Anaemia is a very common condition in elderly patients with hip fracture. The side effects of blood transfusions are well known, and further research on potential alternative therapies is needed.
Objectives and designA non-controlled descriptive study, conducted on 138 patients admitted for hip fracture, aimed at analysing the effects of an anaemia treatment protocol adjunctive to transfusion, based on the use of supra-physiological doses of intravenous iron and erythropoietin (IS/EPOS). The variables collected were, medical history, physical and cognitive status prior to fracture, as well as the need of blood products, medical complications during admission and their functional outcome at three and six months after the fracture were evaluated. Transfusion rates were compared with a historical control group when the only treatment for acute anaemia was transfusion (2011).
ResultsAlmost half (63, 48%) of the patients received blood transfusion, with (91,70%) IS/EPOD. Intravenous iron did not reduce the percentage of transfused patients (56% vs. 44%), but it did reduce the number of blood units required (0.7 units less in IS/EPO group). Patients who required transfusion had a longer hospital stay, (1.7 days; 13.2 vs. 11.5; p<0.005). Patients who received IS had better functional recovery assessed with Barthel index and the Functional Ambulation Categories (FAC scale) at 3 and 6 months after the fracture. Patients with malnutrition or subtrochanteric fracture needed more tabletransfusions (p<0.005). Functional recovery at 3 and 6 months after fracture was better in patients who received intravenous iron. Neither blood transfusions nor intravenous iron were associated with infectious complications or increased mortality. The patient series of this study was compared with a group of patients with hip fracture and similar characteristics seen in 2011, before intravenous iron was available, revealing a 17% reduction in blood transfusion needs (p<0.005).
ConclusionThe use of intravenous iron in elderly patients with hip fracture may help to reduce the number of blood units needed for the treatment of anaemia, although a causal relationship cannot be established due to not having a control group. Transfusions were associated with longer hospital stay in elderly patients with hip fracture.
El síndrome anémico es muy frecuente en el anciano ingresado por fractura de cadera. Los documentados efectos secundarios de la transfusión de hemoderivados hacen necesario investigar otras posibles alternativas terapéuticas.
Material y métodosEstudio descriptivo de 138 pacientes ingresados por fractura de cadera que evalúa el efecto de un protocolo de tratamiento de anemia perioperatoria complementario a transfusión, basado en el empleo de dosis suprafisiológicas de hierro intravenoso y eritropoyetina (FE/EPO). Se trata de un estudio descriptivo sin grupo control. Se evaluaron los antecedentes médicos de los pacientes, y su situación mental y física previas al ingreso, la necesidad de hemoderivados, las complicaciones en el ingreso y su evolución funcional en los 6 meses posteriores a la fractura. Los ratios de transfusión fueron comparados con los de una muestra histórica de similares características sin tratamiento con ferroterapia intravenosa (2011).
ResultadosRecibieron transfusión el 48% de los pacientes (63) y ferroterapia parenteral con eritropoyetina (FE/EPO) el 70% (91). La administración de FE/EPO no disminuyó el porcentaje de pacientes hemotransfundidos (56 vs. 44%) de forma significativa, pero sí redujo el número de unidades de sangre requeridas (0,7 unidades menos en grupo de FE/EPO). Los pacientes que recibieron ferroterapia intravenosa tuvieron una estancia hospitalaria de 1,7 días inferior que los transfundidos (11,5 vs. 13,2; p<0,005). La administración de hierro parenteral se relacionó con tendencia a una mejor recuperación de capacidad de autocuidados y deambulación medidos mediante el índice de Barthel (IB) y la escala de deambulación de Holden (FAC) a los 3 y 6 meses de la fractura. La anemia y la malnutrición al ingreso, así como el tipo de fracturas subtrocantérea se relacionaron de forma independiente a mayor necesidad de transfusión (p<0,005). Ni las transfusiones ni el tratamiento con ferroterapia parenteral se asociaron a más complicaciones infecciosas ni a mayor mortalidad. Al comparar la muestra actual con un control histórico de pacientes con fractura de cadera sin disponibilidad de tratamiento con hierro intravenoso, se observa reducción actual del porcentaje de pacientes transfundidos en un 17% (p<0,005).
ConclusiónEl empleo de ferroterapia intravenosa en pacientes con fractura de cadera puede reducir el número de unidades de hemoderivados necesarias, sí bien no se puede establecerse una relación causal al no ser un estudio controlado. La transfusión se asocia con una prolongación de la estancia hospitalaria en el anciano ingresado por fractura de cadera.
In lower limb surgery in elderly patients, blood loss, blockage of erythropoiesis by inflammatory factors, and frequent haematinic deficiencies result in anaemia in over 90% of cases. The treatment of anaemia with therapies other than blood transfusion is currently under investigation.
The high prevalence of anaemia in patients with hip fracture and the proven deleterious effects of treatment with transfusion alone prompted us to design a treatment protocol with intravenous iron aimed at reducing blood transfusion needs in these patients. Even though this therapy has been evaluated in younger patients undergoing other surgical procedures1,2 specific recommendations for frail elderly patients cannot yet be easily drawn from the available scientific evidence.3 In Spain, several studies have been recently published about treatment of anaemia in orthopaedic surgery.4–7 But the results of use of intravenous iron therapy in semi-urgent surgical procedures, the importance of haemoglobin level on discharge in patients who are generally very old, frequently malnourished, with a high comorbidity burden, and its effects in mortality or functional recovery, still remain controversial.8–10
Based on recent evidence from literature, a treatment protocol was established in 2013 for treating anaemia with therapy adjunctive to transfusion in elderly patients with hip fracture admitted in the Guadalajara University Hospital,11 following the recent recommendations for Patient Blood Management (PBM) published for hip fracture and other types of orthopaedic surgery. The outcomes of the implementation of this protocol were subsequently evaluated.
Material and methodsStudy designThis was a descriptive study to analyse the effects and usefulness of a treatment protocol for anaemia secondary to hip fracture with 3 and 6 month follow-ups. It is a descriptive trial with no control group. The Hospital Ethics Committee approved the conduct of the study, and all patients provided their informed consent to take part. For the calculation of the sample size, it was interesting to know specifically if the application of a protocol with IS/EPO on patients with hip fracture was able to reduce transfusion of red blood cell concentrates in comparison to a standard. With an alfa error of 5% and a beta error of 20% (an 80% of acceptability), and a bilateral test, it is estimated that 45% of the patients would need transfusion, in contrast with 65% of those on whom such protocol was not applied (according to a study of historical cohorts). Bearing in mind these assumptions the estimate of patients per cohort is of 96 (historical cohort and prospective cohort). If we apply the Fleiss correction to obtain a more conservative estimation and we assume a 15% marginal loss then we would need 113 patients for each cohort. The dependent variables were haemoglobin level on discharge, medical complications during hospital stay and functional status three and six months after the fracture.
ObjectiveTo evaluate the effects of an anaemia treatment protocol in elderly patients admitted for hip fracture. The main objective was to identify transfusion related factors. Secondary objectives were evaluation of transfusion requirements in patients treated with intravenous iron and the effect of this protocol in mortality functional outcomes 3 and 6 months after the fracture.
Target populationPatients included were subjects over 75 years of age, with osteoporotic proximal femoral fracture consecutively admitted at the Orthopaedic Surgery Department of the Guadalajara University Hospital (Castilla-La Mancha Health Service, Spain) between November 2014 and June 2015. The Geriatric Department assessed all patients at admission and monitored them daily.
Inclusion criteriaPatients with osteoporotic proximal femoral fracture over 75, admitted for surgical treatment in the University Hospital of Guadalajara between November 2014 and June 2015.
Exclusion criteriaPatients with pathological fractures or fractures due to a high-impact injury, iron storage disorders or intolerance to intravenous iron therapy were excluded, as were patients with absolute contraindication for erythropoietin and those who did not sign the informed consent.
Data collection and description of variablesPatient data were obtained from their medical records, and geriatric researchers assessed patients within three days after fracture. Patients were initially assessed during hospital stay and, subsequently, telephone follow-ups were conducted after 3 and 6 months.
Demographic data, medical history, and previous physical, cognitive, and social status were collected. The following scales were used: the Barthel index (BI) to assess self-care ability,12 the Holden Functional Ambulation Scale (FAC) to assess walking ability13 and the Reisberg Global Deterioration Scale (GDS) to measure cognitive impairment.14 The type of fracture, surgical treatment, rehabilitation therapy, complications during hospital stay, length of hospital stay, mortality, and functional outcome at the time of discharge were also assessed. Previous drug treatments potentially associated with the fall causing the fracture or with perioperative complications were also evaluated. On the first day after hospital admission, anaemia-related laboratory tests and nutritional status assessments (laboratory tests, Mininutritional Assessment test [MNA]),15 and vitamin deficiency assessments were conducted. Haemoglobin levels were measured on admission and then every 48h until postoperative stability. We evaluated haemoglobin level with which transfusion was prescribed to detect protocol deviations. Functional outcome-related data (BI and FAC), subsequent hospital admissions, and mortality were recorded 3 and 6 months after fracture. Four patient groups were determined according to the therapy prescribed for anaemia: transfusion alone, transfusion plus IS/EPO, IS/EPO alone, or no therapy.
In order to assess more accurately the influence of the protocol for erythropoiesis stimulation in our study patients, we subsequently decided to compare the transfusions administered to our series with a historical control group of patients over 75 years of age with hip fracture who had been admitted to our hospital between December 2010 and June 2011 (n=165), before intravenous iron was available, and when the only treatment for acute anaemia was transfusion.
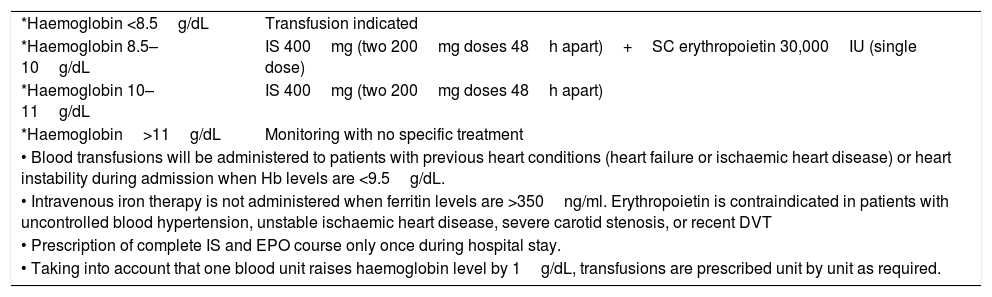
Treatment protocol for anaemia with adjunctive therapies to transfusionThe treatment protocol for anaemia is shown in Table 1. The protocol was based on the use of supra-physiological doses of intravenous iron and erythropoietin to overcome functional iron deficiency and decreased erythropoietin production and activity due to inflammation caused by the injury.1,2,16,17 It was designed according to others used for preoperative anaemia in our institution and was made by a consensus group of haematologists, geriatricians, anesthesiologists and orthopaedic surgeons, for frail elderly surgical patients. The transfusion could be prescribed by the geriatrician from the Orthogeriatrics team, the orthopaedic surgeon treating the patient or the anaesthesiologist. No autologous blood recovery was performed during surgery in any case. Haemoglobin was evaluated on admission and every 48h until postoperative stability. The administration con FE/EPO could be applied only once during the hospital stay, in relation to haemoglobin levels detected in each determination as previously described.
Anaemia treatment protocol.
| *Haemoglobin <8.5g/dL | Transfusion indicated |
| *Haemoglobin 8.5–10g/dL | IS 400mg (two 200mg doses 48h apart)+SC erythropoietin 30,000IU (single dose) |
| *Haemoglobin 10–11g/dL | IS 400mg (two 200mg doses 48h apart) |
| *Haemoglobin>11g/dL | Monitoring with no specific treatment |
| • Blood transfusions will be administered to patients with previous heart conditions (heart failure or ischaemic heart disease) or heart instability during admission when Hb levels are <9.5g/dL. | |
| • Intravenous iron therapy is not administered when ferritin levels are >350ng/ml. Erythropoietin is contraindicated in patients with uncontrolled blood hypertension, unstable ischaemic heart disease, severe carotid stenosis, or recent DVT | |
| • Prescription of complete IS and EPO course only once during hospital stay. | |
| • Taking into account that one blood unit raises haemoglobin level by 1g/dL, transfusions are prescribed unit by unit as required. | |
IS: intravenous iron sucrose; EPO: epoetin zeta; DVT: deep venous thrombosis.
All patients received individualised doses of low molecular weight heparin in accordance with their past history and renal function at admission. Perioperative antiplatelet and anticoagulant therapies were administered based on hospital clinical guidelines.18 All patients received perisurgical antibiotic prophylaxis with 2g of cefazolin 30min before the surgical procedure and every 24h for two days, as indicated by the hospital infection commission. Centre for Disease Control and Prevention diagnostic criteria19 were used for the diagnosis of hospital-acquired infections, and infections detected at the time of admission were excluded.
Statistical analysisIn the descriptive statistical analysis, median values and interquartile ranges were used to express continuous variables, and percentages to express categorical variables. Univariate statistical analyses were initially conducted. The Chi-squared test and the Fisher's exact text were used, as applicable, for comparisons between categorical variables. The Student's t test, analysis of variance, or linear regression, were used for comparisons between continuous variables means when values were normally distributed. In case of important asymmetry or lack of normality, a non-parametric test was used. Significant variables in the univariate analysis were included in linear regression or multiple logistic regression models with stepwise exclusion. Dependent variables evaluated were haemoglobin on discharge, need of transfusion, Barthel index and FAC scale three and six months after the fracture. p-Values <0.05 were considered statistically significant.
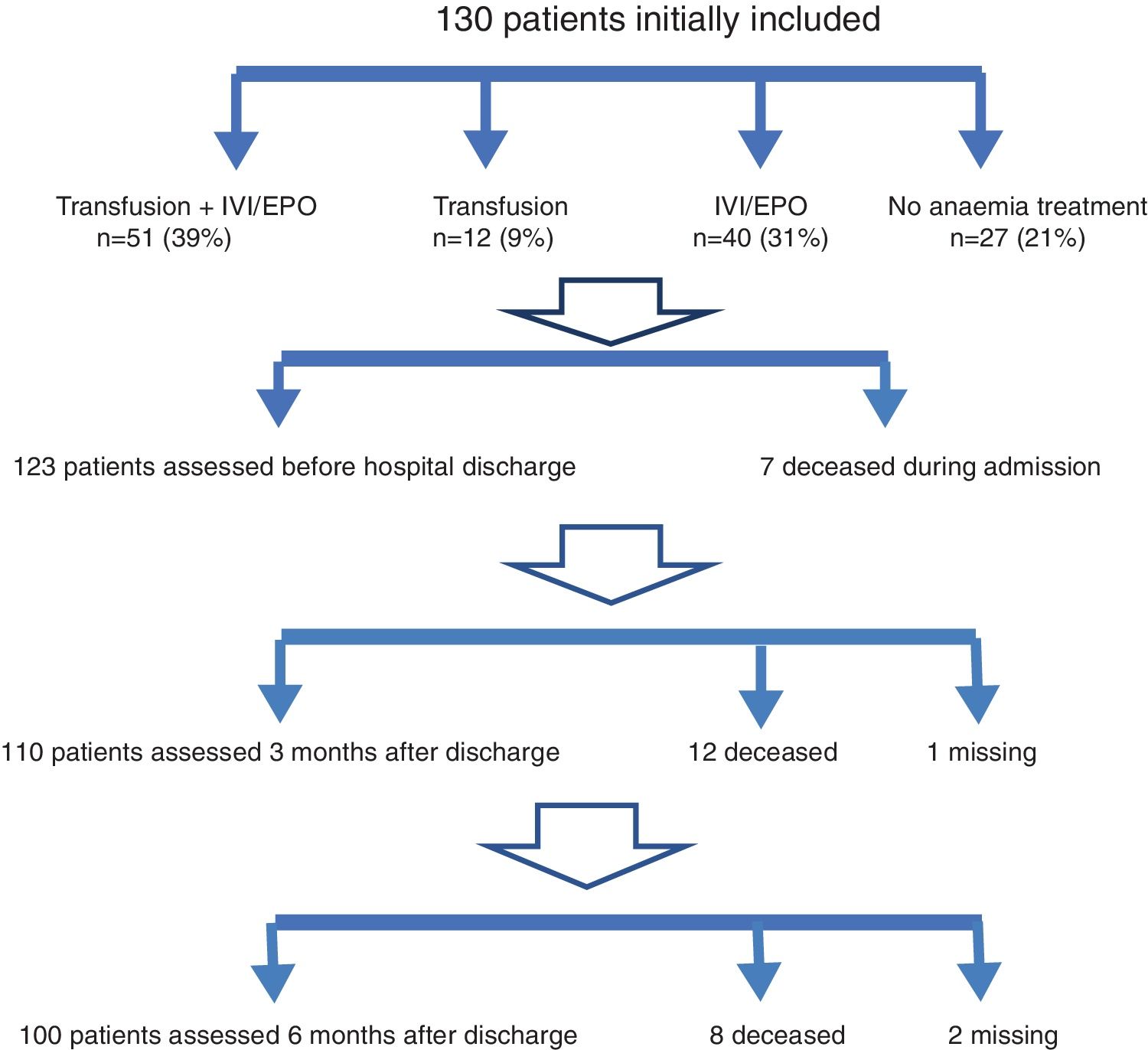
ResultsPatient characteristicsThe study sample included 138 patients. Eight patients were initially excluded because informed consent could not be obtained due to patient's mental disability or/and to the absence of a caregiver or responsible person during hospital stay.
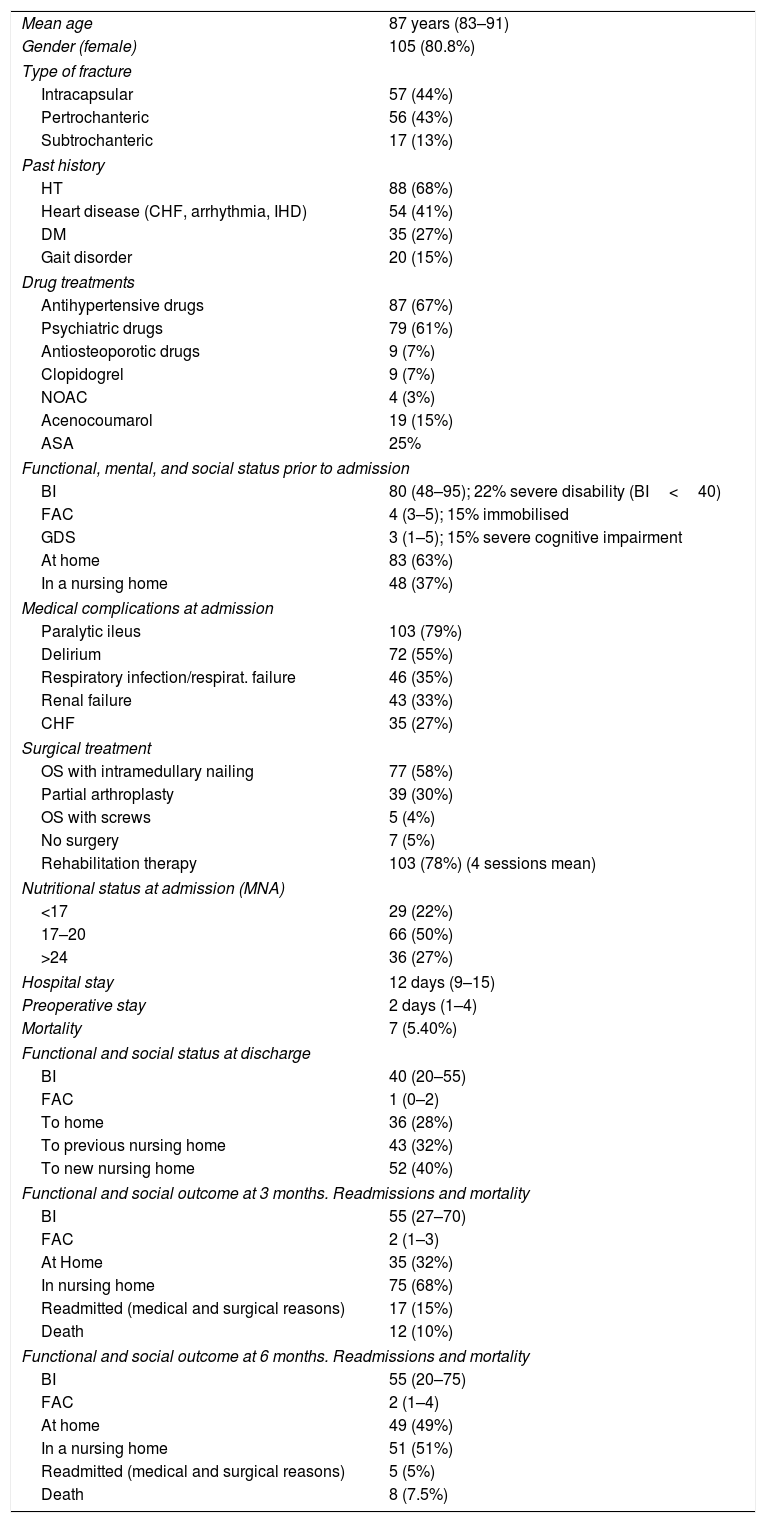
Patients characteristics and outcomes during hospital stay are shown in Table 2.
Patient Characteristics.
| Mean age | 87 years (83–91) |
| Gender (female) | 105 (80.8%) |
| Type of fracture | |
| Intracapsular | 57 (44%) |
| Pertrochanteric | 56 (43%) |
| Subtrochanteric | 17 (13%) |
| Past history | |
| HT | 88 (68%) |
| Heart disease (CHF, arrhythmia, IHD) | 54 (41%) |
| DM | 35 (27%) |
| Gait disorder | 20 (15%) |
| Drug treatments | |
| Antihypertensive drugs | 87 (67%) |
| Psychiatric drugs | 79 (61%) |
| Antiosteoporotic drugs | 9 (7%) |
| Clopidogrel | 9 (7%) |
| NOAC | 4 (3%) |
| Acenocoumarol | 19 (15%) |
| ASA | 25% |
| Functional, mental, and social status prior to admission | |
| BI | 80 (48–95); 22% severe disability (BI<40) |
| FAC | 4 (3–5); 15% immobilised |
| GDS | 3 (1–5); 15% severe cognitive impairment |
| At home | 83 (63%) |
| In a nursing home | 48 (37%) |
| Medical complications at admission | |
| Paralytic ileus | 103 (79%) |
| Delirium | 72 (55%) |
| Respiratory infection/respirat. failure | 46 (35%) |
| Renal failure | 43 (33%) |
| CHF | 35 (27%) |
| Surgical treatment | |
| OS with intramedullary nailing | 77 (58%) |
| Partial arthroplasty | 39 (30%) |
| OS with screws | 5 (4%) |
| No surgery | 7 (5%) |
| Rehabilitation therapy | 103 (78%) (4 sessions mean) |
| Nutritional status at admission (MNA) | |
| <17 | 29 (22%) |
| 17–20 | 66 (50%) |
| >24 | 36 (27%) |
| Hospital stay | 12 days (9–15) |
| Preoperative stay | 2 days (1–4) |
| Mortality | 7 (5.40%) |
| Functional and social status at discharge | |
| BI | 40 (20–55) |
| FAC | 1 (0–2) |
| To home | 36 (28%) |
| To previous nursing home | 43 (32%) |
| To new nursing home | 52 (40%) |
| Functional and social outcome at 3 months. Readmissions and mortality | |
| BI | 55 (27–70) |
| FAC | 2 (1–3) |
| At Home | 35 (32%) |
| In nursing home | 75 (68%) |
| Readmitted (medical and surgical reasons) | 17 (15%) |
| Death | 12 (10%) |
| Functional and social outcome at 6 months. Readmissions and mortality | |
| BI | 55 (20–75) |
| FAC | 2 (1–4) |
| At home | 49 (49%) |
| In a nursing home | 51 (51%) |
| Readmitted (medical and surgical reasons) | 5 (5%) |
| Death | 8 (7.5%) |
Data as median for cuantitative variables (IQR) except for age (Mean –SD) or n (percentage) for cualitative variables.
ASA: salicilic acid; HT: hypertension; DM: diabetes mellitus CHF: cronic heart failure; IHD: ischaemic heart disease NOAC; new anticoagulants; BI: Barthel index; FAC: Functional Ambulation Scale; GDS; Global Deterioration Scale; OS: osteosynthesis; MNA: Mininutritional Assessment Test; IQR: interquartile range; SD: standard deviation.
The mean haemoglobin value at admission was 12.6g/dL. Haematinic analysis showed that 33% of patients had ferritin values <120ng/ml in spite of the associated inflammatory component. Folic acid deficiency (<3.5ng/nl) was detected in 37% of patients, and vitamin B12 deficiency (<245pg/ml) in 30%.
Mean haemoglobin loss between admission and the time of surgery was 2g, due to loss secondary to bone fracture secondary losses and contusion and bruising of soft tissues.
Forty-eight percent of all patients received a transfusion (63), with a mean of 2.21 units of packed red blood cells, 58% of cases required two units and 23% one, while 19% received three or more
On average, transfusion was indicated on the 5th day after admission. As patients underwent surgery on the second day after admission, 67% of transfusions were administered during the postoperative period.
Globally, 38% of the transfusions were prescribed for haemoglobin levels >8.5g/l, outside the limits of the proposed protocol, although patients in this group did not present a higher prevalence of cardiologic history (39.1% vs. 36.8% p=NS). These transfusions to patients with haemoglobin levels above the proposed limit were administered on days 5 or 6 after admission, and were not related to instability during surgery or to postoperative acute cardiorespiratory disorders.
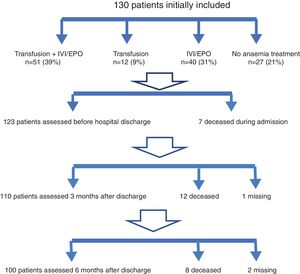
Seventy percent of patients received treatment with intravenous iron and/or EPO (91). Treatment was rarely contraindicated due to high ferritin levels (ferritin >350ng/ml: 11%), in spite of the inflammatory component caused by the acute fracture. Erythropoietin was simultaneously administered to 60% of all patients. No patients received oral iron therapy during hospital stay (Fig. 1).
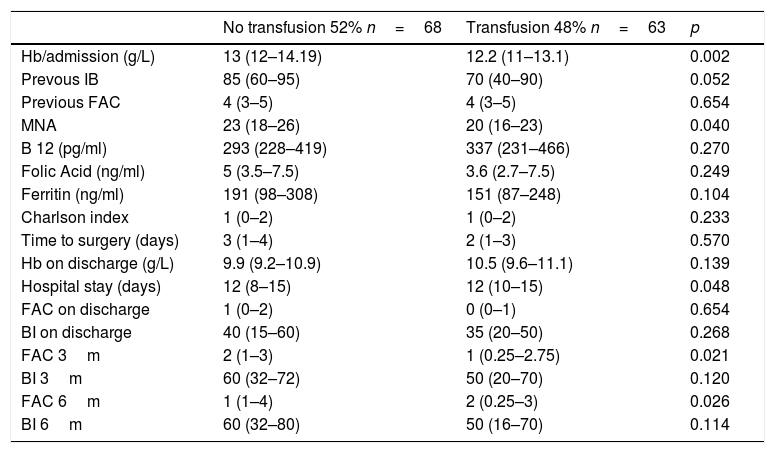
Transfusion-related factors during hospital stayIn the univariate analysis, the haemoglobin level at admission was revealed to be a driving factor of the need for transfusion during hospital stay. Thus, for each additional gram of haemoglobin at admission, the possibility of transfusion decreased by 34% (p<0.05). Transfused patients did not have the best haemoglobin values at discharge. Transfusion was not more common in patients with poorer previous functional status or with more history of heart disease, and was not associated with more cardiologic complications during hospital stay. Low levels of vitamin B12, folic acid, or ferritin at admission did not confer greater blood transfusion needs. Infection and mortality rates were not higher in transfused patients during hospital stay, and 3 and 6 months after fracture. A longer preoperative stay did not entail a greater need for transfusion. Total hospital stay of patients who received blood products was 1.65 days longer (13.2 vs. 11.5 days; p<0.05) (Table 3).
Univariate analysis of transfusion related factors.
| No transfusion 52% n=68 | Transfusion 48% n=63 | p | |
|---|---|---|---|
| Hb/admission (g/L) | 13 (12–14.19) | 12.2 (11–13.1) | 0.002 |
| Prevous IB | 85 (60–95) | 70 (40–90) | 0.052 |
| Previous FAC | 4 (3–5) | 4 (3–5) | 0.654 |
| MNA | 23 (18–26) | 20 (16–23) | 0.040 |
| B 12 (pg/ml) | 293 (228–419) | 337 (231–466) | 0.270 |
| Folic Acid (ng/ml) | 5 (3.5–7.5) | 3.6 (2.7–7.5) | 0.249 |
| Ferritin (ng/ml) | 191 (98–308) | 151 (87–248) | 0.104 |
| Charlson index | 1 (0–2) | 1 (0–2) | 0.233 |
| Time to surgery (days) | 3 (1–4) | 2 (1–3) | 0.570 |
| Hb on discharge (g/L) | 9.9 (9.2–10.9) | 10.5 (9.6–11.1) | 0.139 |
| Hospital stay (days) | 12 (8–15) | 12 (10–15) | 0.048 |
| FAC on discharge | 1 (0–2) | 0 (0–1) | 0.654 |
| BI on discharge | 40 (15–60) | 35 (20–50) | 0.268 |
| FAC 3m | 2 (1–3) | 1 (0.25–2.75) | 0.021 |
| BI 3m | 60 (32–72) | 50 (20–70) | 0.120 |
| FAC 6m | 1 (1–4) | 2 (0.25–3) | 0.026 |
| BI 6m | 60 (32–80) | 50 (16–70) | 0.114 |
| % (n) | % (n) | p | |
|---|---|---|---|
| IS/EPO treatment | 44 (32) | 56 (44) | 0.05 |
| Cardiopathy | 44 (38) | 56 (44) | 0.171 |
| ASA | 41 (13) | 59 (25) | 0.155 |
| Clopidogrel | 67 (6) | 33 (3) | 0.317 |
| Anticoagulation | 42 (8) | 58 (11) | 0.370 |
| HF | 43 (15) | 57 (20) | 0.229 |
| Infection | 54 (26) | 46 (22) | 0.717 |
| Hospital mortality | 57 (4) | 43 (3) | 0.539 |
| Mortality 3 months | 50 (6) | 50 (6) | 0.559 |
| Readmission 3m | 60 (6) | 40 (4) | 0.580 |
| Surgical readmission 3m | 14 (1) | 86 (6) | 0.045 |
| Mortality 6m | 37 (3) | 63 (52) | 0.380 |
| Readmission 6m | 40 (2) | 60 (2) | 0.555 |
| Surgical readmission 6m | 60 (3) | 40 (3) | 0.557 |
Data as median for cuantitative variables (IQR) or n (percentage) for cualitative variables.
Hb: haemoglobin; BI: Barthel index; FAC: Functional Ambulation Scale; MNA: Mininutritional Assessment Test; OR: odds ratio; CIOR: confidence interval for odds ratio CIMD: confidence interval for means difference; IS: intravenous iron sucrose; EPO: epoetin; ASA: salicilic acid; HF: heart failure; m: month.
Nutritional status at admission, assessed using the MNA test, was directly related to an indication for blood transfusion. Transfusions were more frequently administered to malnourished patients. This association was not random, and a linear trend was observed between the degree of malnutrition and transfusion needs during admission. A 5-point increase in MNA score corresponded with a 40% decrease in transfusion needs (68% of patients with MNA scores <17 vs. 35% with MNA score >24 received a transfusion; p<0.05).
Furthermore, a higher percentage of transfused patients received treatment with IS and EPO compared with non-transfused patients. According to the protocol, IS and EPO should be concomitantly administered with transfusion in case of progressive loss of haemoglobin during hospital stay.
Subtrochanteric fracture was the fracture that required more transfusions. The likelihood of receiving a transfusion was 4.67-fold higher in patients with subtrochanteric fracture (71% in subtrochanteric fractures vs. 37% in intracapsular fractures; p<0.05). No significant differences in transfusion needs were seen between different types of surgical treatment.
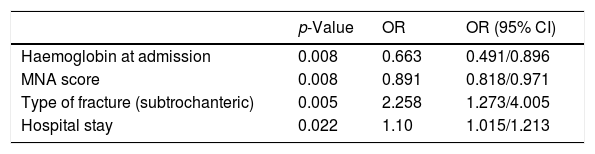
In the multivariate analysis, some pre-existing conditions as low haemoglobin values and poor nutritional status at admission and subtrochanteric fracture were found to be independent factors related to transfusion (Table 4).
Multivariate analysis of transfusion-associated factors.
| p-Value | OR | OR (95% CI) | |
|---|---|---|---|
| Haemoglobin at admission | 0.008 | 0.663 | 0.491/0.896 |
| MNA score | 0.008 | 0.891 | 0.818/0.971 |
| Type of fracture (subtrochanteric) | 0.005 | 2.258 | 1.273/4.005 |
| Hospital stay | 0.022 | 1.10 | 1.015/1.213 |
MNA: Mininutritional Assessment Test.
The cost of each blood unit was estimated in 150€, not including expenses related to blood processing and administration. In economic terms, the most expensive treatment for perioperative anaemia is transfusion alone as, in addition to its cost, it is independently associated with a longer hospital stay (1.7 days p<0.005). Savings related to the reduced length of hospital stay associated with the use of IS/EPO are much higher compared to the cost of drugs (in 2014, the estimated mean cost of hospital stay for a patient over 75 years of age with a proximal femoral fracture was €661/day, while the cost of IS/EPO therapy was €48). Patients treated with intravenous iron received less blood units, and had a shorter hospital stay, what implied lower costs.
Effects of the anaemia treatment protocol in functional recoveryIn our study, the protocol for erythropoiesis stimulation was initially related to better mid-term functional recovery (Barthel index and FAC 3–6 months after fracture) and this effect persisted, although to a lower extent, if a patient also needed transfusion. This relation was not statistically significant in the multivariable analysis.
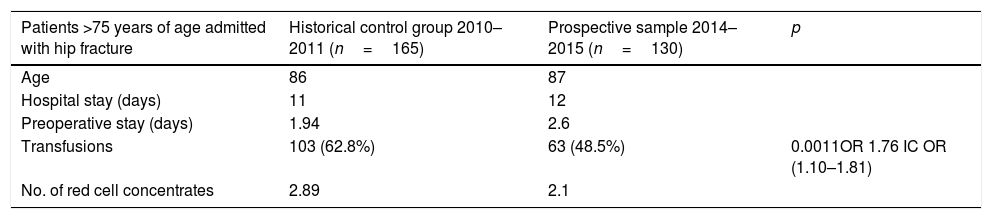
Comparison with historical group with no IS/EPO treatmentWe compared the transfusions administered to our series with a historical control group of patients over 75 years of age with hip fracture who had been admitted to our hospital between December 2010 and June 2011 (n=165), before intravenous iron was available, when the only treatment for acute anaemia was transfusion. Age and length of hospital stay were similar in both patient series. However, the more recent group of patients, who received IS/EPO, showed a statistically significant reduction in transfusion rates (63% vs. 48%; p<0.005; OR=1.76 OR CI=1.10–1.81), as well as lower transfusion volumes, in spite of a longer preoperative stay, which is an important predisposing factor for severe anaemia. Data are shown in Table 5.
Comparison between prospective and historical patient groups.
| Patients >75 years of age admitted with hip fracture | Historical control group 2010–2011 (n=165) | Prospective sample 2014–2015 (n=130) | p |
|---|---|---|---|
| Age | 86 | 87 | |
| Hospital stay (days) | 11 | 12 | |
| Preoperative stay (days) | 1.94 | 2.6 | |
| Transfusions | 103 (62.8%) | 63 (48.5%) | 0.0011OR 1.76 IC OR (1.10–1.81) |
| No. of red cell concentrates | 2.89 | 2.1 |
Data as median for quantitative variables.
Transfusion is the most widely used treatment for acute anaemia. However, its effect on haemoglobin values is only temporary. Numerous studies have related blood transfusions with infectious and cardiologic complications, as well as with increased dose-dependent mortality.6,20–27 In hip fracture in particular, some authors have named respiratory infection and transfusion-related heart overload as the primary causes of acute mortality.28
Microbiological studies have evidenced a close relationship between iron availability and bacterial virulence, as iron is considered essential for microorganism proliferation.29 This association means that iron supplementation is inadvisable when transferrin saturation levels are >50% and ferritin levels are >800ng/ml.24 However, these results were observed in haemodialysis patients, in whom chronic administration of iron may result in the presence of small amounts of free iron in plasma, a major source of toxicity.30,31 This is far less likely in surgical patients who only occasionally receive parenteral iron therapy administration during admission.
In this study, blood transfusion was not associated with higher rates of cardiologic or infectious complications at admission; however, transfusion was associated with a 1.65-day increase in hospital stay. It is difficult to determine whether this association is related to the transfusion itself or to increased patient complexity and frailty. In our study, transfusions were not associated with mortality, probably due to an insufficient sample size and a limited number of deaths.
In our setting, no association was found between intravenous iron and infection or mortality rates in surgical patients.17,32 In fact, iron deficiency is considered a risk factor for hospital-acquired infection,33 due to its association with immunosuppression and increased transfusion needs.34,35 Recent publications have indicated lower transfusion rates, fewer infectious complications, shorter hospital stay, and lower mortality rates at 30 days in patients with hip fracture treated with IS and EPO, with or without transfusion.6,7 In our experience, when the protocol restrictions were applied, no adverse reactions to intravenous therapy with IS were observed.
As the current protocol design considers IS and EPO as adjunctive therapy, instead of replacement therapy for transfusion, and the administration of these drugs depends on the progress of haemoglobin levels, most patients requiring transfusion also receive parenteral iron therapy. Almost half of the patients received a transfusion, with a mean of 2 units of packed red blood cells. These figures are similar to those from other published studies that assessed the efficacy of intravenous iron to reduce the transfuse on index (40–60%).6 Thus, the use of IS and EPO did not reduce the risk of transfusion, but resulted in a lower requirement for blood (0.7 units less).
Reducing the number of transfusions is a process with little room for manoeuvre, as our patients are elderly subjects with a high comorbidity burden who require semi-urgent surgery. Transfusions are needed to raise arterial oxygen levels and to improve tissue perfusion and raising haemoglobin levels is not the only aim. These results suggest that clinical instability appears to be one of the primary factors driving the prescription of transfusions, instead of basing the decision only in the lab tests.
The administration of iron and EPO may contribute to increased and maintained erythropoiesis over a longer period and it could have positive functional effects. In our study, the protocol for erythropoiesis stimulation did not reach a difference of statistical significance in terms of a better mid-term physical recovery, probably due to the sample size. Several recent papers have evaluated the benefits of liberal transfusion policies and higher haemoglobin thresholds but the relation between haemoglobin levels at discharge and functional evolution is still controversial.8–10,36,37,3,38–41 Cochrane reviews, moreover, do not support liberal transfusion policies.42
When we compared the patients analysed in this study with a similar group of patients admitted for hip fracture in 2011, when there was no IS available, the rate of transfusions was reduced in 17%. The use of intravenous iron can reduce the number of patients that require transfusion and the number of blood units needed. This suggests the potential benefit of IS treatment.
As a limitation of this study, almost 40% of the transfusions were prescribed outside the protocol, to patients with haemoglobin values >8.5g/dL, and transfusions were not associated with the acute surgical phase or with a past history of cardiologic instability. If the clinician is uncomfortable or unwilling to accept low transfusion thresholds, it is very likely that transfusions will be overused.8
In our opinion, the treatment protocol used in this study could be improved. As other authors have pointed out, the transfusion threshold after surgical treatment could probably be lower than we the proposed one.6 Moreover, the iron dose proposed was insufficient to cover the overall deficiency and the deficit should be calculated individually (Ganzoni Formula). Newer studies are being developed with doses of 500–1000mg, using different types of iron supplements as ferric carboximaltose.
More multi-centre controlled trials are needed to assess the new treatments for surgical anaemia in complex elderly patients, specific for their peculiarities, counting on the fact this group of population each time has more importance in the Orthopaedic wards of our hospitals.
ConclusionsLower haemoglobin rates on admission, malnutrition and subtrocanteric fractures are admission are predisposing factors for transfusion after hip fracture. Treatment of perioperative anaemia with transfusion alone was associated with longer hospital stay, making this the most burdensome therapy. The greater complexity of the transfused patients is probably related with this results. Patients treated with IS/EPO had a shorter hospital stay and this may represent savings higher than the mere cost of the drugs.
The administration of IS/EPO as an alternative therapy to blood transfusion is related to reduction of the number of packed red blood cells transfused and may be associated with mid-term functional improvement in frail elderly patients with hip fracture, without an increased number of complications. Hence, recommending their use seems reasonable, but further controlled studies are needed to move towards a universal indication. Subtrochanteric fractures more commonly require transfusion, and may benefit from routine IS/EPO administration initiated at the time of admission.
Conflict of interestsThe authors declare no conflict of interests.