Polypharmacy is a common condition among older adults and is associated with adverse drug reactions and health outcomes, including falls, functional and cognitive impairment, and frailty.
MethodsA prospective observational study will be conducted on older adults with polypharmacy. The aim is to assess the impact of a specialized outpatient clinic focused on pharmacotherapy optimization recently integrated into daily clinical practice in a Spanish public tertiary teaching hospital on patients’ functional and cognitive abilities. Patients who attend a first consultation and meet inclusion criteria (≥75 years old, have a life expectancy≥3 months, and polypharmacy (≥5 prescribed medications) will be invited to participate in the study, until reach a calculated sample size of 104 participants. Patients will be excluded if they are enrolled in a clinical trial related to medication or in the event of a no-show or cancellation of the appointment at the first visit. Participants will receive usual care: a first consultation including multidisciplinary pharmacological optimization in the context of a CGA and subsequent face-to-face and/or telephone follow-up (∼3 and ∼6 months). The primary endpoint will be the functional (Barthel index) and cognitive change in capacities (IPCR – Índice de Incapacidad psíquica de la Cruz Roja). Secondary endpoints include medication changes, changes in patients’ quality of life, rate of falling, and use of healthcare resources.
DiscussionWe expect that the close collaboration between professionals from different disciplines working together will be an effective strategy to improve the functional and cognitive abilities of older adults.
Trial registrationClinicalTrials.gov: NCT05408598 (March 1, 2022).
La polifarmacia es una condición común entre los adultos mayores, y se asocia a reacciones adversas a medicamentos y a resultados negativos en la salud como caídas, deterioro funcional y cognitivo, y fragilidad.
MétodosSe realizará un estudio observacional prospectivo en adultos mayores con polifarmacia. El objetivo es evaluar el impacto de una consulta especializada ambulatoria centrada en la optimización farmacológica y recientemente integrada en la práctica clínica habitual en un hospital universitario público español, sobre las capacidades funcionales y cognitivas de los pacientes. Los pacientes que acudan a una primera consulta y cumplan los criterios de inclusión (≥75 años, tengan una esperanza de vida ≥3 meses, y polifarmacia (≥5 medicamentos prescritos) serán invitados a participar en el estudio, hasta alcanzar un tamaño muestral calculado de 104 participantes. Los pacientes serán excluidos si ya participan en un ensayo clínico relacionado con medicación o en caso de no presentarse o cancelar la consulta inicial. Los participantes recibirán la atención habitual: una primera consulta que incluirá la optimización farmacológica en el contexto de una valoración geriátrica integral (VGI) y un seguimiento posterior presencial y/o telefónico (∼3 y ∼6 meses). La variable principal será el cambio en las capacidades funcionales (índice de Barthel) y cognitivas (Índice de Incapacidad Psíquica de la Cruz Roja [IPCR]) medidas al inicio y durante el seguimiento. Las variables secundarias incluyen cambios en la medicación, en la calidad de vida de los pacientes, en la tasa de caídas y en el uso de recursos sanitarios.
DiscusiónEsperamos que esta estrecha colaboración entre profesionales de diferentes disciplinas que trabajan conjuntamente sea una estrategia eficaz para mejorar las capacidades funcionales y cognitivas de los adultos mayores.
Registro del ensayoClinicalTrials.gov: NCT05408598 (1 de marzo de 2022).
The ageing of the world's population and its increasing longevity challenges the healthcare system with a higher prevalence of chronic diseases and prescription expenditures. The simultaneous involvement of multiple physicians in patient care1 and the follow-up of disease-specific guidelines in multi-morbid patients often leads to an increased number of prescribed medicines per patient2 complicating their clinical management.
Polypharmacy is a common condition among older adults. In and of itself, it increases the likelihood of adverse drug reactions (ADRs), especially in this population due to age-related changes in drug pharmacokinetics and pharmacodynamics and other factors that influence patients’ prognosis (frailty, dementia, limited life expectancy). ADRs place many patients in the hospital every year and can have life-threatening consequences; one in ten hospital admissions in older patients is due to ADRs3 in a physical, psychological, and social vulnerability context and they can also be a major contributor to the development of functional and cognitive impairment, falls, frailty, and increased use of healthcare resources.4
Thus, polypharmacy has become one of the most significant public health challenges in older adults and is an important focus of integrated care. In 2017, the WHO set its third Global Patient Safety Challenge on Medication Safety to gain worldwide commitment and action to reduce severe, avoidable medication-related harm by 50% in the next five years, specifically by addressing harm resulting from errors or unsafe practices due to weaknesses in health systems.5 Thereby, all professionals must be involved in the adequate care of older patients and the proper management of their medication.1
Knowledge gaps in the field of medication optimization in older patients are well recognized due to the complexity of these patients and the limited scientific evidence because of their under-representation in pharmacological trials.6 Medication management in older adults usually involves various healthcare specialists, thus optimal interprofessional collaboration is needed.
In this sense, care by geriatric specialists may be associated with more appropriate use of medicines in older patients, especially in frail patients.7,8 Additionally, many studies have shown that pharmacists can positively impact the multidisciplinary team for comprehensive geriatric assessment CGA and patient management.8–14 However, these studies are mostly limited to hospitalized patients8–12,15 or institutionalized settings,13,14 with little evidence remaining for geriatric outpatient settings.
In this regard, our research group has been working along these lines for the last few years with geriatric acute inpatients and frailty. The conclusions reached from these studies showed that a systematic pharmacist-led intervention at hospital admission to an acute geriatric unit (AGU) within a CGA was associated with a decrease in polypharmacy and that this reduction may be an effective strategy to prevent and manage frailty.1,4,16
Regarding geriatric outpatients, studies in which medication reviews have been conducted by a multidisciplinary team (at least comprising a physician and a pharmacist) have not usually reported patients’ functional and cognitive capacities,17–21 so previous examples are very scarce.22,23 Lozano-Montoya et al. analyzed the relationship between STOPP recommendations and physical and mental disabilities in patients aged 80 years or over but the setting was an AGU and they did not find an association between both variables. In the case of the START criteria, not following START criteria was significantly associated with being dependent for basic ADLs (OR: 0.66 for compliance with the recommendation; 0.49–0.89), dependent for instrumental ADLs (OR: 0.64; 0.48–0.85), or unable to walk (OR: 0.72; 0.54–0.98).24 In short, there is a need to study the impact of multidisciplinary interventions to address medication in older adults’ functional and cognitive status, as these are probably the most important variables in this population, and however, they have been very little studied.
Recently, a geriatric outpatient consultation focused on polypharmacy has been implemented in our hospital, integrating multidisciplinary medication review into clinical practice in the context of a CGA. We expect that this new initiative could improve patients’ outcomes, including functional and cognitive performance.
Methods/designAimThe aim is to assess the impact of a specialized outpatient clinic focused on pharmacotherapy optimization recently integrated into daily clinical practice as standard care in a Spanish public tertiary teaching hospital on patients’ functional and cognitive abilities.
Participant selection, eligibility criteria, and consent processThis observational prospective study will be conducted on geriatric outpatients in the University Hospital of Navarre (Spain). Potential participants are those with a planned appointment for a first clinical consultation. The patients who attend the first consultation are invited by a geriatrician to enter the study if they meet the inclusion criteria. The geriatrician explains the study and gives a written information sheet to potential participants. Those who agree to participate must sign the informed consent form and will be informed that they have a waiver form so that they can withdraw from the study at any time if they wish. Consent may be given by the patient or the accompanying relative in case of disability. Patients will be eligible for study inclusion if they are ≥75 years old, have a life expectancy ≥3 months, and have polypharmacy (defined as ≥5 prescribed medications at the time of inclusion). Patients will be excluded if they are enrolled in a clinical trial related to medication or in the event of a no-show or cancellation of the appointment at the first visit. Study participants will receive usual care consultation and their data will be registered.
Ethical considerationsThe protocol of this clinical trial was approved by the Ethics Committee of the Navarra Government (CEIM) with registration number EO_2021/16. All participants will be informed verbally during the process of inclusion in the study by the geriatrician of the outpatient consultation and will be given the information sheets with all the detailed aspects and the informed consent. The participants’ identity in the study documents will be coded, and only authorized persons will have access to identifiable personal details if data verification procedures require inspection of these details. The data obtained will be stored in an electronic database with access restricted to the research team.
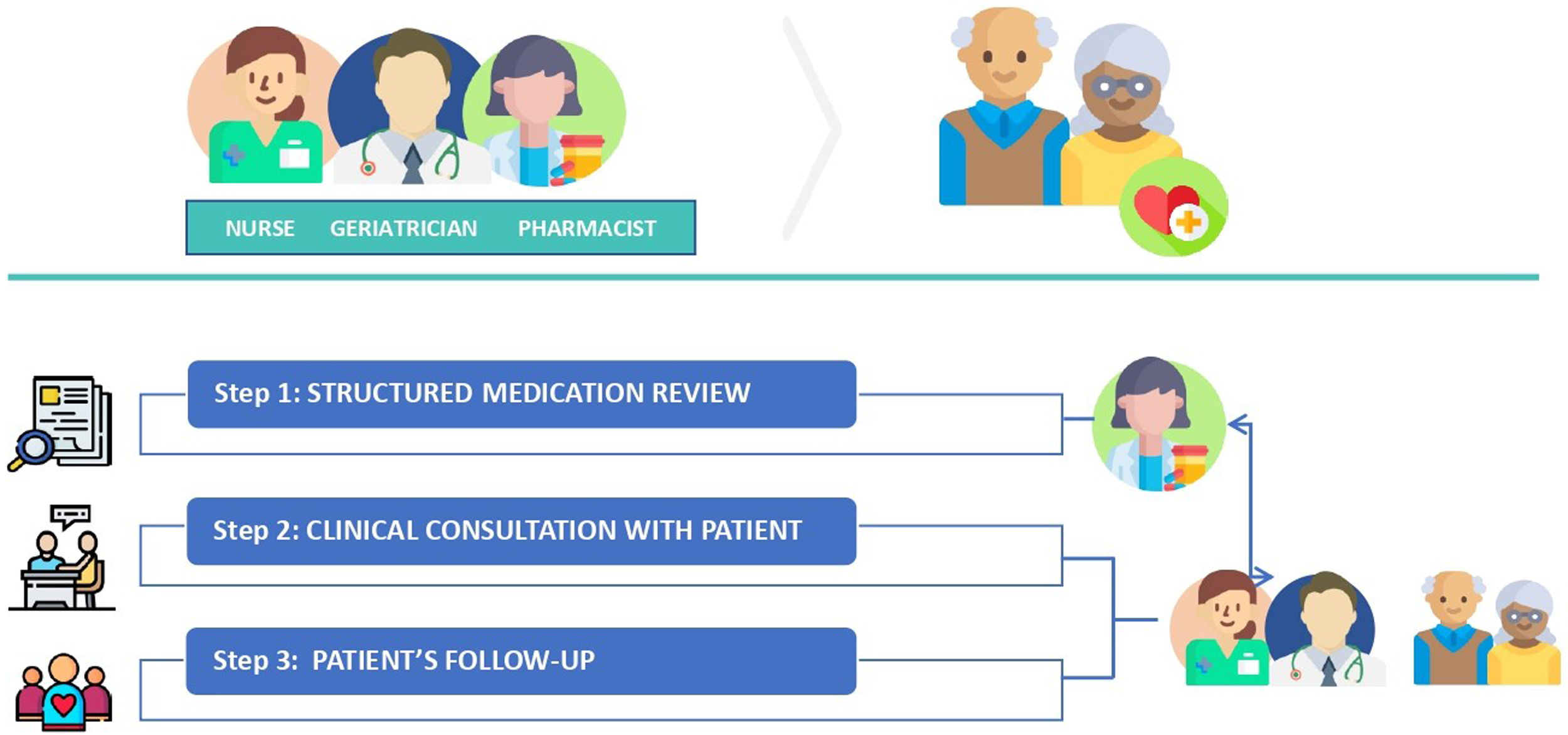
ProcedureThe outpatient consultation team consists of a clinical pharmacist, a geriatrician, and a nurse. Patients enrolled in the study will have an appointment date for the first visit to the outpatient clinic. The workflow is described in the following figure (Fig. 1).
Step 1: Medication reviewAbout a week before a patient's first visit, the clinical pharmacist will check medication records, background information, dispensing records, and recent laboratory data. Then, based on the available information, a structured medication review will be performed on a drug-by-drug basis, assessing the need, effectiveness, appropriateness, and safety of each drug with the aid of clinical guidelines, implicit tools such as the Medication Appropriateness Index (MAI)25,26 and explicit tools such as STOPP/START27,28 and Beers criteria.29 Recommendations on medication changes will be recorded and shared in the patient's digital file, which is accessible to the whole team.
Step 2: Clinical consultation with patientsOn the first visit (baseline), the geriatrician and the nurse will perform CGA on a regular basis through a medical care interview with the patient or a relative or caregiver, and a physical examination. Then, the medication will be reviewed by the geriatrician considering the pharmacist's recommendations as part of the CGA. In case of any discrepancy, this will be discussed among the team. As a result, a treatment plan will be implemented and agreed on with the patient.
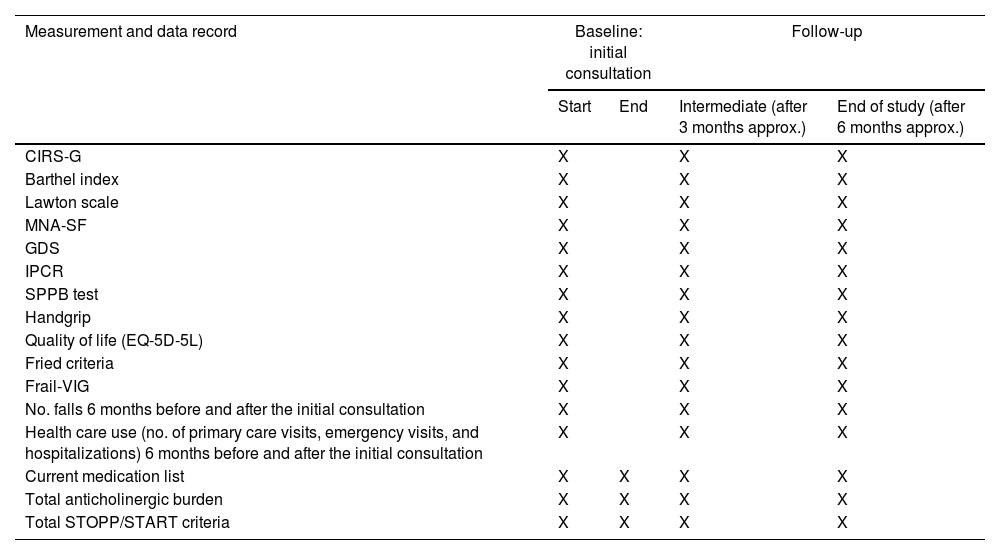
Data registration: CGA data will be registered as follows (Table 1): Cumulative Illness Rating Scale for Geriatrics (CIRS-G), Barthel index for ADL; Lawton Instrumental Activities of Daily Living Scale (IADL), Mini Nutritional Assessment-Short Form (MNA-SF), Reisberg's Global Deterioration Scale (GDS) and social status, “Índice de Incapacidad psíquica de la Cruz Roja (IPCR)” for cognitive status, Short Physical Performance Battery (SPPB) test, Handgrip measurement (measured via dynamometer), quality of life by self-reported and proxy-reported (in cases of severe cognitive impairment) EQ-5D-5L questionnaire, and frailty (Fried Criteria, Frail-VIG). The number of falls in the preceding 6 months, and health care use (no. of primary care visits, emergency visits, and hospitalizations) will be also quantified. The current medication list will be recorded, as well as any changes in medication, the total anticholinergic burden (drug burden index [DBI] and Anticholinergic Cognitive Burden [ACB] scales) and total STOPP/START criteria to measure Medication Appropriateness.
Assessment measures at baseline, post-intervention, and follow-up evaluations.
| Measurement and data record | Baseline: initial consultation | Follow-up | ||
|---|---|---|---|---|
| Start | End | Intermediate (after 3 months approx.) | End of study (after 6 months approx.) | |
| CIRS-G | X | X | X | |
| Barthel index | X | X | X | |
| Lawton scale | X | X | X | |
| MNA-SF | X | X | X | |
| GDS | X | X | X | |
| IPCR | X | X | X | |
| SPPB test | X | X | X | |
| Handgrip | X | X | X | |
| Quality of life (EQ-5D-5L) | X | X | X | |
| Fried criteria | X | X | X | |
| Frail-VIG | X | X | X | |
| No. falls 6 months before and after the initial consultation | X | X | X | |
| Health care use (no. of primary care visits, emergency visits, and hospitalizations) 6 months before and after the initial consultation | X | X | X | |
| Current medication list | X | X | X | X |
| Total anticholinergic burden | X | X | X | X |
| Total STOPP/START criteria | X | X | X | X |
CIRS-G, Cumulative Illness Rating Scale for Geriatrics; MNA-SF, Mini Nutritional Assessment-Short Form; GDS, Global Deterioration Scale for dementia; IPCR, “Índice de Incapacidad psíquica de la Cruz Roja”; SPPB test, Short Physical Performance Battery; EQ-5D-5L, the 5-level EQ-5D version; Frail-VIG, Frail-Valoración Geriátrica Integral; STOPP criteria, Screening Tool of Older Persons’ Prescriptions; START criteria, Screening Tool to Alert to Right Treatment.
Drug optimization intervention will be accompanied by subsequent face-to-face and/or telephone follow-up, with patients or relatives. Follow-up frequency (3 and 6 months) will depend on monitoring needs according to the patient's clinical condition and treatment plan. The geriatric assessments performed at baseline will be repeated in the follow-up consultations to analyze changes. Patients will be followed-up for 6 months, and lost to follow-up and dead will be analyzed.
Additional researchers’ registersThe number of primary care visits, emergency visits, and hospitalizations of the patient in the last year will also be registered from patients’ electronic health records. The anticholinergic burden will be calculated in all medication lists obtained for each patient (baseline and follow-up).
Outcomes assessmentThe primary endpoint will be the change in functional and cognitive capacities measured at baseline and follow-up. The functional capacity will be evaluated by: Barthel index, to assess basic ADLs (Spanish version; the scale of 100 [functional independence] to 0 [severe functional dependence]), an international, validated, and the most used tool to measure disability and which allows us to detect changes at follow-up points (considering a change of 5 points clinically relevant)30; Lawton scale, to assess IADLs (Spanish version), which assesses a person's ability to perform tasks such as using a telephone, doing laundry, and handling finances30 (considering a change of 0.5 points clinically relevant); and SPPB test (Spanish version; total score ranging from 0 [worst] to 12 points [best]) which includes balance, gait, and rising from a chair test and which can predict disability.31 Patients’ strength will be registered whenever a measurement is possible by the use of a handgrip.
Patients’ cognitive function will be assessed by IPCR (Spanish version),32 which stands out for the ease of its application, making it very practical. It is graded in the form of stages or degrees of disability, from “0” (no cognitive impairment) to “5” (maximum degree of impairment, advanced dementia) (considering a change of 0.5 points clinically relevant).
Secondary endpoints will include quality of life, assessed by the questionnaire EQ-5D-5L (Spanish version), which consists of a classification system with five dimensions (mobility, self-care, usual activities, pain or discomfort, and anxiety or depression) and a subjectively rated visual analogue scale (EQ VAS). This questionnaire has been validated to measure perceived health in the Spanish-speaking population.33 Frailty will be assessed by Fried criteria and Frail-VIG. Fried's phenotype method classifies older adults as frail, pre-frail or non-frail based on five criteria.34 The Frail-VIG is a multidimensional index designed by Amblàs-Novellas et al. to assess frailty simply and quickly.35 It is based on the accumulation of deficits extracted from the CGA, measuring 22 variables grouped in 8 domains: functional, nutritional, cognitive, emotional, social, and geriatric syndromes before admission, symptoms with criteria of severity and the presence of chronic diseases.36 The rate of falling, and healthcare use (hospital admissions, primary care, and emergency visits) will also be compared between baseline and follow-up consultations. Mortality will be registered as an endpoint.
The analysis of medication changes between baseline and follow-up will comprise the number of medications and drugs (active substance), type of intervention, anticholinergic burden assessed by DBI and ACB scales,37 and the number of STOPP/START criteria to determine the prescribing quality. The medication list will be collected from primary care medical records. Pharmacist recommendations will be classified according to the drug-related problem classification defined by the Pharmaceutical Care Network Europe (PCNE)38 and the type of medication classified by the anatomical therapeutic chemical (ATC) classification.
All data will be recorded in case report forms and collected in a database accessible only to the research team. All data collection will be identified by a coded ID to maintain participant confidentiality.
Planned statistical analysisSociodemographic and clinical data will be described as frequencies and percentages for categorical variables and using mean and standard deviations or medians and interquartile range in the case of continuous variables.
Changes from baseline to, after medication review, and to 3-month and 6-month-follow-up in outcome variables, will be computed and tested using paired t-tests or Wilcoxon signed-rank test. The effect of sociodemographic and baseline clinical status variables (i.e., sex, age, CIRS-G) on the change in outcome variables will be analyzed by fitting linear or Poisson regression models. Statistical analysis will be performed with SPSS statistics and R software.
Assuming a standard deviation of the change after a baseline of 16 for a power of 0.8, 83 patients will be necessary to detect a statistically significant mean increase of 5 points in the Barthel index. This value for meaningful change (i.e., clinically significant) is set based on literature39 and previous exercise intervention works of our team, since there is scarce previous evidence in Barthel index directly related to drug optimization interventions. Assuming a 20% loss of patients at follow-up, a total sample size of 104 patients will be required.
DiscussionThere is growing recognition of drug inappropriateness as a public health problem, given its potential impact on morbidity, mortality, and healthcare resource use.40 Current models of care should be oriented towards prolonging the autonomy of older adults so that they remain as long as possible in their homes and delaying their admission to nursing homes. With an ageing population, the real challenge for the healthcare system is the increasing burden of chronic diseases and ongoing chronic medication.41
Potentially inappropriate medication in community-dwelling older adults is highly prevalent. However, limited evidence exists regarding clinically relevant improvements as highlighted before. Drug treatment can be either the facilitator which gives the opportunities, or the opposite, an intensifier of problems by occurrence of unacceptable side effects leading to decreased quality of life and cognitive or functional impairment.40 This study will analyze brings forward the impact of a specialized consultation team focused on pharmacotherapy optimization on the functional and cognitive abilities of community-dwelling older adults with polypharmacy.
Through this multidisciplinary intervention recently integrated into daily clinical practice and focused on pharmacotherapy optimization, an improvement in the quality of prescriptions is expected, with a reduction of drugs associated with falls and impairment (sedatives and hypnotics, antidepressants, etc.). Medications that possess anticholinergic activity are a class of potentially inappropriate medications (PIMs) widely prescribed for various clinical conditions in older adults, being older adults particularly vulnerable to their adverse effects.42 These medications are associated with lower objective physical function, worsen cognitive impairment, and increased risk of falls resulting in problems in activities of daily living (ADL) in older people.43 Other therapeutic groups commonly implicated in inappropriate prescribing in the Spanish population are benzodiazepines (well known to be linked with cognitive impairment), nonsteroidal anti-inflammatory drugs (NSAIDs) and proton inhibitors,44 increasing the risk of adverse drug reactions with a negative clinical impact. These medication changes could be translated into an improvement in patient functionality and cognitive status or, at least, a limitation in the progression of any impairment.
The study has the strength of analysing this new multidisciplinary management approach in a real clinical practice setting, thus ensuring that the patients are representative and that it is feasible on a day-to-day basis. On the other hand, this also presents some limitations, since depending on the patient's clinical situation and needs, some consultations will be by telephone, which may lead to some missing data. On the other hand, the sample size is only calculated based on the functional impairment variable, measured by means of the Barthel score.
Through this collaborative model, professionals from different disciplines coordinate patient care and put their own specialities and skills to use to improve patients’ outcomes. Given the complexity of these patients, it is expected that the association of different roles working together in multidisciplinary teams will have a synergistic effect and will prove to be an effective strategy. The challenge of this study is to prove an improvement in patients’ functional or cognitive status as well as an improvement in quality of life in a follow-up period of up to 6 months. We expect as well that the total number of medications per patient, anticholinergic drug burden, and the number of falls per patient will decrease. This project will not analyze variation in drug costs. However, a lower use of resources and prescription of drugs may lead to a lower cost for the health care system.
Authors’ contributionsThe protocol was developed by VR-B, MG-V and NM-V. BC-V, RSE and VR-I and contributed to study conception and design, and project planning. VR-B, IM-E, IG-E, MS-L and KG-A are in charge with study protocol implementation. AG provided advice on the statistical analysis and writing. VR-B, MG-V and IEB prepared the initial manuscript. All authors reviewed, read, and approved the final manuscript prior to submission.
Ethics approval and consent to participateThe study follows the principles of the Declaration of Helsinki and has been approved by the Clinical Research Ethics Committee (EO_2021/16) of the University Hospital of Navarre. All patients or their legal representatives will provide written informed consent.
Consent for publicationNot applicable.
Availability of data and materialsNot applicable.
Dissemination policyThe study results will be released to the participating physicians and other professionals, patients, and the general medical community.
FundingThis study is partially funded by the research project Optimage INTERREG-POCTEFA in which the research team is involved. This study protocol has not undergone peer-review by the funding body, and it had no role in study conception and design, writing, project planning, study protocol implementation or in the decision to submit the paper for publication.
Conflict of interestsThe authors declare that they have no competing interests.
Co-authored statementGiven his role as Editorial Board Member in the journal, Nicolás Martínez-Velilla had no involvement in the peer review of this article and has no access to information regarding its peer review.
Not applicable.