Candidemia is one of the most common nosocomial infections globally and it is associated with considerable excess mortality and costs. Abreast, biofilm-forming strains are associated with even higher mortality rates and poor prognosis for the patient.
AimsTo evaluate a possible association between the biofilm-forming capability of Candida bloodstream isolates and the clinical evolution in patients with candidemia.
MethodsAn observational, retrospective study was conducted at a tertiary care university hospital during 9 years (2006–2015). The biofilm quantitation of the Candida bloodstream isolates was determined by crystal violet staining and XTT reduction assay.
ResultsA total of 218 cases of candidemia had been diagnosed and 89 isolates were obtained. The mortality rate was 36% and the main risk factors were antibiotic exposure and the use of catheters. Candida tropicalis (52.8%) was the most frequent species, followed by Candida albicans (30.4%), Candida parapsilosis sensu stricto (10.1%), Candida orthopsilosis (3.4%), Candida krusei (2.2%) and Candida glabrata sensu stricto (1.1%). All the strains were biofilm producers, which is an important contribution to the patient's mortality. C. tropicalis showed the highest production of biomass biofilm, whereas C. glabrata exhibited the highest metabolic activity.
ConclusionsThis study contributes to expand the knowledge about the local epidemiology of candidemia and highlights the impact of Candida biofilm on patient's outcome.
La candidemia es una de las infecciones nosocomiales más frecuentes globalmente y se encuentra asociada con una elevada mortalidad y coste económico. Las cepas productoras de biopelícula se asocian con elevadas tasas de mortalidad y mal pronóstico para el paciente.
ObjetivosEvaluar una posible asociación entre la capacidad de formación de biopelícula de aislamientos sanguíneos de Candida y la evolución clínica de pacientes con candidemia.
MétodosDurante 9 años (2006-2015) se ha llevado a cabo un estudio observacional y retrospectivo en un hospital universitario de tercer nivel de atención. La cuantificación de biopelícula de los aislamientos sanguíneos de Candida se determinó por tinción con cristal violeta y ensayo de reducción de XTT.
ResultadosSe diagnosticó un total de 218 casos de candidemia y se obtuvieron 89 aislamientos. La tasa de mortalidad fue del 36% y los principales factores de riesgo fueron la exposición a antibióticos y el uso de catéteres. Candida tropicalis (52,8%) fue la especie más frecuente, seguida por Candida albicans (30,4%), Candida parapsilosis sensu stricto (10,1%), Candida orthopsilosis (3,4%), Candida krusei (2,2%) y Candida glabrata sensu stricto (1,1%). Todas las cepas produjeron biopelícula, una contribución importante a la mortalidad de los pacientes. C. tropicalis mostró la producción más alta de biomasa de biopelícula, mientras que C. glabrata exhibió la actividad metabólica más alta.
ConclusionesEste estudio contribuye a expandir el conocimiento de la epidemiología local de la candidemia y resalta el impacto de las biopelículas de Candida en el pronóstico del paciente.
Candidemia is the most important fungal infection in hospitalized individuals worldwide.10,29 It is a cause of late sepsis, and risk factors include broad-spectrum antibiotic therapy, ICU stay for more than 72h, immunosuppressive therapy, parenteral nutrition, and multiple invasive medical procedures.1,29 Attributable mortality associated with invasive candidiasis has been reported to range from 5% to 71%,3,37 and crude mortality rates can be as high as 76%.13 Each episode contributes to prolonged hospital stay of 3–30 days and to additional medical expenses amounting to nearly USD $40,000,22 representing one of the highest financial burden to health care systems of any healthcare-associated infection.12,16
The epidemiology of candidemia varies according to geographical regions,3,37 and despite it has been extensively studied in United States and Europe there is a huge knowledge gap in Latinamerica. Recently, there has been an increase in infections due to non-Candida albicans Candida species (NAC) that may present resistance to some antifungals,15,25,29 which is a serious medical concern. For this reason, continuous surveillance studies to monitoring incidence, species distribution and antifungal susceptibility profiles are mandatory.
Biofilm formation is one of the most extensively investigated virulence factor of those Candida species associated with bloodstream infections (BSI), and also contributes to pathogenicity in catheter related candidemia.36Candida biofilm is known to be highly resistant to antifungal agents, being a key attribute to the mortality in these infections.33 Furthermore, it has been reported that patients with candidemia caused by biofilm-forming strains have a worse outcome than those infected with non-biofilm-forming strains.38
In the present study we performed a 9-year observational retrospective analysis of the candidemia cases in our hospital in order to investigate the association between the biofilm forming capability of hematogenous Candida isolates and the 30-day mortality.
Materials and methodsEthics statementThis study was reviewed and approved by the Ethics and Research Committee of the School of Medicine of the Universidad Autónoma de Nuevo León under the following registration number: IF15-009. Informed consent was not required because of the observational nature of the study.
Study population, design and data collectionThis is an observational, transversal and retrospective analysis of a collection of culture-proven candidemia cases registered during the period from July 2006 to June 2015 at the University Hospital “Dr. José E. González” in Monterrey, Mexico, which is an academic tertiary care center with 450 beds and ∼20,000 hospital admissions annually. A case of candidemia was defined as the presence of Candida in one or more blood cultures. Only the first episode of candidemia was reported in patients with recurrent episodes of the infection. Patients with more than one Candida species in the cultures were excluded from the study. Only those patients with clinical data and a viable Candida isolate were included in the study.
We considered several variables, such as demographic characteristics, microbiological parameters (Candida species isolated and biofilm formation), comorbid conditions, invasive procedures, use of immunosuppressive agents, surgery, bacteremia, or exposure to any antibiotic within the 3 weeks before the onset of the candidemia; all of them were recorded and evaluated. Severity of illness was measured using the Acute Physiology and Chronic Health Evaluation (APACHE) II score, according to which the candidemia cases were stratified as with low severity (0–15) or high severity (>15), while the Charlson's score was used as a composite index of comorbidities (0–5). The outcome variable was the 30-day mortality. The infection-related mortality was defined as the mortality with the symptoms and signs of the infection unresolved at the time of death, being no other reason for it. On the other hand, in order to define a catheter-related or catheter-associated infection, we used the criteria previously established.27
Identification of the isolatesThe isolates were subcultured onto Sabouraud-dextrose agar (SDA) (Difco, Detroit, MI, USA) and identified by standard mycological procedures as germ tube test, morphology on corn-meal agar with Tween-80, and a carbohydrate assimilation test using a commercially available API 20C AUX kit (bioMérieux, Mexico). For the molecular identification of Candida parapsilosis and Candida glabrata species complex, sequencing of the ITS1-5.8S-ITS2 non-coding region was performed.40 Type strains ATCC 22019, ATCC 96139, ATCC 96144, CBS 10154 and CBS 9983 were used as quality controls for C. parapsilosis sensu stricto, Candida orthopsilosis, Candida metapsilosis, Candida bracarensis and Candida nivariensis, respectively.
Biofilm induction and quantitationThe growth conditions were established according to the standardized methodology previously conducted by Melo et al., as well as the biofilm quantitation by crystal violet staining and XTT reduction assays.21 Strains were initially grown on SDA at 37°C for 24h, and one colony of each strain was further subcultured in RPMI 1640 broth medium with L-glutamine (Hardy Diagnostics, Santa Maria, CA, USA) at 37°C for 18h at 120–200rpm. The cells were harvested, washed twice with PBS, and adjusted to a concentration of 1×107 cells/ml in RPMI 1640 medium. An aliquot of 100μl of the cell suspensions were transferred into each well of a sterile flat-bottomed 96-well polystyrene Corning Costar 9018 plate (Costar, Corning Incorporated, NY, USA). The plates were incubated at 37°C for 1.5h at 75rpm, washed with 150μl of PBS, and then 100μl of fresh RPMI 1640 medium was added to each well. The plates were finally incubated at 37°C for 72h at 75rpm to allow biofilm growth. A negative control (RPMI medium without cells) was added to the final well of each plate.
To determine the biofilm biomass, the crystal violet staining assay was performed. Briefly, after biofilm formation, each well was washed twice with 200μl of PBS and the plates were dried at 37°C for 20min. The washed biofilms were stained with 110μl of 0.4% aqueous crystal violet solution for 45min. The wells were then washed three times with 200μl of sterile distilled water and then faded with 200μl of 95% ethanol. After 45min, 100μl of the destaining solution from each sample was transferred to a new plate and measured with a spectrophotometer plate reader at 595nm. The optical density (OD) values of the negative controls were subtracted from the values of the test wells in order to eliminate background interference. On the other hand, biofilm metabolic activity was determined by the XTT (tetrazolium salt 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-(phenylamino)-carbonyl-2H-tetrazoliumhydroxide) (Sigma, St Louis, MO, USA) reduction assay. After biofilm formation, the wells were washed twice with 200μl of PBS, and 200μl of PBS and 12μl of XTT-menadione solution were added to each well. The XTT-menadione solution was prepared on each day of testing by adding 1.5ml of XTT (1mg/ml in sterile saline) to 300μl of menadione solution (0.4mM in acetone; Sigma, St Louis, MO, USA). Plates were incubated at 37°C in darkness for 5h. Afterwards 100μl of the reaction solution was transferred to a new plate and the concentration of the formazan product was spectrophotometrically determined at 490nm. The OD values of the negative control wells were subtracted from the values of the test wells. Type strain C. parapsilosis ATCC 22019 was used as good biofilm former control. The amount of biofilm formed by an isolate was categorized as high (OD490≥0.1), low (0.025≤OD490<0.1) and no biofilm formation (OD490<0.025), according to the criteria previously established by Pannanusorn et al.28
StatisticsDescriptive data are presented. The biofilm quantifications were expressed as OD means±standard deviation (SD) for each set of data determined, and were compared using one-way ANOVA test. An additional T-test comparison of the OD means between the isolates recovered or not from a central venous catheter (CVC) was made; a p-value ≤0.05 was considered significant. Statistics and graphics were performed on GraphPad Prism v5.03 for Windows (GraphPad Software, San Diego, CA, USA).
ResultsCharacteristics of the populationA total of 218 cases of candidemia were found in the clinical records of the database of our hospital during the study period; among them, 89 bloodstream isolates were available and provided by the Mycology Laboratory of the Microbiology Department, but only 56 isolates had complete clinical information of every patient.
Of the 56 patients with complete clinical information, 64% (n=36) were adults and 36% (n=20) pediatric patients. The 39% and 40% of these isolates were obtained in the intensive care unit for adults and pediatrics, respectively. The rest of the isolates from adults were obtained of surgical (36%), medical (22%) and the emergency (3%) areas. Conversely, the distribution of the isolates by hospital area from pediatrics showed the same tendency than the adults, being the surgical area the one that had the highest percentage of isolation (40%), followed by medical (15%) and emergency (5%) areas, respectively.
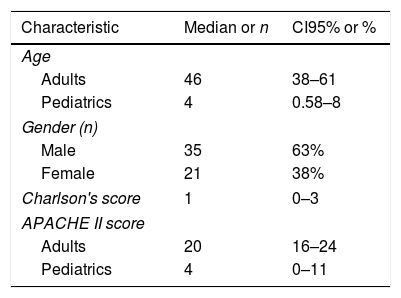
The demographic characteristics of the patients are depicted in Table 1. The mean of hospital length of stay (LOS) prior to the isolation of Candida was 18 days (SD±17) and 46% of the patients had been hospitalized at least 30 days before candidemia was diagnosed. The mean of LOS after the isolation of Candida from blood was 18 days (SD±18.4) and, importantly, 64% (n=36) of the cases were catheter-associated candidemia.
Demographic characteristics of the study population (n=56).
| Characteristic | Median or n | CI95% or % |
|---|---|---|
| Age | ||
| Adults | 46 | 38–61 |
| Pediatrics | 4 | 0.58–8 |
| Gender (n) | ||
| Male | 35 | 63% |
| Female | 21 | 38% |
| Charlson's score | 1 | 0–3 |
| APACHE II score | ||
| Adults | 20 | 16–24 |
| Pediatrics | 4 | 0–11 |
CI95%: confidence interval 95% of the median, APACHE II: acute physiology and chronic health evaluation.
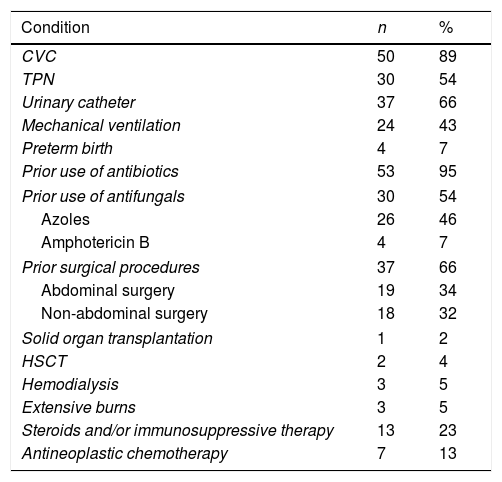
The antibiotic exposure (95%), the use of CVC (89%), urinary catheter (66%), surgical procedures (64%) and the utilization of total parenteral nutrition (54%) were the main risk factors associated to candidemia in our study population (Table 2). It is worth noting that these factors were present simultaneously in some patients. Forty four percent (n=24) of the patients presented also Candida isolates in another anatomical site, being the urine isolates the most frequent (38%, n=21), with the 81% (n=17) of them coming from urinary catheter users (data not shown). In all the patients with CVC, the latter was removed or replaced unless there were contraindications. Antifungals had been prescribed to 54% (n=30) of the patients prior to the Candida bloodstream isolation, being the azoles the ones more used. The treatment of choice was amphotericin B (45%) and the median of the treatments followed by the patients was 13 days. Regarding candidemia complications, 23% of the patients presented septic shock and 4% developed retinitis by Candida. Of the total cases analyzed, 33 patients (59%) died; 20 (36%) of these deaths were attributable to the candidemia episode. We found that the mortality rate increases nearly two-fold in patients with CVC-associated candidemia (65%) in comparison with those episodes not associated with CVC (35%).
Baseline conditions of the 56 patients with candidemia.
| Condition | n | % |
|---|---|---|
| CVC | 50 | 89 |
| TPN | 30 | 54 |
| Urinary catheter | 37 | 66 |
| Mechanical ventilation | 24 | 43 |
| Preterm birth | 4 | 7 |
| Prior use of antibiotics | 53 | 95 |
| Prior use of antifungals | 30 | 54 |
| Azoles | 26 | 46 |
| Amphotericin B | 4 | 7 |
| Prior surgical procedures | 37 | 66 |
| Abdominal surgery | 19 | 34 |
| Non-abdominal surgery | 18 | 32 |
| Solid organ transplantation | 1 | 2 |
| HSCT | 2 | 4 |
| Hemodialysis | 3 | 5 |
| Extensive burns | 3 | 5 |
| Steroids and/or immunosuppressive therapy | 13 | 23 |
| Antineoplastic chemotherapy | 7 | 13 |
CVC, central venous catheter; TPN, total parenteral nutrition; HSCT, hematopoietic stem cell transplant.
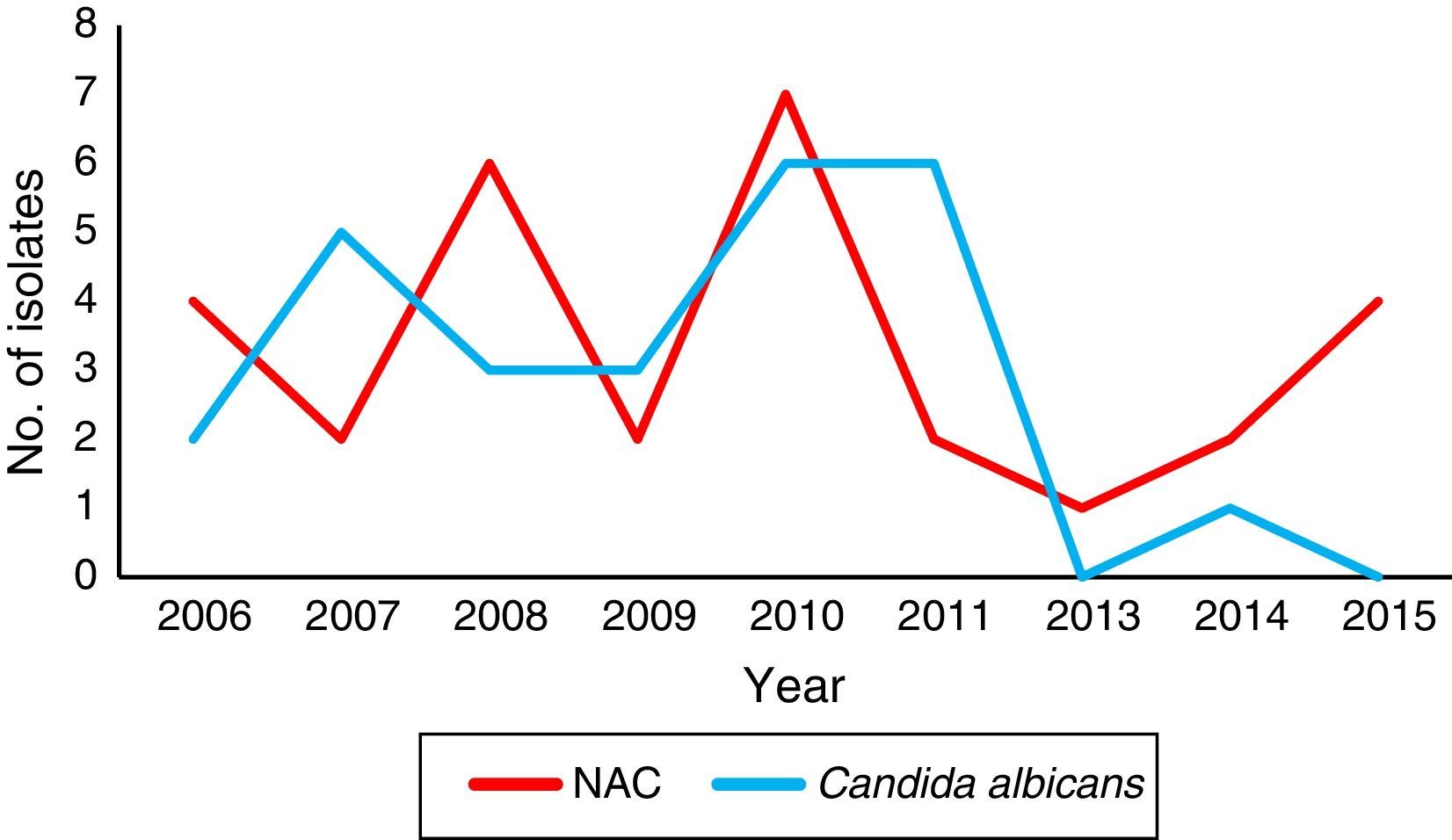
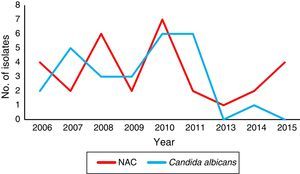
Concerning the local species distribution, we considered a total of 89 bloodstream isolates. Candida tropicalis (52.8%, n=47) was the leading agent, followed by C. albicans (30.4%, n=27), C. parapsilosis sensu stricto (10.1%, n=9), C. orthopsilosis (3.4%, n=3), Candida krusei (2.2%, n=2), and C. glabrata sensu stricto (1.1%, n=1). The temporal species distribution in our center showed a marked shift, being C. albicans the predominant species until 2011 (Fig. 1).
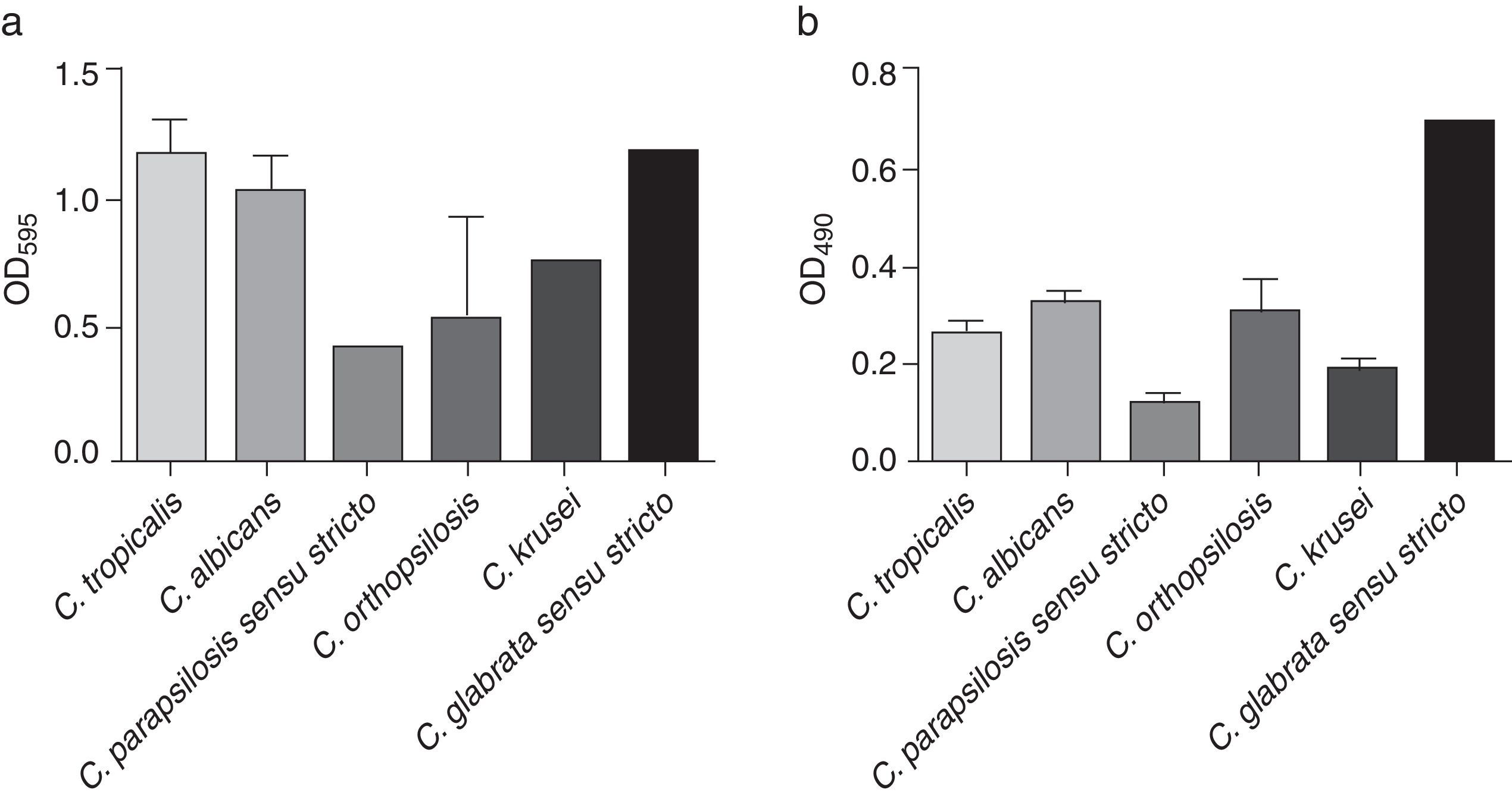
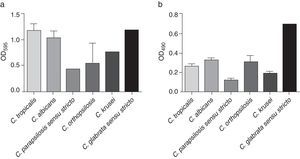
All the analyzed strains were biofilm producers; this production was evaluated using two standardized methodologies, showing important variations between species and strains (Fig. 2). According to the crystal violet staining, C. tropicalis exhibited the highest biofilm formation (mean OD=1.171±0.858), followed by C. glabrata sensu stricto (OD=1.169), C. albicans (mean OD=1.034±0.792), C. krusei (mean OD=0.773±0.021), C. orthopsilosis (mean OD=0.540±0.682) and C. parapsilosis sensu stricto (mean OD=0.439±0.520) (Fig. 2a). On the other hand, C. glabrata sensu stricto (OD=0.694) showed the highest metabolic activity, followed by C. albicans (mean OD=0.324±0.101), C. orthopsilosis (mean OD=0.300±0.118), C. tropicalis (mean OD=0.264±0.120), C. krusei (mean OD=0.189±0.029) and C. parapsilosis sensu stricto (mean OD=0.115±0.053) (Fig. 2b). The OD mean values of the metabolic activity were significantly different between the species (p<0.0001), as opposed to those OD means of crystal violet staining, which did not showed significant differences between the species. A statistical association between the biofilm-forming capability of the Candida bloodstream isolates and the 30-day mortality could not be calculated as all the analyzed strains were biofilm producers.
Due to the marked difference in mortality between CVC associated-candidemia and non-CVC associated-candidemia, a complementary comparison between the biofilm formation in Candida isolates recovered from patients with CVC and the non-CVC related isolates was performed. Those isolates from CVC exhibited more production of biofilm in vitro (mean OD595=1.047±0.828; mean OD490=0.275±0.130) compared with their counterparts (mean OD595=1.027±0.818; mean OD490=0.272±0.130), but this difference was not statistically significant.
DiscussionCandida is the second leading cause of BSI in rank-order (after Staphylococcus aureus) in Europe and North America, and is presently the third foremost cause of catheter-associated BSI in United States.17,39Candida infections are considered high-morbidity infections, with significant hospital costs, largely due to increased hospital LOS and costs for the antifungal therapy.29 In general, risk factor of candidemia include neutropenia, use of broad-spectrum antimicrobial agents, CVC, Candida colonization in multiple sites, hemodialysis, abdominal surgery, intravenous feeding, use of immunosuppressant agents, intensive care unit hospitalization, organ or stem-cell transplants, malignant tumors and severe burns. However, findings vary significantly between studies, probably due to heterogeneity in pathogens and study populations.
In the present study, we observed a 30-day mortality rate of 36%, which is close to the overall rate of 39% reported by Bassetti et al.,4 and the rate of 38% communicated by Wisplinghoff et al. on nosocomial BSIs due to Candida in 52 hospitals in the United States.41 In a recent Italian survey, a crude mortality rate of 31% was reported in 2015.24 It is worth mentioning that the overall mortality rates of patients with candidemia are usually higher in Latinamerica than in the northern hemisphere,5,26 thus a debate is ongoing regarding the real magnitude of the mortality attributable to the candidemia, ranging from 14.5% to 49%.14 It should be noted that the data on the epidemiology and prognostic factors associated with candidemia in patients admitted to intensive care units in Latinamerica are scarce.6,7 Nucci et al.26 reported an incidence of candidemia in Latinamerica of 1.18 cases per 1000 admissions, with considerable variations across countries, being Colombia and Chile the countries with the highest and the lowest incidence, respectively. They reported an overall 30-day survival of 59.3% and a high percentage (44.2%) of candidemia in children.26 In this sense, Santolaya et al.31 conducted an active surveillance of candidemia in children in 8 Latinamerica countries, finding a median incidence of 0.81 cases per 1000 admissions and a 30-day survival rate of 60% in neonates and 72% in children. All these shocking findings led to the establishment in 2013 of important recommendations for the diagnosis of candidemia in Latinamerica by the Latin America Invasive Mycosis Network, leadered by Arnaldo L. Colombo.8
It is well known that C. krusei cause a disproportionate number of infections in hematopoietic stem cell transplant recipients, while C. parapsilosis causes catheter-related BSIs, especially among neonates receiving total parenteral nutrition.29 Moreover, C. glabrata is commonly associated with BSI among those individuals older than 70 years of age, whereas C. tropicalis causes infection in oncological patients receiving chemotherapy.29 It is important to consider that the exact species distribution may vary by geographic location and risk group. Precisely for this reason, the knowledge of the epidemiology of Candida infections is important to support institutional, national, and regional guidelines that establish the empirical treatment of suspected infections.18
Unfortunately, in Mexico there is scarce information regarding the epidemiology of candidemia and local trends in species distribution. Ayala-Gaytán et al.2 conducted a prospective study from 2004 to 2008 in a population of hospitalized patients who required the use of CVC, and found that Candida species were the most frequently isolated microorganisms with the predominance of C. parapsilosis. Later, González et al.15 performed a 3-year surveillance program (2004–2007) in Monterrey, Mexico, and established that the species distribution differs according to the age of the patients, being C. parapsilosis particularly frequent among infants of <1 year old, and that the proportion of candidemias caused by C. glabrata increases with patient age (>45 years old). We found that 50% of the strains identified as C. parapsilosis and C. glabrata corresponded to pediatric and elderly patients, respectively.
Although there is an increasing evidence of the progressive rise of NAC species, C. albicans still represents the most isolated species in various geographical regions.4,42 In the present study, NAC accounted for a high proportion of all BSI isolates collected (70%), of which C. tropicalis was the most frequent (associated with the 55% of the fatal cases of candidemia). These results are in accordance with those previously reported in some regions of Asia,34,35 and are in contrast to those from Europe and Latinamerica, where C. glabrata and C. parapsilosis were the most common NAC in bloodstream isolates.2,30
Candida species, mainly C. albicans, C. glabrata, C. parapsilosis, Candida dubliniensis and C. tropicalis, are responsible from diverse forms of infections that are associated with the formation of biofilms.19 Biofilms confer significant resistance to antifungal therapy, resulting in persistent infections.9 In the present study, all the analyzed strains were in vitro biofilm producers. C. glabrata exhibited the highest metabolic activity, agreeing with Ferreira et al. and Marcos-Zambrano et al.11,20 In contrast, C. tropicalis showed by crystal violet staining quantitation the highest biomass production of biofilm, as previously reported.20 These differences between the biofilms formed by C. glabrata and those of C. tropicalis could be explained by their differences in the biofilm matrix structure.20 Notoriously, the biofilm formation capability of the bloodstream isolates we analyzed had an important contribution to the patient's mortality observed, since the latter was significantly higher in patients with isolates recovered from CVC when compared with other anatomical sites. In this sense, a recent report showed that the production of biofilm has a marked clinical impact, since mortality is greater in patients infected by biofilm-forming isolates than in those infected by non-biofilm-forming isolates.38 Furthermore, the species that produced biofilms with the highest biomass (C. tropicalis) or with the highest metabolic activity (C. glabrata) were previously correlated with poor outcome of the infected patients.23,32
The main limitations of this study were, on one hand, the retrospective design which may have involved a selection bias, and on the other hand the single-center nature that may limit the generalization of the results to other centers. In addition, the relatively small number of strains and the fact that all of them were biofilm producers compromise the statistical power of any potential association. Nevertheless these results are in agreement with the literature, and could serve as a basis for future larger-scale studies.
In conclusion, our data expand the current knowledge of the local epidemiology of candidemia and highlight the impact of biofilms on the clinical evolution of patients with this invasive fungal disease.
Funding sourcesThis work was supported by internal resources of the Infectology Service of the Internal Medicine Department in University Hospital “Dr. José E. González” and Department of Microbiology, School of Medicine, UANL.
Conflict of interestThe authors have no conflict of interest. The authors alone are responsible for the content and the writing of the paper.