Candida parapsilosis may acquire resistance to echinocandins, a fact that prompts the search for new therapeutic options.
AimsThe present study aimed to evaluate the in vitro activity of antifungal agents, alone and in combination, against four groups of C. parapsilosis strains: (1) echinocandin-susceptible (ES) clinical isolates (MIC ≤ 2μg/ml), (2) anidulafungin-resistant strains (MIC ≥ 8μg/ml), (3) caspofungin-resistant strains (MIC ≥ 8μg/ml), and (4) micafungin-resistant strains (MIC ≥ 8μg/ml).
MethodsAntifungal interactions were evaluated by a checkerboard micro-dilution method. The determination of the MIC to each drug for every isolate according to the Clinical and Laboratory Standards Institute documents M27 (2017) and M60 (2017) was also done.
ResultsThe echinocandins-resistant (ER) strains showed higher MICs to the tested antifungals than the ES strains, except for amphotericin B, for which the ER groups remained susceptible.
ConclusionsMost combinations showed indifferent interactions. The use of monotherapy still seems to be the best option. As resistance to echinocandins is an emergent phenomenon, further studies are required to provide clearer information on the susceptibility differences between strains to these antifungal agents.
Candida parapsilosis puede volverse resistente a las equinocandinas, lo que requiere la búsqueda de nuevas opciones terapéuticas.
Objetivos: El presente estudio tenía como objetivo evaluar la actividad in vitro de algunos antifúngicos, solos y en combinación, frente a cuatro grupos de cepas de C. parapsilosis: 1) cepas clínicas sensibles a las equinocandinas (SE) (CIM ≤ 2 μg/ml), 2) cepas resistentes a la anidulafungina (CIM ≥ 8 μg/ml), 3) cepas resistentes a la caspofungina (CIM ≥ 8 μg/ml) y 4) cepas resistentes a la micafungina (CIM ≥ 8 μg/ml).
MétodosSe evaluaron las interacciones de los antifúngicos con el método de microdilución en damero. También se determinó el valor de la CIM de cada cepa en cada antifúngico de acuerdo con los documentos Clinical and Laboratory Standards Institute M27 (2017) y M60 (2017).
ResultadosLas cepas resistentes a las equinocandinas (RE) presentaron los valores de CIM más altos a los antifúngicos probados que las cepas SE a excepción de la anfotericina B, frente a la cual los grupos RE se mantuvieron sensibles.
ConclusionesLa mayoría de las combinaciones evidenciaron interacciones indiferentes. El uso de monoterapias aún parece la mejor opción. Puesto que la resistencia a las equinocandinas es un fenómeno emergente, se requieren estudios adicionales con el fin de proporcionar una información más clara acerca de las diferencias de sensibilidad de diferentes cepas a estos antifúngicos.
Candida parapsilosis is one of the most common Candida species isolated in Latin America, Europe, and Asia.5,12,19 Echinocandins are recommended as the first-line treatment for invasive candidemia.20,27 However, C. parapsilosis naturally requires higher concentrations of echinocandins in clinical treatments than other species, and has additionally been reported to gain resistance after continuous treatment with these drugs.18 The substantial increase in fungal infections that are refractory to current therapies has aroused a great interest in combined treatments with antifungal agents.13 However, the activities of antifungal combinations against echinocandins-resistant (ER) C. parapsilosis strains have not yet been studied.
In this study, we evaluated the in vitro activity of amphotericin B, flucytosine, fluconazole, and voriconazole, alone and in combination, against echinocandin-susceptible and resistant C. parapsilosis strains.
We studied four groups of C. parapsilosis strains: the first included 30 clinical echinocandin-susceptible (ES) isolates obtained from the Mycological Research Laboratory at the Federal University of Santa Maria, Brazil, identified by standard methods15 and molecular methods.26 The second group was named anidulafungin-resistant (AR; n=10), the third group was caspofungin-resistant (CR; n=10), and the fourth group was micafungin-resistant (MR; n=10). Resistance was induced in the 30 clinical isolates of C. parapsilosis susceptible to echinocandins (endpoint ≥8μg/ml).7 Each isolate of the ES group was challenged to grow at increasing (two-fold) concentrations of anidulafungin, caspofungin or micafungin, based on the method described by Fekete-Forgács et al.9 The initial concentration of the drug was 0.03μg/ml, and the final one 8μg/ml. Afterwards, resistance-induced clinical isolates were maintained in distilled water with 10% glycerol in concentration of resistance (≥8μg/ml) for each echinocandin at −80°C.
Amphotericin B (AMB), flucytosine (5FC) (Sigma–Aldrich, St. Louis, MO); fluconazole (FLZ), voriconazole (VCZ), and anidulafungin (Pfizer Pharmaceutical Group, New York, NY); caspofungin (Merck, Darmstadt, Germany), and micafungin (Astellas, Chuo, Tokyo, Japan) were obtained as standard powders and the dilutions were prepared in accordance with the guidelines of the Clinical and Laboratory Standards Institute (CLSI).7,8 Antifungal susceptibility tests were performed according to the CLSI M27-A3 micro-dilution technique and the results were interpreted as described in the M27-S4 document.7,8 Antifungal interactions were evaluated by the broth micro-dilution checkerboard method.13 A synergistic interaction was defined as a fractional inhibitory concentration index (FICI)≤0.5, additivity 0.5<FICI<1.0, indifference 1.0≤FICI<4.0 and, antagonism FICI≥4.0.16 The C. parapsilosis strain ATCC 22019 and the C. krusei strain ATCC 6258 were used as quality controls. All assays were performed in duplicate. MICs were analyzed by the non-parametric Wilcoxon's paired t-test.
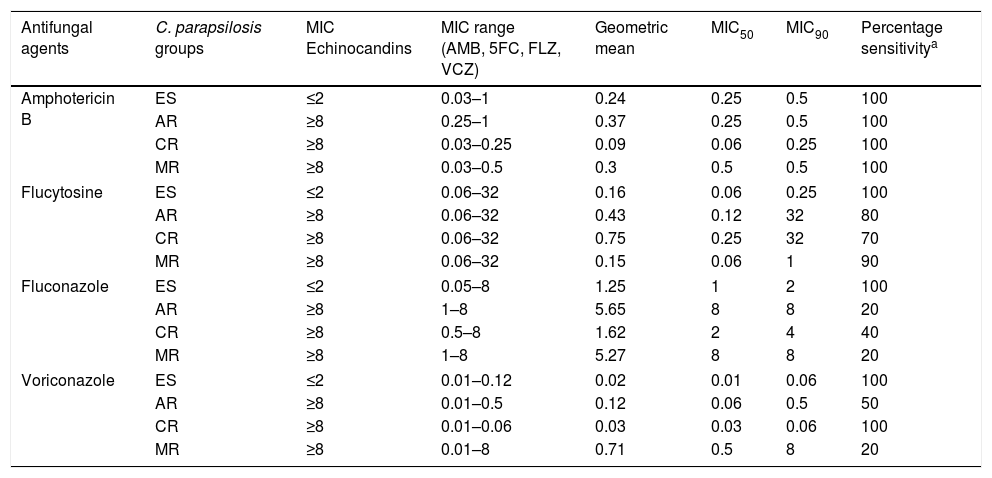
The susceptibilities described for the ES and ER groups are presented in Table 1. ER strains were statistically less susceptible to 5FC (MIC≤4μg/ml) and azoles (FLZ MIC ≤2μg/ml and VCZ MIC ≤0.12μg/ml) than the ES strains (MIC ≤2μg/ml) (p<0.05). The only exception was the CR group, which did not show any change in the susceptibility profile to VCZ. All ER strains remained susceptible to AMB.
In vitro susceptibility (μg/ml) of echinocandin-susceptible and echinocandin-resistant C. parapsilosis strains to amphotericin B, flucytosine, fluconazole and voriconazole.
| Antifungal agents | C. parapsilosis groups | MIC Echinocandins | MIC range (AMB, 5FC, FLZ, VCZ) | Geometric mean | MIC50 | MIC90 | Percentage sensitivitya |
|---|---|---|---|---|---|---|---|
| Amphotericin B | ES | ≤2 | 0.03–1 | 0.24 | 0.25 | 0.5 | 100 |
| AR | ≥8 | 0.25–1 | 0.37 | 0.25 | 0.5 | 100 | |
| CR | ≥8 | 0.03–0.25 | 0.09 | 0.06 | 0.25 | 100 | |
| MR | ≥8 | 0.03–0.5 | 0.3 | 0.5 | 0.5 | 100 | |
| Flucytosine | ES | ≤2 | 0.06–32 | 0.16 | 0.06 | 0.25 | 100 |
| AR | ≥8 | 0.06–32 | 0.43 | 0.12 | 32 | 80 | |
| CR | ≥8 | 0.06–32 | 0.75 | 0.25 | 32 | 70 | |
| MR | ≥8 | 0.06–32 | 0.15 | 0.06 | 1 | 90 | |
| Fluconazole | ES | ≤2 | 0.05–8 | 1.25 | 1 | 2 | 100 |
| AR | ≥8 | 1–8 | 5.65 | 8 | 8 | 20 | |
| CR | ≥8 | 0.5–8 | 1.62 | 2 | 4 | 40 | |
| MR | ≥8 | 1–8 | 5.27 | 8 | 8 | 20 | |
| Voriconazole | ES | ≤2 | 0.01–0.12 | 0.02 | 0.01 | 0.06 | 100 |
| AR | ≥8 | 0.01–0.5 | 0.12 | 0.06 | 0.5 | 50 | |
| CR | ≥8 | 0.01–0.06 | 0.03 | 0.03 | 0.06 | 100 | |
| MR | ≥8 | 0.01–8 | 0.71 | 0.5 | 8 | 20 | |
ES, echinocandin-susceptible; AR, anidulafungin-resistant; CR, caspofungin-resistant; MR, micafungin-resistant; AMB, amphotericinB; 5FC, flucytosine; FLZ, fluconazole; VCZ, voriconazole; MIC50, minimal inhibitory concentration for 50% of the strains; MIC90, minimal inhibitory concentration for 90% of the strains.
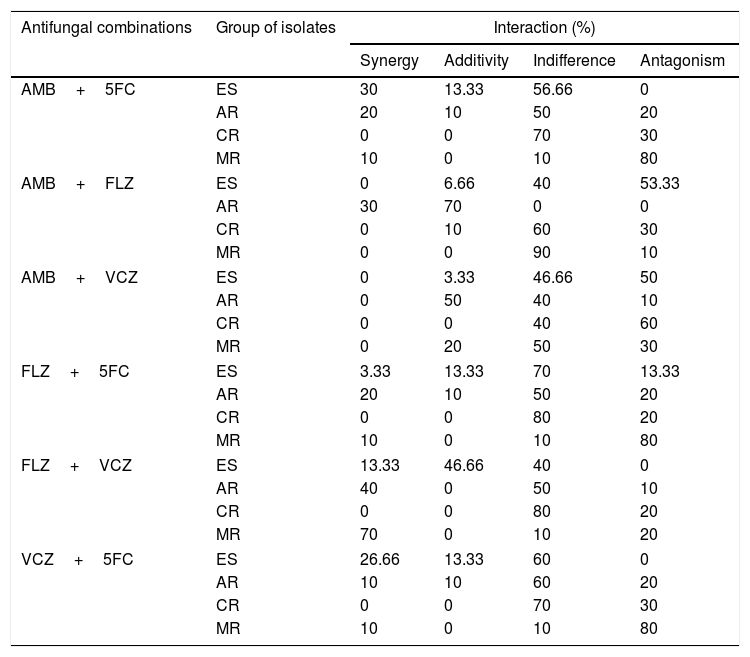
Table 2 shows the percentages of antifungal agents interactions. Indifferent activity was the main interaction shown. The interactions of 5FC with AMB or VCZ and FLZ+VCZ did not exhibit antagonism and showed similar percentages of synergism and indifference when testing them against ES strains. Antagonistic interactions were shown with the combinations AMB+FLZ (53.3%), AMB+VCZ (50%), and FLZ+5FC (13.3%). All combinations for the ER group showed percentages of antagonism, except the combination AMB+FLZ against the AR group which showed synergism (30%) and additivity (70%).
In vitro antifungal interactions of amphotericin B (AMB), flucytosine (5FC), fluconazole (FLZ) and voriconazole (VCZ) against echinocandin-susceptible and echinocandin-resistant C. parapsilosis strains.
| Antifungal combinations | Group of isolates | Interaction (%) | |||
|---|---|---|---|---|---|
| Synergy | Additivity | Indifference | Antagonism | ||
| AMB+5FC | ES | 30 | 13.33 | 56.66 | 0 |
| AR | 20 | 10 | 50 | 20 | |
| CR | 0 | 0 | 70 | 30 | |
| MR | 10 | 0 | 10 | 80 | |
| AMB+FLZ | ES | 0 | 6.66 | 40 | 53.33 |
| AR | 30 | 70 | 0 | 0 | |
| CR | 0 | 10 | 60 | 30 | |
| MR | 0 | 0 | 90 | 10 | |
| AMB+VCZ | ES | 0 | 3.33 | 46.66 | 50 |
| AR | 0 | 50 | 40 | 10 | |
| CR | 0 | 0 | 40 | 60 | |
| MR | 0 | 20 | 50 | 30 | |
| FLZ+5FC | ES | 3.33 | 13.33 | 70 | 13.33 |
| AR | 20 | 10 | 50 | 20 | |
| CR | 0 | 0 | 80 | 20 | |
| MR | 10 | 0 | 10 | 80 | |
| FLZ+VCZ | ES | 13.33 | 46.66 | 40 | 0 |
| AR | 40 | 0 | 50 | 10 | |
| CR | 0 | 0 | 80 | 20 | |
| MR | 70 | 0 | 10 | 20 | |
| VCZ+5FC | ES | 26.66 | 13.33 | 60 | 0 |
| AR | 10 | 10 | 60 | 20 | |
| CR | 0 | 0 | 70 | 30 | |
| MR | 10 | 0 | 10 | 80 | |
ES, echinocandin-susceptible; AR, anidulafungin-resistant; CR, caspofungin-resistant; MR, micafungin-resistant.
The ES C. parapsilosis strains showed marked sensitivity to all the antifungal agents tested alone, as previously described.1,3,21,22 ER C. parapsilosis strains were less susceptible to the antifungal agents tested than the sensitive strains, except for AMB, for which the ER groups remained susceptible. To our knowledge, there is only one reported case of multiple resistance involving echinocandins and azoles for C. parapsilosis.18 This study shows that multidrug resistance involving echinocandins and 5FC can also occur in C. parapsilosis, whereas previous studies have reported that this phenomenon is emerging for C. glabrata.6 Failure in the detection of C. parapsilosis multi-drug resistance involving AMB and echinocandins corroborates previous studies that confirm the potent in vitro activity of AMB.18 The CLSI breakpoints used in this study in the echinocandins and azoles for C. parapsilosis are in accordance with the new antifungal breakpoints on antifungal resistance proposed by Fothergill et al.10
Investigation of the combination of AMB with 5FC has yielded mixed results, depending on the conditions and test employed. The predominant interaction observed in the Candida species is synergism16,17; however, indifference has also been observed by Kelle et al.14 Our results showed predominance of indifference for the ES, AR, and CR groups, and antagonism for the MR group. The mechanism of the antagonistic effect to polyenes and 5FC is still unknown. Shadomy et al.23 suggested that antagonistic interactions between these agents might be related to changes in fungal cell membrane functions due to the effects of AMB.
The combination of 5FC with VCZ or FLZ showed higher antagonism against the ER strains than against the ES strains. In vitro testing of VCZ+5FC by Barchiesi et al.4 showed 5% synergy, 95% indifference, and no antagonism against C. glabrata isolates. However, previous studies observed that 5FC+azole combinations are antagonistic in C. glabrata.2,24 Steier et al.25 observed that mitochondrial dysfunction resulting in Pdr1 (positive regulator of proteins involved in permeability) activation is the likely basis for 5FC antagonism of azole activity against C. glabrata.
Although the majority of reports mention antagonism between AMB and azoles, data from in vitro studies remain controversial as indifferent, additive and antagonistic interactions have also been described.2,4 Here, the combination of AMB and azoles resulted in antagonism against the ES group and mostly indifference against the ER groups. The combination of FLZ+VCZ against the ER groups showed increased antagonism, and the additivity percentage in the ES group (46.66%) was similar to that found by Ghannoum and Isham11 (42%) against Candida isolates.
In summary, the combined activities of antifungal agents against C. parapsilosis strains were disappointing if we consider that a high percentage of indifference was observed in most cases and that, in some of these cases, the antagonism was quite high. The use of antifungal agents alone seems to be the best option currently. However, as resistance to echinocandins and cross-resistance to some antifungal agents increase, the treatment of infections by such isolates will be more daunting. In vivo studies are required to further elucidate the antifungal susceptibility differences between strains that are susceptible or resistant to echinocandins.
Conflict of interestAll authors agree that no conflict of interest exists for the publication of this paper.
This study was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico – (CNPq; process #470229/2012-8) and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES).