Candidemia is one of the most frequent opportunistic mycoses worldwide. Limited epidemiological studies in Latin America indicate that incidence rates are higher in this region than in the Northern Hemisphere. Diagnosis is often made late in the infection, affecting the initiation of antifungal therapy. A more scientific approach, based on specific parameters, for diagnosis and management of candidemia in Latin America is warranted.
‘Recommendations for the diagnosis and management of candidemia’ are a series of manuscripts that have been developed by members of the Latin America Invasive Mycosis Network. They aim to provide a set of best-evidence recommendations for the diagnosis and management of candidemia.
This publication, ‘Recommendations for the management of candidemia in neonates in Latin America’, was written to provide guidance to healthcare professionals on the management of neonates who have, or who are at risk of, candidemia.
Computerized searches of existing literature were performed by PubMed. The data were extensively reviewed and analyzed by members of the group. The group also met on two occasions to pose questions, discuss conflicting views, and deliberate on a series of management recommendations.
‘Recommendations for the management of candidemia in neonates in Latin America’ includes prophylaxis, empirical therapy, therapy for proven candidemia, patient work-up following diagnosis of candidemia, central venous catheter management, and management of complications.
This manuscript is the fourth of this series that deals with diagnosis and treatment of invasive candidiasis. Other publications in this series include: ‘Recommendations for the diagnosis of candidemia in Latin America’, ‘Recommendations for the management of candidemia in adults in Latin America’, and ‘Recommendations for the management of candidemia in children in Latin America’.
This article is also published in Spanish in this issue. It can be found inhttp://dx.doi.org/10.1016/j.riam.2013.06.002
La candidemia es una de las micosis oportunistas más frecuentes en todo el mundo. El escaso número de estudios epidemiológicos llevados a cabo en América Latina indica que las tasas de incidencia en esta región son mayores que las descritas en el hemisferio norte. A menudo el diagnóstico de la infección se establece tardíamente, lo que afecta el inicio del tratamiento antimicótico. Por esta razón, para el diagnóstico y el manejo de la candidemia está justificada una estrategia más científica, basada en parámetros específicos.
Recomendaciones para el diagnóstico y manejo de la candidemia constituye una serie de artículos preparados por miembros del grupo Latin America Invasive Mycosis Network. Su objetivo es proporcionar las mejores evidencias disponibles para el diagnóstico y el manejo de la candidemia.
El presente artículo, Recomendaciones para el manejo de la candidemia en neonatos en América Latina, ha sido redactado con el objetivo de orientar a los profesionales de la salud en el manejo de los neonatos que padecen, o pueden padecer, candidemia.
Mediante la base de datos PubMed se emprendió una búsqueda informatizada de los estudios publicados. Los miembros del grupo revisaron y analizaron exhaustivamente los datos. El grupo también se reunió en dos ocasiones para proponer preguntas, abordar los puntos de vista conflictivos y deliberar sobre las recomendaciones terapéuticas.
Recomendaciones para el manejo de la candidemia en neonatos en América Latina incluye aspectos sobre profilaxis, terapia empírica, tratamiento de la candidemia demostrada, evaluación y seguimiento del paciente después del diagnóstico de candidemia, manejo de los recién nacidos con infección por Candida del catéter venoso central y manejo de otras complicaciones.
Este manuscrito es el cuarto de los artículos de esta serie dedicada al diagnóstico y tratamiento de las candidiasis invasoras. Otras publicaciones de esta serie son Recomendaciones para el diagnóstico de la candidemia en América Latina, Recomendaciones para el manejo de la candidemia en adultos en América Latina, y Recomendaciones para el manejo de la candidemia en niños en América Latina.
Este artículo está publicado en español en este mismo número. Puede encontrarlo enhttp://dx.doi.org/10.1016/j.riam.2013.06.002
There is limited information on the epidemiology of candidemia in pediatric populations. One retrospective study of the incidence of candidemia in the USA in 2000 reported 43 pediatric cases (<18 years of age) per 100,000 hospital admissions.103 Candidemia-associated mortality in pediatric patients is generally lower than in adults, ranging from 13% to 23% in children and 43% to 54% in infants.31,102,103
Candida is the third most common cause of late-onset sepsis in preterm neonates.57,91 Results from a worldwide survey (1997–2000) on Candida isolates, which included hospitals from the USA, Canada, Latin America, and Europe, demonstrated that Candida albicans was the most common cause of infection with among all age groups investigated (≤1, 2–15, 16–64, and ≥65 years), with uniform rates of infection observed across the ages.73 Among infant and pediatric patients (children ≤1 year and 2–15 years of age), the dominant causes of infections were C. albicans and Candida parapsilosis; very few infections were due to Candida krusei and Candida glabrata (3%). In neonates, higher mortality has been reported with infections due to C. albicans compared with those due to other Candida species16,27,91: 43% in C. albicans, compared with 20% C. parapsilosis, and 0% Candida tropicalis candidemia16; 24% in C. albicans, compared with 0% in C. parapsilosis invasive candidiasis27; 44% in C. albicans, compared with 16% in C. parapsilosis sepsis.91
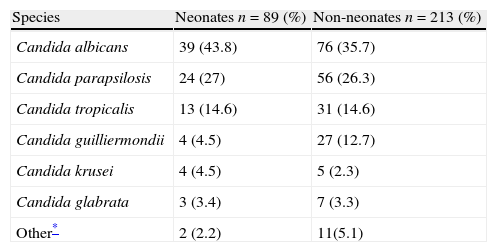
Latin American dataEpidemiologic information on pediatric candidemia in Latin America is limited.70,76,79 In a prospective surveillance study in children and adults from 23 hospitals throughout eight Latin American countries (Argentina, Brazil, Chile, Colombia, Ecuador, Honduras, Mexico and Venezuela) between November 2008 and October 2010, 302 of the 752 clinical isolates (40%) were from patients <18 years of age, with a mean incidence of 0.98/1000 admissions. Of these 302 episodes of candidemia, 89 (29%) occurred in neonates (≤28 days), with a median age at candidemia presentation of 16 days (range 1–28 days). Among the 213 children, median age was 2 years (range 0.2–18 years). The main species isolated in neonates and children were C. albicans (44% and 36%, respectively), C. parapsilosis (27% and 26%), C. tropicalis (15% and 15%), and Candida guilliermondii (5% and 13%) (Table 1).85 Overall mortality was 31% in the pediatric population: 41% in neonates, 26% in children from 1 month to 1 year, 24% in children from 1 to 12 years, and 35% in patients between 13 and 18 years of age (p=0.049 comparing neonates with non-neonates).85
Species distribution in 302 episodes of candidemia in children in 23 hospitals from eight countries in Latin America.
| Species | Neonates n=89 (%) | Non-neonates n=213 (%) |
| Candida albicans | 39 (43.8) | 76 (35.7) |
| Candida parapsilosis | 24 (27) | 56 (26.3) |
| Candida tropicalis | 13 (14.6) | 31 (14.6) |
| Candida guilliermondii | 4 (4.5) | 27 (12.7) |
| Candida krusei | 4 (4.5) | 5 (2.3) |
| Candida glabrata | 3 (3.4) | 7 (3.3) |
| Other* | 2 (2.2) | 11(5.1) |
Additional data are needed to better characterize candidemia in neonates and children. Such data include: demographics, clinical presentation, risk factors, treatment, outcome and mortality, microbiology issues, antifungal susceptibility, the affected population (neutropenic vs. non-neutropenic children, intensive care unit [ICU] vs. non-ICU patients), species distribution, epidemiology of resistant isolates, the effects of prophylaxis on resistance,6 mortality and long-term outcomes (particularly neurodevelopmental consequences),22 timing of infection, and characteristics of late-onset vs. early-onset infection.6 Large, randomized controlled trials are also needed to evaluate the efficacy of different treatments.6
A better understanding of candidemia in neonates and children will help to define the best practices for management. Currently, there are no separate treatment guidelines for children and neonates with candidemia, and no consensus treatment guidelines exist in Latin America exclusively for candidemia. The following guidelines provide recommendations for the treatment of candidemia in neonates in Latin America and are based on current clinical evidence, the regional situation regarding candidemia in pediatric patients, and the expert opinions of the authors.
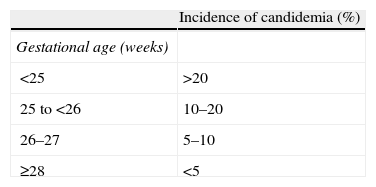
Unique clinical features that affect management of candidemia in neonatesRisk factors for Candida infectionThe clinical features of neonates born prematurely and with low birth weights (particularly those with extremely low birth weight [ELBW], weighing less than 1000g and born before 28 weeks gestation) increase their susceptibility to Candida infections7 and are important considerations in the management of infections (Table 2). Premature newborns are born without fully competent immune systems; thus, they may lack basic immunologic functions, such as chemotaxis, cytokine production, antibody production, and phagocytosis.61 Premature newborns are often in need of aggressive supportive care and medical interventions and, as a result, may have many risk factors for candidemia, including total parenteral nutrition (TPN), central venous catheters (CVCs), mechanical ventilation, broad-spectrum antibiotics, H2 blockers, steroids, prolonged neonatal intensive care unit (NICU) stays, and abdominal or thoracic surgery.16,24,72,84
Incidence of candidemia in neonates by gestational age and weight at birth.
| Incidence of candidemia (%) | |
| Gestational age (weeks) | |
| <25 | >20 |
| 25 to <26 | 10–20 |
| 26–27 | 5–10 |
| ≥28 | <5 |
| Weight at birth (g) | |
| <750 | >10 |
| 750–999 | 5–10 |
| 1000–1500 | <5 |
Neonates frequently have persistent candidemia; that is, positive blood cultures (BCs) for more than 72h in a patient receiving effective therapy. Neonates with candidemia may develop serious complications resulting from spread of infection to the central nervous system (CNS), heart, eyes, kidneys, spleen, and liver. Those with CNS complications may experience neurodevelopmental impairment, which may continue following resolution of the Candida infection. In one study, 73% of ELBW neonates with candidemia had died or showed signs of neurodevelopmental impairment by 18–22 months follow-up.16 Compared with neonates not infected with Candida, those with Candida infections were more likely to have moderate or severe cerebral palsy, and to be blind or deaf.16
Summary of unique clinical features of neonates
Risk factors for infection with Candida spp.
- 1.
Gestational age of <28 weeks.
- 2.
Weigh at birth <1000g.
- 3.
TPN.
- 4.
CVC.
- 5.
Mechanical ventilation.
- 6.
Broad-spectrum antibiotics.
- 7.
H2 blockers.
- 8.
Steroids.
- 9.
Prolonged neonatal intensive care unit stays.
- 10.
Abdominal or thoracic surgery.
In neonates with invasive candidiasis:
- 1.
Persistent candidemia can be common.
- 2.
There is the possibility of false-negative BCs.
- 3.
Infection may spread to the CNS, heart, eyes, kidneys, spleen, and liver, resulting in serious complications.
The high risk of candidemia in neonates provides a strong rationale for prophylaxis. Candidemia is highly prevalent in ICU-admitted ELBW neonates and very low birth weight (VLBW) neonates (weighing less than 1500g).44,50 Up to 60% of VLBW neonates may become colonized with Candida in their first month in the NICU, and up to 20% of these infants may develop an invasive fungal infection.44 Invasive candidiasis can be difficult to diagnose in neonates and may be advanced by the time of diagnosis, owing to the non-specific clinical features of the disease and the poor sensitivity of diagnostic tests leading to late recognition of infection.60
The high incidence of complications associated with Candida infection in neonates, including neurodevelopmental impairment, to which neonates are already prone, further supports the use of prophylaxis. In addition, ophthalmologic, cardiac, and visceral involvement, are also associated with Candida infections in neonates.53,68 At the time of hospital discharge, Candida-infected ELBW neonates, compared with non-infected ELBW neonates, were associated with higher rates of chronic lung disease, periventricular leukomalacia, and severe retinopathy.33
The risk–benefit ratio of prophylaxis in neonatesThe potential benefits of Candida prophylaxis must be weighed against the risks by considering the efficacy of prophylaxis, incidence of candidiasis, associated mortality, short- and long-term safety, potential for the development of resistant pathogens, and possible alternatives (Table 3).
Efficacy of Candida prophylaxis in neonates.
| Trial | Methods | Intervention | Results | Conclusion |
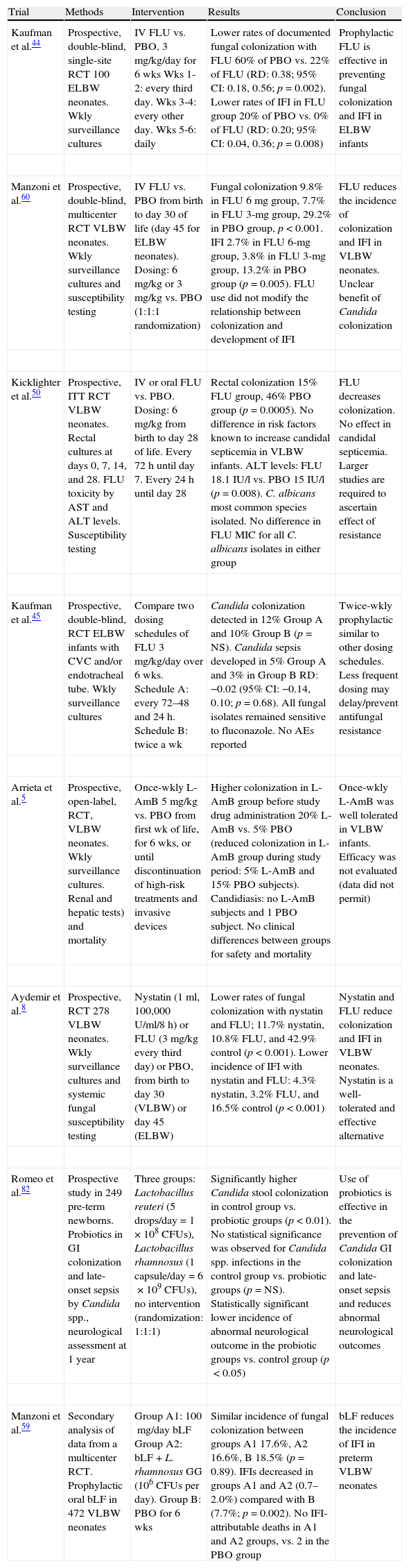
| Kaufman et al.44 | Prospective, double-blind, single-site RCT 100 ELBW neonates. Wkly surveillance cultures | IV FLU vs. PBO, 3mg/kg/day for 6wks Wks 1-2: every third day. Wks 3-4: every other day. Wks 5-6: daily | Lower rates of documented fungal colonization with FLU 60% of PBO vs. 22% of FLU (RD: 0.38; 95% CI: 0.18, 0.56; p=0.002). Lower rates of IFI in FLU group 20% of PBO vs. 0% of FLU (RD: 0.20; 95% CI: 0.04, 0.36; p=0.008) | Prophylactic FLU is effective in preventing fungal colonization and IFI in ELBW infants |
| Manzoni et al.60 | Prospective, double-blind, multicenter RCT VLBW neonates. Wkly surveillance cultures and susceptibility testing | IV FLU vs. PBO from birth to day 30 of life (day45 for ELBW neonates). Dosing: 6mg/kg or 3mg/kg vs. PBO (1:1:1 randomization) | Fungal colonization 9.8% in FLU 6mg group, 7.7% in FLU 3-mg group, 29.2% in PBO group, p<0.001. IFI 2.7% in FLU 6-mg group, 3.8% in FLU 3-mg group, 13.2% in PBO group (p=0.005). FLU use did not modify the relationship between colonization and development of IFI | FLU reduces the incidence of colonization and IFI in VLBW neonates. Unclear benefit of Candida colonization |
| Kicklighter et al.50 | Prospective, ITT RCT VLBW neonates. Rectal cultures at days 0, 7, 14, and 28. FLU toxicity by AST and ALT levels. Susceptibility testing | IV or oral FLU vs. PBO. Dosing: 6mg/kg from birth to day 28 of life. Every 72h until day 7. Every 24h until day 28 | Rectal colonization 15% FLU group, 46% PBO group (p=0.0005). No difference in risk factors known to increase candidal septicemia in VLBW infants. ALT levels: FLU 18.1 IU/l vs. PBO 15 IU/l (p=0.008). C. albicans most common species isolated. No difference in FLU MIC for all C. albicans isolates in either group | FLU decreases colonization. No effect in candidal septicemia. Larger studies are required to ascertain effect of resistance |
| Kaufman et al.45 | Prospective, double-blind, RCT ELBW infants with CVC and/or endotracheal tube. Wkly surveillance cultures | Compare two dosing schedules of FLU 3mg/kg/day over 6wks. Schedule A: every 72–48 and 24h. Schedule B: twice a wk | Candida colonization detected in 12% Group A and 10% Group B (p=NS). Candida sepsis developed in 5% Group A and 3% in Group B RD: −0.02 (95% CI: −0.14, 0.10; p=0.68). All fungal isolates remained sensitive to fluconazole. No AEs reported | Twice-wkly prophylactic similar to other dosing schedules. Less frequent dosing may delay/prevent antifungal resistance |
| Arrieta et al.5 | Prospective, open-label, RCT, VLBW neonates. Wkly surveillance cultures. Renal and hepatic tests) and mortality | Once-wkly L-AmB 5mg/kg vs. PBO from first wk of life, for 6wks, or until discontinuation of high-risk treatments and invasive devices | Higher colonization in L-AmB group before study drug administration 20% L-AmB vs. 5% PBO (reduced colonization in L-AmB group during study period: 5% L-AmB and 15% PBO subjects). Candidiasis: no L-AmB subjects and 1 PBO subject. No clinical differences between groups for safety and mortality | Once-wkly L-AmB was well tolerated in VLBW infants. Efficacy was not evaluated (data did not permit) |
| Aydemir et al.8 | Prospective, RCT 278 VLBW neonates. Wkly surveillance cultures and systemic fungal susceptibility testing | Nystatin (1ml, 100,000U/ml/8h) or FLU (3mg/kg every third day) or PBO, from birth to day 30 (VLBW) or day 45 (ELBW) | Lower rates of fungal colonization with nystatin and FLU; 11.7% nystatin, 10.8% FLU, and 42.9% control (p<0.001). Lower incidence of IFI with nystatin and FLU: 4.3% nystatin, 3.2% FLU, and 16.5% control (p<0.001) | Nystatin and FLU reduce colonization and IFI in VLBW neonates. Nystatin is a well-tolerated and effective alternative |
| Romeo et al.82 | Prospective study in 249 pre-term newborns. Probiotics in GI colonization and late-onset sepsis by Candida spp., neurological assessment at 1 year | Three groups: Lactobacillus reuteri (5 drops/day=1×108CFUs), Lactobacillus rhamnosus (1 capsule/day=6×109CFUs), no intervention (randomization: 1:1:1) | Significantly higher Candida stool colonization in control group vs. probiotic groups (p<0.01). No statistical significance was observed for Candida spp. infections in the control group vs. probiotic groups (p=NS). Statistically significant lower incidence of abnormal neurological outcome in the probiotic groups vs. control group (p<0.05) | Use of probiotics is effective in the prevention of Candida GI colonization and late-onset sepsis and reduces abnormal neurological outcomes |
| Manzoni et al.59 | Secondary analysis of data from a multicenter RCT. Prophylactic oral bLF in 472 VLBW neonates | Group A1: 100mg/day bLF Group A2: bLF+L. rhamnosus GG (106CFUs per day). Group B: PBO for 6wks | Similar incidence of fungal colonization between groups A1 17.6%, A2 16.6%, B 18.5% (p=0.89). IFIs decreased in groups A1 and A2 (0.7–2.0%) compared with B (7.7%; p=0.002). No IFI-attributable deaths in A1 and A2 groups, vs. 2 in the PBO group | bLF reduces the incidence of IFI in preterm VLBW neonates |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; bLF, bovine lactoferrin; CFUs, colony-forming units; CI, confidence interval; CVC, central venous catheter; ELBW, extremely low birth weight (<1000g); FLU, fluconazole; GI, gastrointestinal; IU/l, international units/litre; IFI, invasive fungal infection; ITT, intent to treat; IV, intravenous; L-AmB, liposomal amphotericin B; MIC, minimum inhibitory concentration; NS, not significant; PBO, placebo; RCT, randomized controlled trial; RD, risk difference; VLBW, very low birth weight (<1500g); Wk, week; Wkly, weekly.
Prophylaxis in neonates has a favorable risk–benefit ratio when considering the high mortality associated with Candida infection in this population. However, mortality data are limited, as multicenter studies often report only all-cause mortality associated with fungal infections, rather than mortality specifically and directly related to candidemia. Among VLBW neonates with fungal sepsis, death rates from multicenter studies were 28% (odds ratio, fungi vs. other organisms, 1.67; p<0.05) compared with 7% among VLBW neonates without an infection.90 All-cause mortality is higher among ELBW neonates, ranging from 37% to 40%.33,44,46 The number of deaths directly attributable to fungal infections is most likely smaller than the all-cause mortality reported in many studies. Single-center studies with smaller samples of patients have found lower mortality when reporting mortality directly attributable to fungal sepsis.46
The benefits of prophylaxis must also be balanced against the risk of selecting for resistant organisms. Based on studies of neutropenic and HIV-infected adults, the use of antifungal agents is associated with the development of fluconazole resistance in previously susceptible species, as well as with the emergence of intrinsically resistant species.1,41,51,67 A systematic review of randomized clinical trials found that fluconazole prophylaxis increased the risk of colonization but did not significantly affect the risk of invasive infections, with fluconazole-susceptible dose-dependent or resistant Candida species; however, the overall sample size was limited and data came from neonatal, pediatric, and adult patients. The authors noted that breakthrough infection remains a concern.19 By contrast, multiple randomized controlled trials (Table 3) and observational studies in neonates have noted no increase in resistant species; however, these studies may not have been adequately powered to detect such differences.8,34,44,58,60,62,95
Long-term outcomes of Candida prophylaxis have not been studied in a large, multicenter, randomized controlled trial (Table 3).6,22,28 Because neonates are at increased risk of cholestasis, administration of a potentially hepatotoxic drug is of particular concern.2 One retrospective, non-randomized study with historical controls found an increase in conjugated hyperbilirubinemia in ELBW neonates who received fluconazole prophylaxis compared with neonates who did not receive prophylaxis.2 In a similar study, there was an increase in cholestasis in neonates who received fluconazole prophylaxis. However, two-thirds of patients had other predisposing conditions for cholestasis, mainly the duration of TPN.34
Although some studies have reported no significant incidence of fluconazole-related toxicity,8,9,44,95 one study found a temporary elevation of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in fluconazole-treated neonates, which returned to normal levels within 2weeks (Table 3).60 A follow-up study of survivors aged 8–10 years old found that fluconazole prophylaxis for the prevention of invasive Candida infections in ELBW neonates did not appear to be associated with any long-term adverse effects.48
While trials generally show efficacy of prophylaxis with fluconazole, the actual rates of candidiasis and efficacy vary by country and NICU.49 Therefore, an accurate assessment of the risks and benefits of prophylaxis depends on local or at least country-specific information. There are few alternatives to prophylaxis with antifungal agents for the prevention of Candida infections in neonates. One alternative in VLBW neonates was recently reported: prophylactic bovine lactoferrin reduced the incidence of invasive fungal infection in preterm VLBW neonates (Table 3).59 Prevention is otherwise limited to monitoring high-risk patients and using infection control measures to prevent the spread of identified cases; however, data on these methods are limited. Accurate and early diagnosis of invasive Candida disease in neonates remains a challenge, as BCs – the ‘gold standard’ for diagnosis – have low sensitivity.25
Identifying neonates for prophylaxisNot all neonates need prophylaxis for invasive candidiasis. Multiple studies have identified risk factors associated with invasive candidiasis, and these characteristics can be used to identify neonates at high risk of infection as candidates for prophylaxis. As previously mentioned, birth weight, prematurity, intubation, length of hospital or NICU stay, presence of CVCs, and use of broad-spectrum antibiotics (e.g. third-generation cephalosporins), H2 blockers, and TPN are associated with invasive candidiasis.29,57,84 Some studies also report associations with gastrointestinal pathology29 and bacterial sepsis.57
Multiple studies have investigated the use of prophylaxis in select groups of neonates, including VLBW infants,50,56 ELBW neonates (Table 3),2,44,45 VLBW neonates with central vascular access,17 and VLBW neonates with one additional risk factor (treatment with a third-generation cephalosporin, treatment for >10 consecutive days with systemic broad-spectrum antibiotics, or fungal colonization from surface sites and a CVC in situ).62 The Infectious Diseases Society of America (IDSA) recommends prophylaxis for ELBW neonates in nurseries with high rates of invasive candidiasis; but the IDSA also notes that antifungal drug resistance, drug-related toxicity, and neurodevelopmental outcomes should be observed.71
Prophylaxis with fluconazoleDespite potential concerns regarding cost,63,66 antifungal prophylaxis with fluconazole has been found to be inexpensive and cost-effective.49 A comparison of costs before and after initiation of fluconazole prophylaxis in neonates at high risk for invasive fungal infections in a single-institution observational trial found that fluconazole prophylaxis was cost-effective.95 Compared with lower and less frequent dosing, twice-weekly dosing of prophylactic fluconazole can decrease cost and patient exposure to the drug in high-risk, preterm ELBW neonates while also decreasing Candida colonization and invasive infection.45
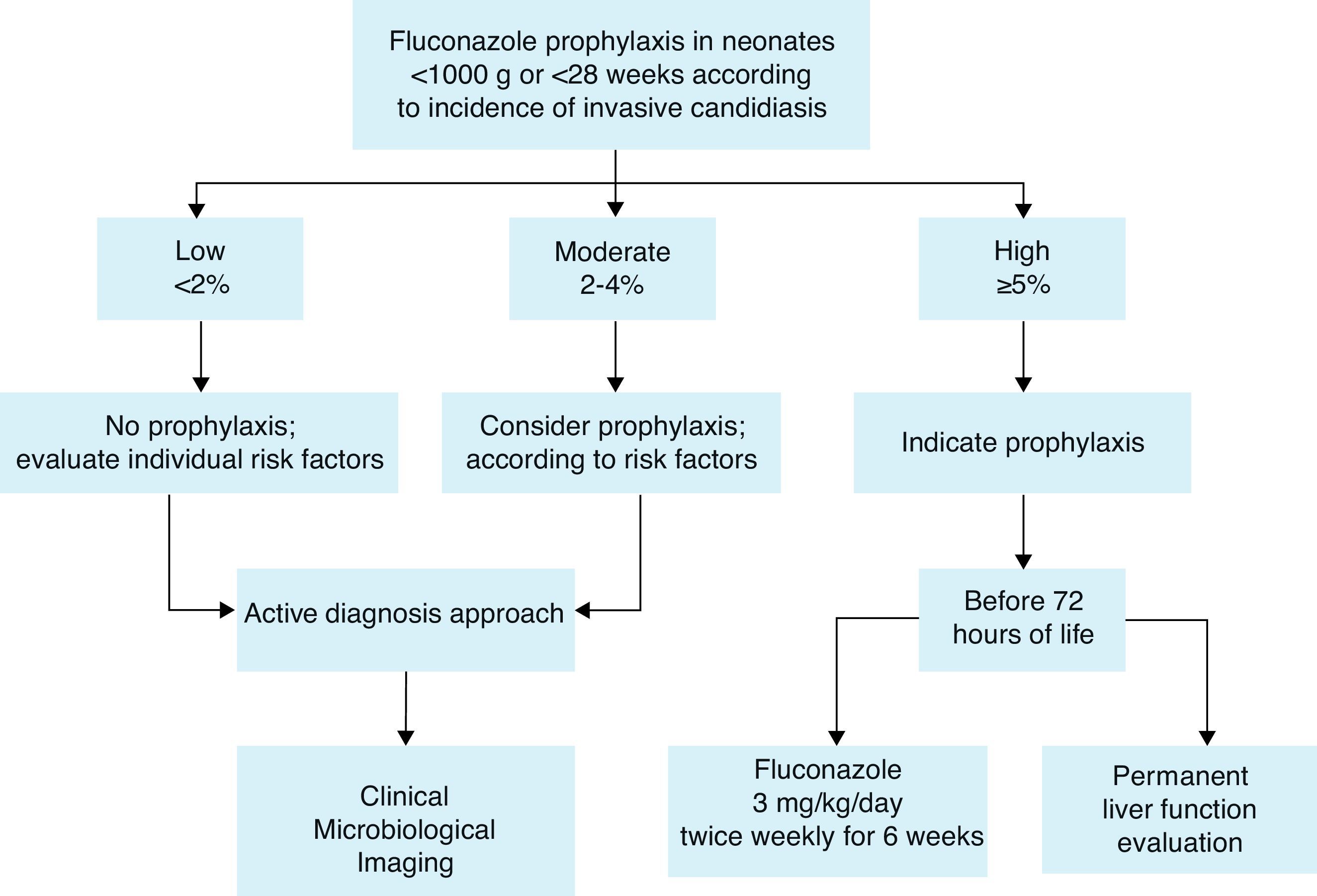
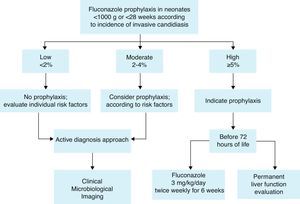
Prophylaxis recommendationBased on these considerations, the Working Group recommends fluconazole prophylaxis (3mg/kg twice weekly for 6weeks) in ELBW neonates44,60 who are in NICUs that have a high incidence of invasive candidiasis defined as ≥5%. If the incidence is not known, fluconazole prophylaxis could be considered (Fig. 1). A summary of efficacy data for Candida prophylaxis in neonates that has been reported in systematic reviews and meta-analyses is presented in Table 4.
Recommendations summary forCandidaprophylaxis in neonates:
Risk factors to identify neonates as candidates for prophylaxis are mainly:
- 1.
Low birth weight (<1000g) and extreme prematurity (<28 weeks).
- 2.
Fluconazole prophylaxis (3mg/kg twice weekly for 6 weeks) is recommended in ELBW neonates who are in NICUs that have a high incidence of invasive candidiasis (≥5%).
- 3.
If the incidence is not known or is <5%, fluconazole prophylaxis could be considered according to risk factors.
Efficacy of Candida prophylaxis in neonates (systematic reviews and meta-analyses).
| Trial | Assessment | Methods | Results | Conclusion |
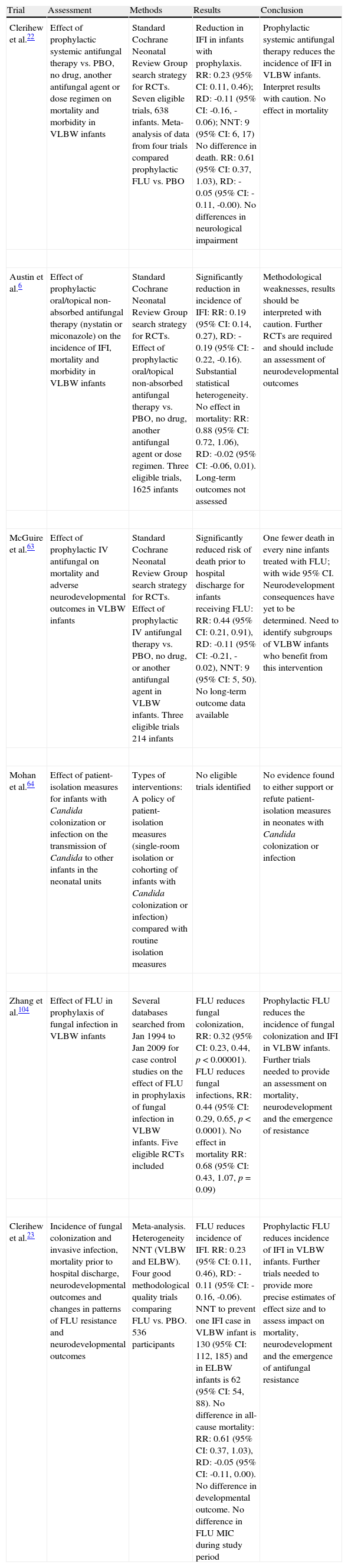
| Clerihew et al.22 | Effect of prophylactic systemic antifungal therapy vs. PBO, no drug, another antifungal agent or dose regimen on mortality and morbidity in VLBW infants | Standard Cochrane Neonatal Review Group search strategy for RCTs. Seven eligible trials, 638 infants. Meta-analysis of data from four trials compared prophylactic FLU vs. PBO | Reduction in IFI in infants with prophylaxis. RR: 0.23 (95% CI: 0.11, 0.46); RD: -0.11 (95% CI: -0.16, -0.06); NNT: 9 (95% CI: 6, 17) No difference in death. RR: 0.61 (95% CI: 0.37, 1.03), RD: -0.05 (95% CI: -0.11, -0.00). No differences in neurological impairment | Prophylactic systemic antifungal therapy reduces the incidence of IFI in VLBW infants. Interpret results with caution. No effect in mortality |
| Austin et al.6 | Effect of prophylactic oral/topical non-absorbed antifungal therapy (nystatin or miconazole) on the incidence of IFI, mortality and morbidity in VLBW infants | Standard Cochrane Neonatal Review Group search strategy for RCTs. Effect of prophylactic oral/topical non-absorbed antifungal therapy vs. PBO, no drug, another antifungal agent or dose regimen. Three eligible trials, 1625 infants | Significantly reduction in incidence of IFI: RR: 0.19 (95% CI: 0.14, 0.27), RD: -0.19 (95% CI: -0.22, -0.16). Substantial statistical heterogeneity. No effect in mortality: RR: 0.88 (95% CI: 0.72, 1.06), RD: -0.02 (95% CI: -0.06, 0.01). Long-term outcomes not assessed | Methodological weaknesses, results should be interpreted with caution. Further RCTs are required and should include an assessment of neurodevelopmental outcomes |
| McGuire et al.63 | Effect of prophylactic IV antifungal on mortality and adverse neurodevelopmental outcomes in VLBW infants | Standard Cochrane Neonatal Review Group search strategy for RCTs. Effect of prophylactic IV antifungal therapy vs. PBO, no drug, or another antifungal agent in VLBW infants. Three eligible trials 214 infants | Significantly reduced risk of death prior to hospital discharge for infants receiving FLU: RR: 0.44 (95% CI: 0.21, 0.91), RD: -0.11 (95% CI: -0.21, -0.02), NNT: 9 (95% CI: 5, 50). No long-term outcome data available | One fewer death in every nine infants treated with FLU; with wide 95% CI. Neurodevelopment consequences have yet to be determined. Need to identify subgroups of VLBW infants who benefit from this intervention |
| Mohan et al.64 | Effect of patient-isolation measures for infants with Candida colonization or infection on the transmission of Candida to other infants in the neonatal units | Types of interventions: A policy of patient-isolation measures (single-room isolation or cohorting of infants with Candida colonization or infection) compared with routine isolation measures | No eligible trials identified | No evidence found to either support or refute patient-isolation measures in neonates with Candida colonization or infection |
| Zhang et al.104 | Effect of FLU in prophylaxis of fungal infection in VLBW infants | Several databases searched from Jan 1994 to Jan 2009 for case control studies on the effect of FLU in prophylaxis of fungal infection in VLBW infants. Five eligible RCTs included | FLU reduces fungal colonization, RR: 0.32 (95% CI: 0.23, 0.44, p<0.00001). FLU reduces fungal infections, RR: 0.44 (95% CI: 0.29, 0.65, p<0.0001). No effect in mortality RR: 0.68 (95% CI: 0.43, 1.07, p=0.09) | Prophylactic FLU reduces the incidence of fungal colonization and IFI in VLBW infants. Further trials needed to provide an assessment on mortality, neurodevelopment and the emergence of resistance |
| Clerihew et al.23 | Incidence of fungal colonization and invasive infection, mortality prior to hospital discharge, neurodevelopmental outcomes and changes in patterns of FLU resistance and neurodevelopmental outcomes | Meta-analysis. Heterogeneity NNT (VLBW and ELBW). Four good methodological quality trials comparing FLU vs. PBO. 536 participants | FLU reduces incidence of IFI. RR: 0.23 (95% CI: 0.11, 0.46), RD: -0.11 (95% CI: -0.16, -0.06). NNT to prevent one IFI case in VLBW infant is 130 (95% CI: 112, 185) and in ELBW infants is 62 (95% CI: 54, 88). No difference in all-cause mortality: RR: 0.61 (95% CI: 0.37, 1.03), RD: -0.05 (95% CI: -0.11, 0.00). No difference in developmental outcome. No difference in FLU MIC during study period | Prophylactic FLU reduces incidence of IFI in VLBW infants. Further trials needed to provide more precise estimates of effect size and to assess impact on mortality, neurodevelopment and the emergence of antifungal resistance |
CI, confidence interval; ELBW, extremely low birth weight (<1000g); FLU, fluconazole; IFI, invasive fungal infection; IV, intravenous; MIC, minimum inhibitory concentration; NNT, number needed to treat; PBO, placebo; RCT, randomized controlled trial; RD, risk difference; RR, relative risk; VLBW, very low birth weight (<1500g).
The Working Group cannot provide a recommendation for the empiric treatment of invasive candidiasis, as there are no validated tools to identify candidates, and only a few studies (none of which were prospective trials) have investigated strategies for empiric treatment. In a retrospective study of neonates at the Hospital de Clínicas de Porto Alegre, Brazil, Candida-related mortality occurred in 11/18 historic control patients compared with 0/6 patients who received empirical treatment for invasive candidiasis.74 In this study, empiric therapy was given to VLBW or very ill neonates who had clinical signs of infection and/or neutropenia and who had been treated with antibiotics (vancomycin or third-generation cephalosporins) for 7 or more days, in association with TPN, mechanical ventilation, postnatal corticosteroids, H2 blockers or mucocutaneous candidiasis. Based on a database of 6172 neonates born at <1250g and with a BC after the third day of life, a predictive multivariate model demonstrated that thrombocytopenia and cephalosporin or carbapenem use in the 7 days prior to BC were risk factors for subsequent invasive candidiasis.13 In addition, neonates who were 25–27 weeks estimated gestational age or born before 25 weeks were also at increased risk.
Treatment of invasive candidiasis in neonatesBased on available data and clinical experience, amphotericin B (AmB), either AmB deoxycholate (AmB-d) or liposomal-AmB (L-AmB) or an echinocandin (micafungin or caspofungin) are the first options recommended for the treatment of neonatal candidiasis (Table 5).
Pharmacological treatment of neonatal candidiasis.
| Drug | Dose | Efficacy/pharmacokinetics | Safety |
| AmB-d | 0.35–0.5mg/kg/day once daily over 2–4h | In a study that compared AmB-d, AmB colloidal dispersion, and L-AmB for treatment in neonates with candidemia there was no difference in time to clear infection observed.55 Pharmacokinetic data in neonates are derived from small studies and exhibit considerable variability.3,32 CSF penetration, which is only 5-10% of serum levels in adults, may reach 40% of serum levels in preterm neonates12 | Side effects observed in VLBW neonates include electrolyte abnormalities and nephrotoxicity.11 There is a trend of more nephrotoxicity with AmB-d than with lipid formulations, with a wide range reported (0–70%).94 Neonates with normal baseline renal function appeared to tolerate AmB-d well; a sodium intake of 4mg/kg/day may significantly reduce AmB-d nephrotoxicity94 |
| L-AmB | 3–5mg/kg/day, once daily | Similar efficacy to AmB-d. In the treatment of systemic candidiasis in VLBW infants, the fungal eradication rate and the time to eradication were 84% and 9±8days, respectively, in the L-AmB group, and 89% and 10±9days in the AmB group (p=0.680 and p=0.712)40 | Well tolerated.40,101 Major adverse effects were lower in the L-AmB-treated than in the AmB-treated VLBW infants (renal toxicity, 21% vs. 55%, p=0.029; hepatotoxicity, 25% vs. 65%, p=0.014)40 |
| Micafungin | 15mg/kg/day once daily | Similar efficacy to L-AmB. Successful treatment of neonates with invasive candidiasis in 7/7 infants who received micafungin and 4/7 (57.1%) infants who received L-AmB.75 Shorter half-life (8h) with more rapid clearance rate (approximately 39ml/h) in premature infants compared with older children and adults.35 Doses up to 15mg/kg/day corresponded to an exposure of 5mg/kg in adults89 | Well tolerated.15,35,89 Among pediatric patients (neonate to 16yrs), those treated with micafungin had a lower incidence of adverse events that led to discontinuation (2/52, 3.8%) compared with those treated with L-AmB (9/54, 16.7%) (p=0.05, Fisher exact test)75 |
| Caspofungin | 25mg/m2 once daily | Superior efficacy to AmB99 and AmB-d.69 In neonates with invasive candidiasis, caspofungin was superior, with a favorable response in 86.7% of patients compared with 41.7% of those who received AmB (p=0.04).99 25mg/m2 once daily was well tolerated in neonates/infants of <3months of age and provided relatively similar plasma exposure to that obtained in adults receiving 50mg/day83 | Well tolerated.69,99 Among term and preterm neonates with invasive candidiasis, there were no clinical or laboratory adverse events related to caspofungin administration.69 In a study that compared caspofungin with AmB in neonates, there were significantly fewer adverse events in the caspofungin group than in the AmB group99 |
| Fluconazole | 12mg/kg/day, once daily | Fluconazole compared favorably with AmB-d.26 Long half-life that decreases with increasing postnatal age.86 A population pharmacokinetic study in 55 neonates and young infants suggests that maintenance fluconazole doses of 12mg/kg/day are necessary to achieve exposures similar to older children and adults98 | Well tolerated.39,87 Among newborns and infants (median birth weight of 1120g and born within gestational weeks 23–38), no serious side effects were observed87 |
AmB, amphotericin B; AmB-d, amphotericin B deoxycholate; CSF, cerebrospinal fluid; L-AmB, liposomal amphotericin B; VLBW, very low birth weight (<1500g).
Data on the treatment of neonatal candidiasis are very limited, and only one trial provides comparative data.75 This multinational, double-blind, randomized controlled trial studied first-line treatment of invasive candidiasis with micafungin (2mg/kg) compared with L-AmB (3mg/kg). There was a sub-study of 106 pediatric patients (ITT population), 19 were premature at birth. Of the 106 pediatric patients, 98 had a confirmed diagnosis of candida at baseline (MITT) and were included in the efficacy analysis. In the MITT population treatment was successful in 35/48 (72.9%) patients who received micafungin and 38/50 (76.0%) patients who received L-AmB. Efficacy was consistent regardless of patient age. Both treatments were well tolerated, but the incidence of adverse events leading to discontinuation was lower in the micafungin group (2/52, 3.8%) compared with the L-AmB group (9/54, 16.7%; p=0.05). Within the subgroup of neonatal patients (0 days to <4 weeks old) only, 4/7 (57.1%) who received L-AmB experienced treatment success.
Despite the efficacy of L-AmB, this therapy is not available in the majority of hospitals in Latin America; however, AmB-d is more freely available, has been widely used in neonates, and is better tolerated in this population than in adults.94 It also appears to be at least as efficacious as L-AmB in the treatment of invasive candidiasis in neonates and infants.94 However, L-AmB has a better safety profile than AmB-d,52 and the long-term consequences of AmB-d treatment in this patient population are not known.
EchinocandinsMicafungin and caspofungin are also recommended for the treatment of neonatal candidiasis. CNS involvement should be ruled out prior to their use owing to unknown efficacy in treating CNS infection (see the management of complications section below). Small trials have shown efficacy of micafungin75 and caspofungin69 in the treatment of neonates with invasive candidiasis. Although data are limited, preliminary investigations have demonstrated that micafungin is well tolerated in neonates.4,35,75,89 Single doses of micafungin (0.75–3.0mg/kg) were well tolerated in premature infants weighing more than 1000g; however, increased clearance of the drug resulted in low plasma concentrations.35 Subsequent investigation demonstrated that repeat 15mg/kg doses were also well tolerated in neonates and resulted in plasma levels equivalent to a dosage of 5mg/kg in adults.89 Data on the use of caspofungin in neonates are also limited; preliminary investigations showed that once-daily caspofungin (25mg/m2) was well tolerated in neonates and infants (<3months), and that this dose provided similar plasma exposure to that obtained in adults receiving 50mg/day.83
FluconazoleFluconazole is efficacious and well tolerated in neonates. However, because this treatment is primary fungistatic rather than fungicidal, it is not considered a first-line option for neonatal invasive candidiasis, except for the case of urinary-tract candidiasis. Additionally, neonates may have already received fluconazole treatment as prophylaxis. De-escalation to fluconazole treatment may be possible when the patient is stable and susceptibility information is known.
Fluconazole (12mg/kg) is recommended for the treatment of urinary tract candidiasis in neonates.97 It is highly water-soluble, primarily excreted in urine in its active form, and easily achieves urine levels exceeding the minimum inhibitory concentration for most Candida strains.18 Small studies have described successful fluconazole treatment of infants and newborns with a C. albicans urinary-tract infection.36,93
Infection controlStudies have found evidence for transmission of Candida through direct and indirect contact and cross-infection by health-care workers; however, in neonates with Candida colonization or infection, there is no evidence to support or refute the use of patient-isolation measures (single-room isolation or cohorting) beyond routine infection control measures (e.g. hand washing) that exist in the neonatal units (Table 4).64,80,92 Infection control measures to prevent invasive candidiasis might include prenatal detection and eradication of maternal vaginal candidiasis, and stewardship programs to limit the use of broad-spectrum antibiotics (specifically third generation cephalosporins and carbapenems), H2 blockers, and postnatal dexamethasone, particularly during high-risk periods for infection (e.g. when neonates require CVCs, TPN, or endotracheal tubes).47 In addition, having feeding protocols and encouraging breastfeeding may help prevent necrotizing enterocolitis, which has been associated with invasive candidiasis. Having standardized protocols for the insertion and management of CVCs may also be an effective infection control measure.49
Recommendations summary for the treatment of invasive candidiasis in neonates:
- 1.
AmB (either AmB-d or L-AmB) or an echinocandin (micafungin or caspofungin) are the first options recommended for the treatment of neonatal invasive candidiasis.
- 2.
CNS involvement should be ruled out prior to echinocandin use.
- 3.
De-escalation to fluconazole treatment may be possible when the patient is stable and susceptibility information is known.
- 4.
Fluconazole is recommended for the treatment of urinary-tract candidiasis in neonates.
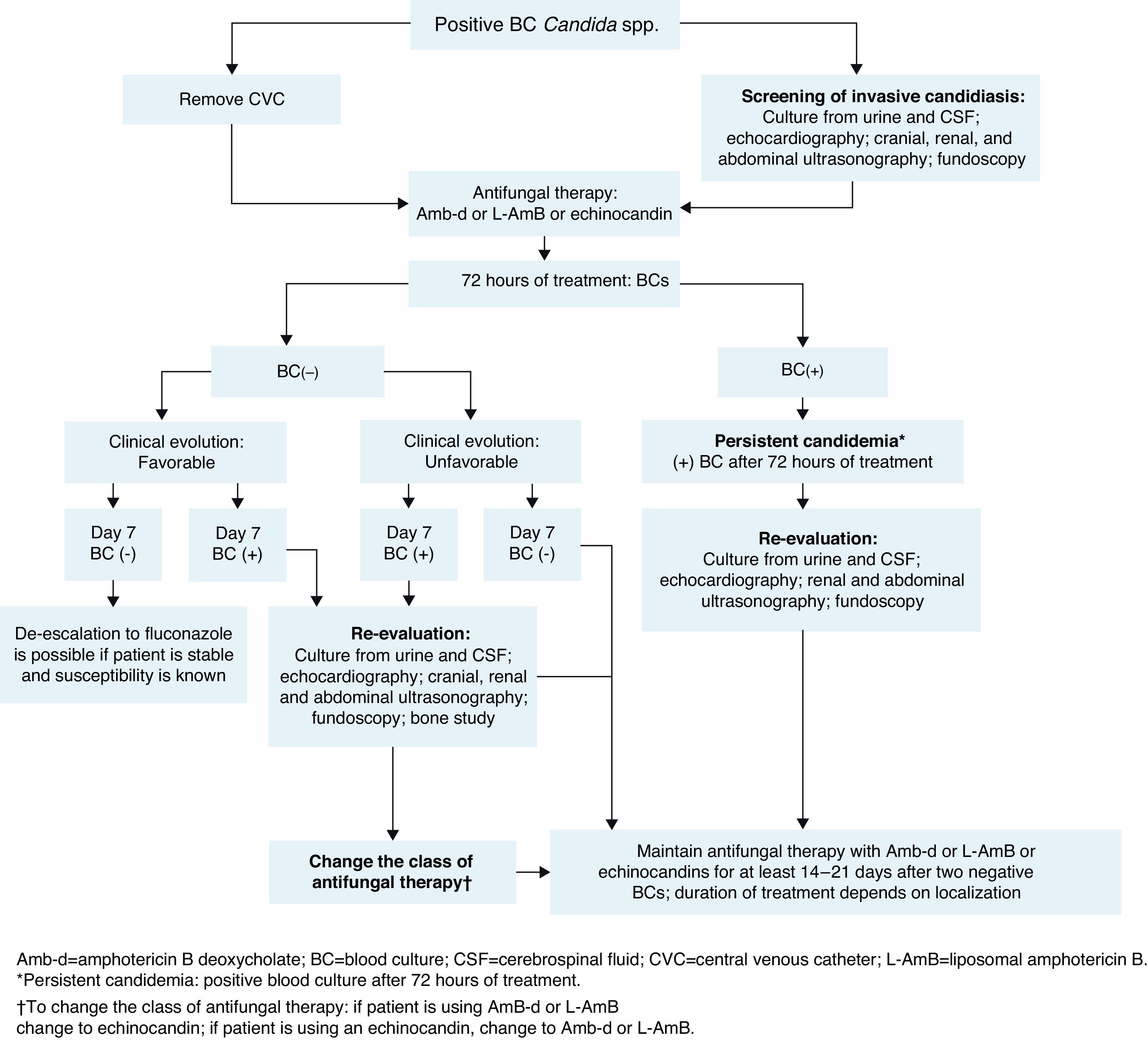
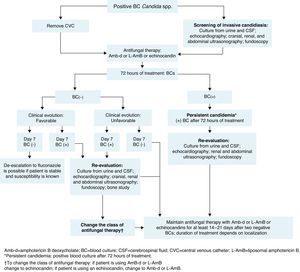
The management of neonates following a diagnosis of candidemia is shown in Fig. 2.
The baseline patient work-up in neonatal invasive candidiasis includes urine culture; lumbar puncture and cerebrospinal fluid (CSF) evaluation (to include culture of CSF, as cultures may be positive despite normal cell counts)14,71; cranial ultrasound; abdominal and renal ultrasound to look for abscesses in the kidney, spleen, and liver (to be carried out in all neonates with candidemia); an echocardiogram14 (recommended in neonates with candidemia to look for endocarditis); and dilated fundoscopic evaluation (ophthalmoscopy – recommended for all neonates with candidemia to rule out retinitis or endophthalmitis).14,71
BC should be done after 72h of treatment and then every 48h until sterilization of the blood is achieved (two consecutive negative BCs). In infants, the volume of blood collected for culture is based on age and body weight, with a blood-to-broth ratio of 1:5 or 1:10, according to technique recommendations.77 If cultures remain positive despite appropriate treatment (≥72h after initiation of antifungal therapy), secondary work-up to evaluate possible focal sites of infection is recommended (similar to baseline work-up). If BC remains positive at day 7 of therapy, an echocardiogram should be performed to rule out endocarditis and imaging of the bones and brain (ideally with magnetic resonance imaging or X-ray computed tomography) to evaluate further spread of the infection.
Recommendations summary for patient work-up after candidemia diagnosis in neonates
- 1.
Baseline patient work-up:
- a.
Urine culture.
- b.
Lumbar puncture and CSF evaluation.
- c.
Cranial ultrasound.
- d.
Abdominal ultrasound.
- e.
Echocardiogram.
- f.
Fundoscopy.
- a.
- 2.
BC should be done after 72h of treatment and then every 48h until sterilization (two consecutive negative BCs)
- 3.
Secondary patient work-up (if cultures remain positive at 72h of treatment):
- a.
The same as baseline work-up.
- a.
- 4.
Secondary patient work-up (if BCs remain positive at day 7 of treatment):
- a.
The same as baseline work-up plus imaging of the bones and brain.
- a.
Neonates with candidemia without septic complications should be treated until at least 2 weeks after two negative BC are obtained (Fig. 2).43 Treatment should be extended in neonates with persistent candidemia or candidemia with septic complications, as discussed below. In these situations, the duration of treatment should be determined using a case-by-case approach based on clinical features and patient characteristics.
Catheter management in neonatesEpidemiologic data related to catheter management and outcomes associated with catheter removal are limited and are not available for the Latin American region. In a study of ELBW neonates with candidemia, the rates of death and neurodevelopment impairment were greater for infants with delayed removal or replacement of catheters (>1 day after initiation of antifungal treatment) compared with infants in whom catheters were removed or replaced promptly.16 Another study found that in neonates with early catheter removal (≤3 days after first Candida-positive BC), candidemia resolved more quickly and the case fatality rate was lower compared with neonates with delayed catheter removal (>3 days).42 The Working Group recommends that catheters should be removed from neonates with candidemia. The decision to remove a catheter from a neonate with candidemia should be carefully considered and weighed against the need for intravenous access, given the potential challenge of finding a new catheter site in these patients.
Management of complications in neonatesPersistent candidemiaIn addition to being linked with high mortality, candidemia is associated with considerable morbidity.14 End-organ damage may involve the CNS, eyes, heart, bones, kidneys, spleen, and liver. Persistent positive cultures are associated with focal complications (ophthalmologic, renal, and cardiac) and/or death. Therefore, serial cultures of the infected site (or sites) should be obtained to predict the need for aggressive surveillance and intervention for focal complications (Fig. 2).21,68 No differences in baseline characteristics were found in two studies comparing neonates with and without persistent candidemia.21,54 In one of these studies, persistent infection was defined as positive repeat BC obtained ≥24h after attaining target dose of antifungal therapy.21 In the other, candidemia was considered persistent when it ranged in duration from 7 to 22 days.54 A duration of >1 day between the time of BC and the initial dose of systemic antifungal treatment places neonates at increased risk of developing persistent candidemia.78 In one prospective, single-institution, cohort study of VLBW neonates diagnosed with candidiasis, 10% had persistent candidemia, defined as positive culture for ≥2 weeks despite antifungal therapy.16 In the same study of 307 neonates with candidemia, up to 21% of infants had intermittent false-negative BCs while receiving antifungal therapy.16 This demonstrates that subsequent cultures in a neonate previously diagnosed with candidemia could yield false-negative results. In this context, one negative BC is not enough to indicate absence of infection in these patients, and at least two negative BCs are required to confirm absence of infection.43
Treating complicationsTreatment of the complications resulting from the spread of Candida infection is not well defined by clinical trials; however, the tissue penetration of potential therapies is an important consideration. Fluconazole has excellent tissue penetration and approximately 70% of it is excreted unchanged in the urine; therefore, fluconazole is the best choice for isolated renal infections due to Candida.18,88,100 An alternative to fluconazole is AmB-d. L-AmB is not indicated in renal infection by Candida, owing to limited tissue penetration.71 Endocarditis is most often associated with prolonged candidemia;53,54,81 however, fungal endocarditis has also been observed in a patient with only a single positive BC.14,68 The treatment approach for neonates with endocarditis due to Candida includes treatment with echinocandins (micafungin or caspofungin) or L-AmB (as these therapies can penetrate biofilms), extended duration of treatment, prompt removal of CVCs, and probably surgery. Owing to the high mortality risk, it is difficult to decide whether to perform surgery in neonates and, usually, surgeons prefer that patients could be treated for 2–3 weeks prior to considering surgery.
Treatment of osteomyelitis due to Candida requires surgery and prolonged duration of therapy for at least 4–6 weeks. Treatment options include 2–4 weeks of lipid formulation AmB (3–5 mg/kg/day), AmB-d (0.5–1mg/kg/day), or an echinocandin (micafungin or caspofungin), followed by fluconazole (12mg/kg/day).96
Infants with candidemia lasting ≥5 days may be more likely to develop ophthalmologic abnormalities. Endophthalmitis may occur as early as the first day of infection; however, it becomes a more probable complication with prolonged candidemia. Systemic antifungal therapy is usually adequate to successfully treat this complication.10 There is a widely reported range of ocular involvement in infants with invasive candidiasis (0–44%). In one retrospective study, retinal abnormalities were observed in 6% (4/67) of infants when indirect ophthalmoscopy examination was performed. In cases of ocular involvement in infants with invasive candidiasis, prolonged treatment with AmB-d or L-AmB is recommended.20 The possible benefits of echinocandin use might be limited, owing to their undetectable vitreous concentrations.96
Central nervous system infectionCNS infection is a relatively frequent complication of candidemia; however, CSF culture findings are varied, and normal CSF may not exclude CNS infection.30 In neonates, concentrations of AmB-d in the CSF may reach 40–90% of serum values (i.e. it penetrates reasonably well).12 For CNS infection, treatment with AmB-d should be considered, as the efficacy of this therapy has been demonstrated in the treatment of Candida meningitis in neonates.30 Lipid formulations of AmB can be administered at higher doses owing to their decreased renal toxicity, and they may have better CNS penetration compared with AmB-d.100 In a multicenter observational study, clearance from CSF was longer among neonates who received AmB-d and flucytosine, compared with those who received only AmB.16
Data to guide the use of other treatments for Candida meningitis are lacking. Preclinical data suggest that micafungin penetrates most sub-compartments of the CNS; however, clinical investigations are needed to establish the efficacy in treatment of neonatal CNS infection. Higher doses of micafungin may be needed to achieve adequate concentrations within the CNS.37 In a study of the safety and pharmacokinetics of micafungin in infants, a dosage of 10mg/kg/day resulted in 82.6% of patients having a maximal decline in fungal burden within the CNS.38 Two studies of the treatment of candidemia with caspofungin demonstrated efficacy of caspofungin in a few neonates with Candida meningitis.65,69 Although further investigation is needed, echinocandins are not indicated to treat CNS complications.
Recommendations summary for management of complications in neonates:
Renal infection:
- 1.
Fluconazole, 12mg/kg/day
Endocarditis:
- 1.
Prolonged treatment with L-AmB or echinocandins
- 2.
Prompt removal of CVCs
- 3.
Surgery (after antifungal treatment)
Osteomyelitis:
- 1.
Surgery
- 2.
Prolonged treatment with AmB-d, L-AmB or echinocandins, followed by fluconazole
Ocular involvement:
- 1.
Prolonged treatment with AmB-d or L-AmB
CNS infection:
- 1.
Prolonged treatment with AmB-d or L-AmB
A.L. Colombo has received research grants from Pfizer, MSD, United Medical and Luminex, medical education grants from Pfizer, MSD, United Medical and Astellas. Moreover, he has also been a consultant for MSD, Pfizer and Gilead. J.A. Cortes has received research grants and support to attend educational meetings from Pfizer and MSD. M. Nucci has received research grants from Pfizer and MSD, and has acted as a consultant and speaker for Pfizer, MSD, Astellas and Gilead. F de Queiroz-Telles has participated in Continuing Education activities in laboratories for Astellas, MSD, Pfizer and United Medical, and in research activities in laboratories for Astellas, MSD and Pfizer. I. N. Tiraboschi has been a speaker for Pfizer and Gilead. J. Zurita has been advisory board member and consultant for Pfizer, and has received research grants from Wyeth and MSD for participating in the SMART study.
Editorial support in the form of assistance with the first draft, collating author comments and editorial suggestions to draft versions of this manuscript was provided by Meredith Kalish, MD, and Brigitte Teissedre, PhD, of Choice Healthcare Solutions and was funded by Pfizer. Responsibility for opinions, conclusions and recommendations lies with the authors.