, determinations of β(1-3)-d-glucan in patient 1 sera showed very high values, while galactomannan tests for patients 2 and 3 were negative, reflecting the ‘conventional’ serological pattern of DF. In our opinion, the value of these tests for DF still remains to be determined.
The first cases of DF in hematological patients were described in children in the 1970s,10 and later in adults. Despite the medical developments in subsequent years and the use of new antifungal compounds, no significant changes in its high mortality rate have been observed. Recent studies claim values between 63% and 79% on days 90 and 120, respectively, after the microbiological diagnosis. The variables influencing the mortality rate include persistent neutropenia, pulmonary or CNS involvement, fungemia and recent treatment with corticosteroids.9,2233,34 According to the series of Nucci et al.33,34 patients undergoing HSCT achieve even higher mortality rates of 87%, 90 days after DF diagnosis. The species most frequently involved are F. solani species complex (50% of cases), F. oxysporum species complex (20%) and Gibberella fujikuroi species complex (20%), where Fusarium verticillioides and Fusarium proliferatum are the most representative of the latter.28,29,32 Other species more rarely involved in DF are F. dimerum, Fusarium chlamydosporum, Fusarium sacchari, Fusarium nygamai, Fusarium napiforme, Fusarium antophilum and Fusarium vasinfectum (the latter is a formae specialis of F. oxysporum so not a separate species).32 The low frequency of DF and its relative resistance to most antifungal compounds have not allowed an ideal treatment to be established. However, the observed susceptibility of the isolates to various antifungal agents and the accumulated clinical experience have led to a series of recommendations. Terbinafine has proved a high in vitro activity against strains of F. dimerum4 and F. verticillioides5; however, Spader et al.47 did not corroborate this observation for the latter species. The combination of voriconazole and terbinafine exhibited in vitro synergism against 84% of strains of F. chlamydosporum, F. oxysporum, F. proliferatum and F. solani,47 and Inano et al.17 have recently reported the first case of disseminated F. solani infection successfully treated by combination therapy of VRZ and terbinafine accompanied by surgical resection of endocardial lesions. Terbinafine may be administered topically or orally, however it is not recommended for hematology patients due to its toxic effects on the liver and bone marrow.18 Fluconazole and echinocandins have apparently no in vitro activity against most Fusarium spp. while other azoles such as VRZ, posaconazole and itraconazole show a variable activity according to species4,12,43,53; nevertheless, the strains of F. solani SC exhibit high MICs for these azoles.1,56 These data do not match those published for Nucci's series in 2007,32 and we still do not know the true meaning of MIC values in the clinical practice.36 In this way, voriconazole, the most often used azole in recent years, appears clinically effective despite its high MIC values, especially against F. solani,22,35 and even more in combination with AmB.20 AmB inhibits the fungal growth of most Fusarium isolates, including the majority of the strains belonging to the F. solani species complex (MIC ≤1μg/ml) while MICs are higher for F. verticillioides.1,56 Although there are no conclusive data supporting the use of L-AmB together with VRZ, this combination has shown synergistic in vitro activity46 and has been used successfully in severely compromised patients with DF undergoing allogeneic HSCT.16,49,52
Altogether, there is still some degree of consensus that the most active antifungal agents against Fusarium in the clinical setting are L-AmB, VRZ and posaconazole.13,22,26,28,32,40,48,53 The most recent ESCMID & ECMM joint guidelines on diagnosis and management of Fusarium spp. mycoses recommend voriconazole and lipid-based amphotericin B formulations, with strong (AII) and moderate (BII) support of the recommendation, respectively.57 Posaconazole has been proposed in DF patients refractory to conventional therapy, or in patients who show intolerance to AmB44; however, this proposal must be taken with reservations since there have been two recent case reports of DF after prophylactic treatment with this triazole derivative.7
In agreement with the aforementioned studies of antifungal susceptibility, our three isolates showed low susceptibility to most antifungal agents analyzed, except F. dimerum SC (patient 3) to AmB and terbinafine, and F. oxysporum SC (patient 1) to AmB (Table 1). Interestingly, despite the F. solani SC (F. keratoplasticum) isolate exhibiting high MIC values for AmB and VRZ, both drugs were used with good results for the treatment of patient 2. This situation underscores the frequent discrepancy between the clinical evolution of patients and susceptibility test results.
Other relevant aspects to be considered in the treatment of DF are surgically cleaning foci of infection when possible, withdrawing presumably contaminated catheters and recovery from neutropenia with the administration of G-CSF, GM-CSF and granulocyte transfusions. It is noteworthy to highlight the unknown impact that DF pulmonary complications induced by immune reconstitution syndrome may represent for the mortality.42 The inflammatory reaction triggered by a vigorous and rapid recovery from neutropenia in patients with fungal pneumonia due to Aspergillus may cause life-threatening complications such as massive hemoptysis or refractory dyspnea in a significant number of patients.54 Thus, in patient 2, we tried to modulate the recovery from neutropenia by administering G-CSF on alternate days, since this patient had an extensive lung involvement. In addition, frequent transfusions of platelets, achieving figures higher than 50.000/μl, were administered to prevent pulmonary bleeding that otherwise would make gaseous exchange difficult. These measures seemed to contribute to the clinical restitution of the patient.
Muhammed et al.27 remarked that skin lesions in immunocompromised patients should raise the suspicion for skin or disseminated fusariosis. A recent review by Nucci et al.36 stated that 14 out of 21 cases of invasive fusariosis showed a primary cutaneous portal of entry. The frequent involvement of the skin in the development of DF makes prevention a hallmark to be kept in mind with some special considerations. It is essential to warn patients, notably those from temperate or tropical countries, of possible skin wounds derived from inadequate footwear or nail care, especially if there are adjacent areas of paronychia or onychomycosis. If such lesions occur, they require surgical cleaning, the administration of topical antifungal agents against Fusarium and other filamentous fungi, and microbiological culture before undergoing chemotherapy treatment and/or HSCT.31 Performing new chemotherapy rounds or HSCTs is controversial since persistent sources of infection might be responsible for a new episode of dissemination, which unfortunately happened to patient 3. Nonetheless, in this case, the activity of the AML forced the treatment. In such a situation, the patient's clinical stability, an absence of new lesions and an intensive treatment with the appropriate antifungal agent should not delay chemotherapy if required.3,58
Controlling the hematologic disease and recovering the patient's immune competence are critical for the control of DF; otherwise, the fatality rate of this infection is high. Anyway, the mortality rate of this IFI is still very high in patients with hematologic malignancies, especially for those in advanced stages. In our experience, the management of fusariosis is not well defined and is highly individualized. In our opinion, the prevention of infection in colonized patients, the maintenance of a high level of diagnostic suspicion for early diagnosis, and the combined, vigorous and prolonged use of L-AmB and VRZ are essential to decrease the mortality rate of this devastating infection.
Conflict of interestThe authors declare no conflict of interest.
This work was supported by the Departamento de Educación, Política lingüística y Cultura del Gobierno Vasco [Basque Government-Spain, grant number IT788-13 to M.-D.M., J.-C.G.R. and I.O.], and the University of the Basque Country [UPV/EHU, grant number UFI11/25 to M-D.M.].