Candida yeasts are considered the main agents of nosocomial fungal infections.
AimsThis study aimed to establish the epidemiological profile of patients with candiduria hospitalized in the capital of the State of Mato Grosso, in the Central-Western region of Brazil.
MethodsPatients from three private hospitals and a public hospital participated in the study. This was an observational and cross-sectional study including analysis of patients mortality. It was carried out from March to August 2015.
ResultsA total of 93 patients with candiduria were evaluated. Candida tropicalis was found most commonly (37.6%; n=35), followed by Candida albicans (36.6%; n=34), Candida glabrata (19.3%; n=18), psilosis complex (4.3%; n=4), Candida lusitaniae (1.1%; n=1) and Candida krusei (1.1%; n=1). Antibiotic therapy (100%) and the use of an indwelling urinary catheter (89.2%; n=83) were the most frequent predisposing factors. Antifungal treatment was given to 65.6% of the patients, and anidulafungin was the most used antifungal. Mortality rates were 48% higher among patients with candiduria who had renal failure. Micafungin was the antifungal most prescribed among the patients who died. Candidemia concomitant with candiduria occurred in eight (8.6%; n=8) cases. Considering the species recovered in the blood and urine, only one patient had genetically distinct clinical isolates.
ConclusionsNon-C. albicans Candida species were predominant, with C. tropicalis being the most responsible for most cases of candiduria.
Las levaduras del género Candida están consideradas los principales agentes de infecciones micóticas nosocomiales.
ObjetivosEl objetivo del presente estudio fue establecer el perfil epidemiológico de los pacientes con candiduria hospitalizados en la capital de Mato Grosso, estado situado en la Región centro-oeste de Brasil.
MétodosParticiparon en el estudio pacientes de tres hospitales privados y un hospital público. Se trataba de un estudio observacional y transversal que incluía el análisis de la mortalidad de los pacientes. Se llevó a cabo de marzo a agosto de 2015.
ResultadosSe incluyó en el estudio a un total de 93 pacientes con candiduria. Candida tropicalis se encontró con mayor frecuencia (37,6%; n=35), seguida por Candida albicans (36,6%; n=34), Candida glabrata (19,3%; n=18), Candida psilosis complex (4,3%; n=4), Candida lusitaniae (1,1%; n=1) y Candida krusei (1,1%; n=1). El tratamiento antibiótico (100%) y el uso de una sonda urinaria permanente (89,2%; n=83) fueron los factores predisponentes más frecuentes. Se prescribió tratamiento antimicótico al 65,6% de los pacientes y la anidulafungina fue el antimicótico utilizado con más frecuencia. Las tasas de mortalidad fueron un 48% superiores entre los pacientes con candiduria con insuficiencia renal. Los pacientes que murieron presentaron la mayor proporción de prescripción del antimicótico micafungina. La candidemia concomitante con candiduria se produjo en ocho casos (8,6%; n=8). Si se tienen en cuenta las especies recuperadas en sangre y orina, solo en un paciente se encontraron aislamientos clínicos genéticamente diferentes.
ConclusionesLas especies de Candida no C. albicans fueron predominantes. C. tropicalis fue la responsable de la mayoría de los casos de candiduria.
The presence of Candida yeasts in urine (candiduria) has been an increasingly common event in hospitalized patients. Candida is considered the second most frequent microorganism in urinary tract infections in intensive care units.29,35Candida spp. are commensal yeasts of the urogenital tract. Their transformation into pathogenic microorganisms is mainly related to invasive procedures and the extensive use of broad-spectrum antibiotics.15,41,59 It is estimated that 50% of the patients using indwelling urinary catheters for more than five days develop candiduria.19 Women are more affected because, in addition to having a shorter urethra, they may have vulvovaginal colonization by Candida.32,33 The infection commonly occurs by the ascending route, via the migration of yeasts from the periurethral area to the bladder. Kidney infections usually occur via a hematogenic route; however, retrograde infections from the bladder to the kidneys may occur, especially in the presence of urinary outflow obstruction.25,38
Although it is frequently seen in hospitalized patients, there is still no laboratory diagnostic protocol and treatment for candiduria, since the presence of Candida in the urine often represents only colonization.27,49 However, candiduria associated with several underlying conditions in patients of the intensive care unit (ICU) may progress to candidemia, increasing the risk of death.2,33,57 Candiduria, which does not necessarily involve the presence of signs and/or symptoms of urinary tract infection, may be defined as the growth of Candida in culture from urine collected by suitable techniques.21 It is a very common event among patients exposed to risk factors, and 20% of hospitalized patients may have candiduria throughout his hospitalization, particularly patients in intensive care unit.25 This laboratory finding is controversial regarding its interpretation, as may correspond to simple contamination of the urine collection, asymptomatic cystitis or pyelonephritis, primary renal candidiasis, ureteropelvic fungal ball or disseminated candidiasis with renal manifestation.26,53
In view of the controversies regarding the relevance and clinical interpretation of candiduria, it is important to conduct studies aimed at elucidating its global epidemiological presence, and establishing prophylactic and therapeutic actions more specific to each region. This study aimed to evaluate the clinical and epidemiological profile of patients with candiduria hospitalized in four hospitals in the capital of Mato Grosso, a State located in the Central-Western region of Brazil.
MethodsType of study and populationThis was an observational and cross-sectional study including analysis of patients mortality. It was carried out from March to August 2015 and involved four hospitals in the capital of Mato Grosso, a State located in the Central-Western region of Brazil. A total of 93 patients with candiduria were analyzed.
Urinary sediment and culture were requested by physicians as part of the routine care to achieve a diagnosis and provide the proper clinical management to the patients. After the collection of urine samples by the nursing team, they were sent to the clinical analysis laboratory and were examined by the professionals responsible. After the laboratory analysis of each sample, the isolates were sent to the Laboratory of Mycology of the Federal University of Mato Grosso to identify them. Data such as sex, age, and predisposing factors, including comorbidities, length of hospital stay, treatment, and mortality, were obtained from the medical records of each patient. Data on urinary sediment analysis and colony counts were acquired by the operating system of each clinical laboratory.
Patients were included when the number of colony-forming units (CFU) were greater than or equal to 103CFU/ml.20 Patients with more than one positive sample were included only once. Patients who had been treated for genital candidiasis during hospitalization were not considered in the study due to the risk of contaminating the urine sample. Candiduria cases that occurred before the collecting of samples were also excluded from the study.
In compliance with Resolution 196/96 of the National Health Council, this study began after the evaluation and approval of the Research Ethics Committee of Plataforma Brasil under the number 38452914.1.0000.5541.
Microbiological and statistical analysisSamples were cultured on Sabouraud dextrose agar (BD Difco® – USA), and the colonies obtained were transferred to a chromogenic agar (BD Difco® – USA) to check the purity of the colonies and to achieve a presumptive identification. The yeasts were identified by germ tube and micromorphologic analysis in cornmeal agar – Tween 80.28 Confirmation of the species was carried out using the Vitek® system (bioMérieux – France). The species recovered from the blood and urine of patients who had candiduria concomitant with candidemia were identified by PCR (polymerase chain reaction) and sequencing of the ITS (internal transcribed spacer) region.
All statistical analyses were performed using the Stata Statistical Software (v.12). To verify the existence of an association between mortality and categorical variables, Pearson's chi-square test was used. In order to estimate the strength of the association, the prevalence ratio (PR) was used. The independent effect of the exploratory variables on the response variable was determined by Poisson regression with robust variance.4 Variables with p-value <0.2 in the bivariate analysis were included in the model. Results were considered significant when p<0.05 in the two-tailed test. Considering the time elapsed between the diagnosis of candiduria and the death of the patient, a Kaplan–Meier curve was made to evaluate the probability of survival.
ResultsA total of 132 isolates were obtained from the urine of 93 patients with candiduria. Considering the first species recovered from each patient, Candida tropicalis (37.6%; n=35) was the most frequent microorganism isolated, followed by Candida albicans (36.6%; n=34), Candida glabrata (19.3%; n=18), psilosis complex (4.3%; n=4), Candida lusitaniae (1.1%; n=1), and Candida krusei (1.1%; n=1). Mixed infections involving C. glabrata and C. albicans occurred in two cases, with significant growth rates (≥105CFU/ml) for both species.
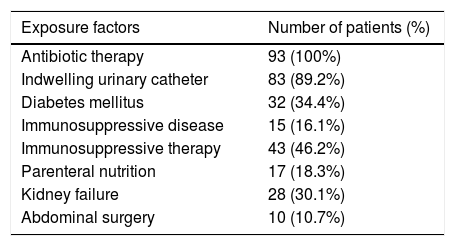
From the patients evaluated, 57% (n=53) were females and 43% (n=40) males. Two patients were from neonatal ICU. The most common species in female patients was C. albicans (37.7%; n=20), and the most common species in male patients was C. tropicalis (45%; n=18). The average age of patients with candiduria was 63.1 years (confidence interval [95% CI]=58.7–67.5). Among the different predisposing factors, the most frequent were antibiotic therapy (100%) and the use of an indwelling urinary catheter (89.2%; n=83) (Table 1).
Frequency distribution of the predisposing factors found among the patients with candiduria.
| Exposure factors | Number of patients (%) |
|---|---|
| Antibiotic therapy | 93 (100%) |
| Indwelling urinary catheter | 83 (89.2%) |
| Diabetes mellitus | 32 (34.4%) |
| Immunosuppressive disease | 15 (16.1%) |
| Immunosuppressive therapy | 43 (46.2%) |
| Parenteral nutrition | 17 (18.3%) |
| Kidney failure | 28 (30.1%) |
| Abdominal surgery | 10 (10.7%) |
Candidemia concomitant with candiduria was observed in 8.6% of cases (n=8). Molecular analysis of the isolates obtained from the blood and urine showed only one case in which the species were different. The species isolated from blood cultures were C. albicans (n=2), C. tropicalis (n=3), Candida parapsilosis (n=2) and Candida orthopsilosis (n=1). Two patients had positive catheter tip cultures for the same species isolated from the blood and urine.
The average time after hospitalization for the occurrence of the first episode of candiduria was 19.9 days (95% CI=15.3–24.5). Nine patients had positive cultures on the first day of hospitalization. The average time for the onset of candiduria after the introduction of an indwelling urinary catheter was 16 days (95% CI=11.2–20.8). The replacement or removal of the catheter occurred in 6.2 days on average (95% CI=4.3–8.2) after the diagnosis of candiduria. The analysis of the urinary sediment was performed in 65 patients. Fifty one (78.4%) of them had leukocyturia and 46 (69.2%) suffered hematuria. The presence of pseudohyphae was reported in only one case.
Antifungal treatment was prescribed in 61 (65.6%) patients. Therapy prescription was 26% higher in patients who had urine cultures with colony counts ≥105CFU/ml (PR=1.26; p=0.035). Anidulafungin was the most commonly used antifungal in private hospitals, and so was micafungin in the public hospital. Among the patients who had an indwelling catheter during candiduria and received treatment, 38.6% (n=22) did not have the catheter replaced or removed when starting the antifungal therapy.
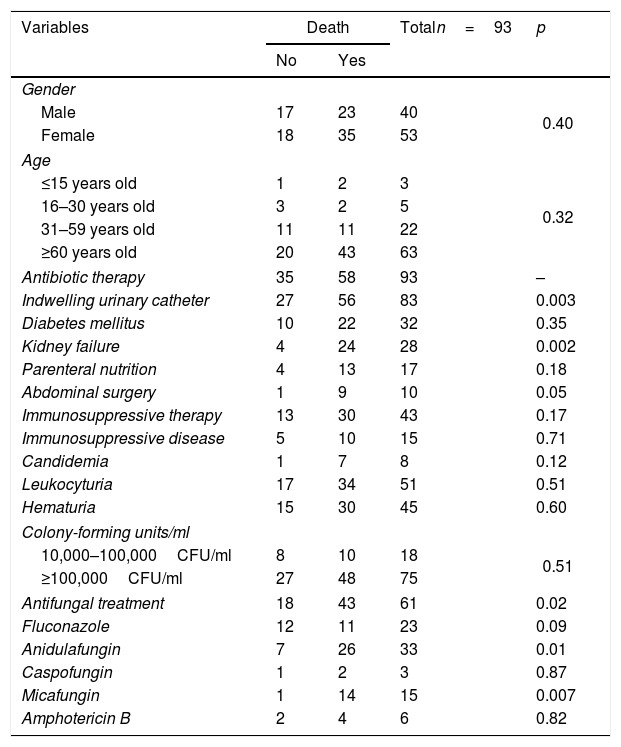
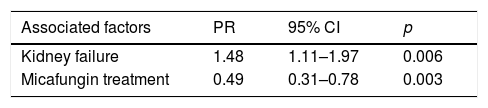
Considering mortality rates in the patients with candiduria, univariate analysis showed that the use of an indwelling catheter (p=0.003), kidney failure (p=0.002), antifungal treatment (p=0.02), use of anidulafungin (p=0.01), and use of micafungin (p=0.007) were statistically significant associations (Table 2). The mortality among patients with candiduria was 48% higher when renal failure was present and 51% lower among those receiving micafungin treatment (Table 3).
Univariate analysis between the explanatory variables and mortality among patients with candiduria admitted to tertiary hospitals in Mato Grosso.
| Variables | Death | Totaln=93 | p | |
|---|---|---|---|---|
| No | Yes | |||
| Gender | ||||
| Male | 17 | 23 | 40 | 0.40 |
| Female | 18 | 35 | 53 | |
| Age | ||||
| ≤15 years old | 1 | 2 | 3 | 0.32 |
| 16–30 years old | 3 | 2 | 5 | |
| 31–59 years old | 11 | 11 | 22 | |
| ≥60 years old | 20 | 43 | 63 | |
| Antibiotic therapy | 35 | 58 | 93 | – |
| Indwelling urinary catheter | 27 | 56 | 83 | 0.003 |
| Diabetes mellitus | 10 | 22 | 32 | 0.35 |
| Kidney failure | 4 | 24 | 28 | 0.002 |
| Parenteral nutrition | 4 | 13 | 17 | 0.18 |
| Abdominal surgery | 1 | 9 | 10 | 0.05 |
| Immunosuppressive therapy | 13 | 30 | 43 | 0.17 |
| Immunosuppressive disease | 5 | 10 | 15 | 0.71 |
| Candidemia | 1 | 7 | 8 | 0.12 |
| Leukocyturia | 17 | 34 | 51 | 0.51 |
| Hematuria | 15 | 30 | 45 | 0.60 |
| Colony-forming units/ml | ||||
| 10,000–100,000CFU/ml | 8 | 10 | 18 | 0.51 |
| ≥100,000CFU/ml | 27 | 48 | 75 | |
| Antifungal treatment | 18 | 43 | 61 | 0.02 |
| Fluconazole | 12 | 11 | 23 | 0.09 |
| Anidulafungin | 7 | 26 | 33 | 0.01 |
| Caspofungin | 1 | 2 | 3 | 0.87 |
| Micafungin | 1 | 14 | 15 | 0.007 |
| Amphotericin B | 2 | 4 | 6 | 0.82 |
Statistically significant results of the Poisson regression with robust variance in relation to mortality among hospitalized patients with candiduria in tertiary hospitals in Mato Grosso.
| Associated factors | PR | 95% CI | p |
|---|---|---|---|
| Kidney failure | 1.48 | 1.11–1.97 | 0.006 |
| Micafungin treatment | 0.49 | 0.31–0.78 | 0.003 |
CI: confidence interval; PR: prevalence ratio. Model adjusted by sex, age, use of indwelling urinary catheter, parenteral nutrition, abdominal surgery, immunosuppressive therapy, candidemia, antifungal therapy, treatment with anidulafungin and fluconazole.
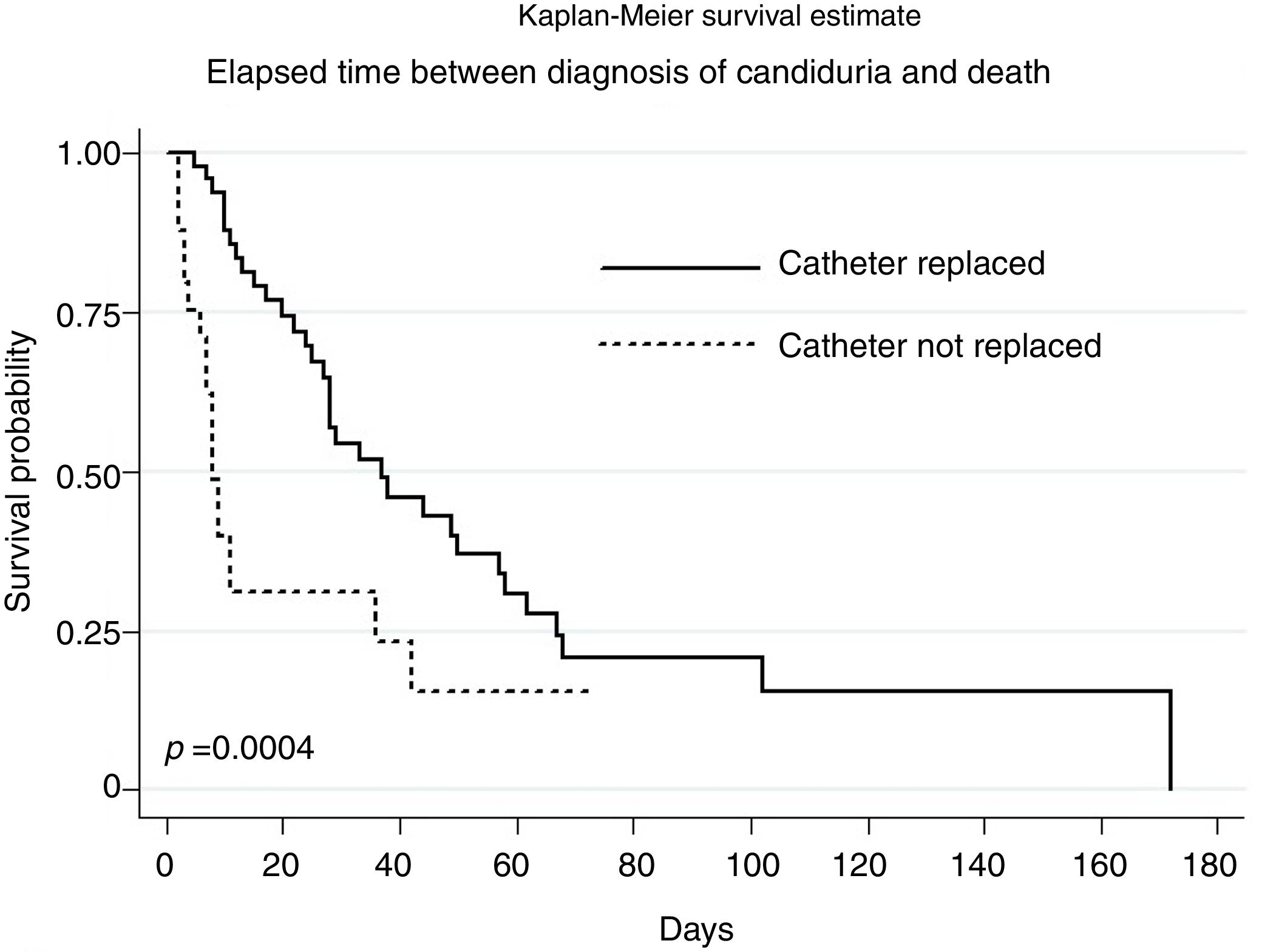
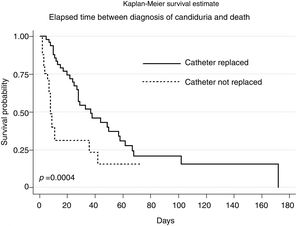
Kaplan–Meier survival analysis showed a statistically significant difference (p=0.0004) between the elapsed time from diagnosis of candiduria to death for patients who underwent the replacement of the indwelling urinary catheter after diagnosing candiduria (Fig. 1). There was no statistical difference between the survival functions for the other variables.
DiscussionDespite the high prevalence in hospitalized patients, the presence of Candida in the urine still has uncertain significance due to the lack of well-established criteria to aid diagnosis.40 Several predisposing factors are related to candiduria. As in several previously published studies, antibiotic therapy and the use of an indwelling urinary catheter were the most frequent predisposing factors in patients with candiduria2,27,41,52 in our study. When suppressing the bacterial flora of the gastrointestinal tract and lower genital tract, antibiotics favor fungal colonization of the epithelial surface, facilitating the entrance to the urinary tract, especially in the presence of permanent catheters.22 According to the literature, cases of candiduria are more frequent in patients above 60 years old (67.7%). The greater predisposition of the elderly is related to immunological factors, long-stay hospitalizations, and clinical procedures that may increase the risk of infection.16,26
Unlike studies conducted in other Brazilian regions in which C. albicans was reported as the most frequent species in cases of candiduria, less cases due to this species were found in our study, with 63.4% of the isolates identified as non-C. albicans Candida species, highlighting C. tropicalis as the most prevalent species in 37.6% of the cases.6,41,51C. tropicalis has been reported in previous studies as the most prevalent species in candiduria with reported prevalences varying from 43% to 57.3%.40,42,52 This difference may be explained by the inversion of the profile of Candida species (C. albicans versus non-C. albicans Candida species) that has occurred over the course of the last few years.36 Moreover, the distribution of species varies according to the characteristics of each demographic region and group of subjects studied. A study in another State in the Central-Western region of Brazil (Mato Grosso do Sul) reported a similar proportion of non-C. albicans Candida species (60.4%), although the frequency of C. albicans (39.6%) was slightly higher than that of C. tropicalis (31.1%).31
As in other studies, candiduria was more frequent in female patients.8,27,41 The higher incidence in women is due to the shorter urethra compared with men, as well as vulvovaginal colonization by Candida, which facilitates reaching the bladder via the ascending route.32,33 This hypothesis was suggested by Febré et al.,14 who found Candida in the urine of five women from a group of eight whose vaginal secretions taken previously were positive for the same species of Candida. According to the literature, there has been an increase in vaginal candidiasis by non-C. albicans Candida species, especially C. glabrata.11,18 In this study, 18 patients were infected with this species, among whom 14 were women. As observed in our study, C. glabrata is reported in the literature as an emerging and frequently species in elderly patients.9,34 Except for one case, all cases of C. glabrata candiduria were diagnosed in patients over 60 years old. Yeasts belonging to the psilosis complex have not been reported as a frequent cause of urinary tract infections. It is a group of yeasts that primarily affects infants and is more related to candidemias.30,44 Although only two infants were included in this study, psilosis complex species were not the etiological agents of candiduria in these patients. The four cases of candiduria caused by this group of yeasts occurred in adult men.
In a multivariate analysis, renal failure increased mortality by 48% in patients with candiduria when compared to patients with candiduria but without renal failure. In pediatric patients hospitalized in an intensive care unit who developed candidemia, candiduria increased mortality by almost five-fold.23 In neonates born with extremely low birth weight, mortality after discharge was two-fold higher among those who developed candiduria when compared to those with no proven infection.60
Candiduria may increase the risk of candidemia by non-C. albicans Candida species by up to 15 times,12 probably due to upper urinary tract involvement and translocation to renal blood vessels. Patients with candiduria and renal failure had a three-fold higher risk of death than patients with candiduria but without renal failure.43 Among patients with candiduria, antifungal treatment with micafungin reduced mortality by 51% in a multivariate analysis. In a study by Gabardi et al., this antifungal was associated with short-term and long-term urine sterilization, regardless the catheter was removed or not, and the cause of candiduria were both C. albicans or non-C. albicans Candida species.17 In a cohort study of 280 patients aged 18–75 years a 6-fold all-cause mortality rate among patients with candiduria was found.46 When compared with the antifungal anidulafungin, micafungin reduced the mortality rate by 27% after 90 days in patients with invasive candidiasis.58
As in other studies, candiduria cases occurred, on average, 20 days after hospitalization.2,27 Some studies have reported the appearance of yeasts in the urine within the first two weeks of hospitalization.39,50 Among patients who used an indwelling urinary catheter, candiduria occurred, on average, 16 days after inserting the device. Another study reported that candiduria developed, on average, 11 days after catheterization.56 Considering the hospitals evaluated in this study, we observed that the catheter was replaced every 20 days in most patients. Thus, it would be possible to reduce colonization by yeasts if the replacements were more frequent. Even if such a practice causes higher costs for the hospital, the potential savings of not requiring antifungal treatment and their consequent potential adverse effects should also be considered. However, in order to assess this change in practice, clinical studies with specific designs for this hypothesis are required, such as randomized controlled trials, cohort studies, or case–control studies aimed at evaluating and comparing groups of patients with candiduria following different replacement intervals for an indwelling catheter.
The higher likelihood of survival among patients who had the urinary catheter replaced after the diagnosis of candiduria, as evidenced by Kaplan–Meier analysis, highlights the benefits that continuous catheter replacement can bring to the patient. Despite the design limitations of this study in testing this hypothesis, a statistically significant difference was found in the mortality rates between patients in whom the catheter was replaced and those in whom the urinary catheter was not replaced after the diagnosis of candiduria. Studies addressing issues specifically related to the urinary catheter replacement regimen in these patients might provide better evidence of the importance of catheter replacement as a non-therapeutic intervention factor for candiduria cases.
Candidemia concomitant with candiduria is reported in the literature as a low occurrence event.8,52,54 In the present study, eight patients (8.6%) out of the 84 patients with candiduria had concomitant candidemia. A similar rate was found by Storfer et al.56 Some genetic studies have been conducted showing identical genotypic patterns among yeasts recovered in the urine and blood.1,5,7 In the present study, the molecular analysis of urine and blood isolates obtained from patients who had candiduria concomitant with candidemia demonstrated only a single case in which Candida species were different in the two samples. However, despite the studies showing the same species of Candida present in the blood and urine, there are difficulties in establishing which event occurred first due to the low sensitivity of blood cultures in detecting candidemias.13,57 Thus, some authors believe that candiduria is an event that should never be ignored because it may be the first indication of systemic or invasive infection.37,51,53
Some studies have reported that most cases of candiduria are untreated.3,6,25 In the present study more than half of the patients (65.6%) received treatment. In most cases, the treatment was based on a single positive culture. Only 34.4% of the treated patients were taken a second sample taken after the detection of candiduria. The same behavior was observed by a group of researchers in Parana, a State located in the Southern Region of Brazil.6 Many authors do not recommend the antifungal treatment in patients with a permanent urinary catheter due to an increase in the resistance to antifungal drugs, since the formation of biofilms complicates the eradication of the microorganisms.47,48 In this study, 22 (38.6%) out of the 57 patients who had indwelling catheter and received treatment had no replacement or removal of the urinary catheter. Similar results were found by Dalen et al.10 who, when assessing the practice in asymptomatic patients using urinary catheters, found in only half of the cases the replacement or removal of the device.
Fluconazole is reported in the literature as the best antifungal drug for the treatment of candiduria due to the high active concentration of the drug in the urine. However, it is known that its use is limited in urinary tract infections caused by C. krusei due to its intrinsic resistance to the mentioned antifungal agent, and C. glabrata due to its dose-dependent susceptibility.24,45,55 However, a major problem faced by most hospitals is the lack of laboratory results enabling an appropriate antifungal drug to treat patients with candiduria. From the hospitals involved in this study, only the public hospital could analyze urine cultures by identifying the yeast species and assessing their susceptibility to antifungal drugs. Thus, anidulafungin was the drug most frequently used by private hospitals since the laboratory results were insufficient to guarantee an optimal antifungal activity of fluconazole.
However, it should be noted that, given the different difficulties related to the diagnosis of candiduria, more discerning measures are required for detecting the presence of Candida in urine. Any event diagnosed as candiduria, considering clinical and laboratory criteria, should be investigated to evaluate the risk of progression from colonization to disease. Thus, any replacement of an indwelling urinary catheter and any antifungal treatment should be evaluated with subsequent urine cultures in order to better monitor candiduria cases.
Competing interestsThe authors declare that they have no competing interests.