Retrospective reviews of patients' records to identify adverse events has been important in providing an indication of the scale of harm as a result of healthcare treatment. This in turn has lead to changes in subsequent studies on how this information can be used to improve patient safety1,2.
Adverse Event studies
An adverse event is defined as 'an injury or complication leading to disability at the time of discharge and/or extended hospital stay caused by healthcare management'. All three components had to be present for the cases identified in these studies. The first, the Harvard Medical Practice study3, was carried out in New York in the mid 1980s and provided evidence of the scale of harm to patients in hospital. These initial findings were substantiated in further studies in the United States4, in Australia5, the UK6, Denmark7, New Zealand8, France9 and Canada10.
The incidence of adverse events in these studies vary, with rates ranging from 2.9-3.7% in the USA to 16.6% in Australia. However, subsequent analysis has revealed that this can be explained to some extent by differences in definitions, exclusion/inclusion criteria and purpose of the studies10-12. Studies which have focused on quality of care have reported that between 37-60% of adverse events were judged to be preventable5,6,9,10,13 and as such have attempted to identify the underlying causes for the adverse events. For example, subsequent US studies have shown how information and decision support systems can reduce medication events14. The Australian team focused on identifying specific errors and failures underlying adverse events and identified the following prevention strategies: better implementation of policies and protocols, better formal quality monitoring, better education and training, and more consultation.
The original review form uses a mixture of taxonomies which are not always clearly distinguished. We found that the structure of the review form meant that results did not always reflect the underlying clinical reality. For example, a post-operative infection is classified as an operative event (because it occurred within 30 days of surgery) and non-technical (because it was not directly related to the operation itself). On subsequent re-examination of the data we identified when in the process of care the adverse event occurred and the nature of the underlying problem. Thus a post-operative chest infection would be classified as a ward based event and the problem as one related perhaps to drug administration (over-sedation), to failure to monitor (such as over a weekend), or to failure to provide physiotherapy15.
Modification of the clinician review form
We modified the review form to produce the Modular Review Form (MRF2) to provide greater emphasis on the causes of adverse events and methods of prevention16. We divided the review form into five sections each with a defined purpose, providing a modular structure to both the form and the review process to provide additional clarity.
The 5 stages of the MRF2 are as follows:
Patient information and background to AE
Disability caused by AE
Phase of care during which AE occurred (e.g. during a procedure including surgery and anaesthesia, during ward care)
Type of error (e.g. diagnostic, operative)
Contributory factors (e.g. poor teamwork) and Preventability of AE
We also gave structure and emphasis on the list of factors that contribute to adverse events. Identifying contributory factors is best done from observation and interview, although this is quite labour and resource intensive. We believe that expert reviewers or those familiar with the working environment can often comment on some of the major contributory factors. This is particularly important as the identification of such factors offers a route to devising methods of prevention.
The MRF2 was piloted by 12 teams from 8 countries (Britain, Italy, France, Spain, Australia, New Zealand, Japan & USA). These teams completed the new forms on example cases. The modular review form was reported to be comprehensive, well structured, and clear. Overall, the modular structure was thought to be helpful. Difficulties reported by the teams were similar to our own experiences of case record review16.
The benefits of this review form include a modular format which enables reviewers or project leaders to select the focus of their review based on resources and the purpose of the review, and to identify contributory factors which indicate areas for improvement and prevention.
Prospective studies and critical incidents
Michel and colleagues have shown that prospective record review is comparable to retrospective record review and in fact identified more preventable adverse events9. Prospective review studies are also being carried out in the UK17. These review studies differ from the earlier, traditional adverse event studies in the following ways. They have widened the definition of adverse events to include critical incidents. These are incidents where any harm due to healthcare treatment did not result in disability at the time of discharge nor extended hospital stay. Another modification was the selective use of the modular review form. One of the main difficulties of conducting case record reviews is the time taken to complete the forms16. The MRF2 was structured in such a way to enable reviewers to focus on a specific aspect of the form.
This work is currently in progress, but preliminary results show that on surgical wards 15% of patients experienced an adverse event and a further 10% experienced a critical incident. 62% of adverse events, and 95% of critical incidents, were judged to be preventable. Most adverse events caused little harm but had a negative impact on hospital resources. Most adverse events in surgical practice occurred in general ward care18.
Examination of cases identified in the preliminary feasibility study suggested that a significant proportion of critical incidents may be specific to a particular hospital, ward or unit17. As such it is amenable to local action and this is a good starting point for strategies to improve patient safety. This could be included as part of the ongoing process of clinical risk management or quality improvement and the reporting of critical incidents which will have impact and greater chances of success at a local level as staff will be able to see the results of any intervention strategies. For example, contributory factors may be difficult to assess in retrospective reviews, but prospective methods would enable the staff to further explore causes for particular types of adverse events or critical incidents.
Case record review remains one of the primary methods for assessing the incidence of adverse events or critical incidents and is suitable for both prospective and retrospective review9,17,18.
References
1. Vincent C. Risk, safety and the dark side of quality. BMJ 1997;314:1775-6.
2. Neale G, Woloshynowych M. Retrospective case record review: a blunt instrument that needs sharpening. Qual Saf Health Care 2003;12:2-3.
3. Brennan TA, Leape LL, Laird NM et al. Incidence of Adverse Events and Negligence in Hospitalised Patients. Results of the Harvard Medical Practice Study I. New Engl. J Med. 1991;324:370-6.
4. Thomas EJ, Studdart DM, Burstin HR et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Medical care 1999;38:261-71.
5. Wilson RM, Runciman WB, Gibberd RW et al. The Quality in Australian Health Care Study. Med J Aust 1995;163:458-471.
6. Vincent C, Neale G, Woloshynowych M. Adverse events in British hospitalised patients: preliminary retrospective record review. BMJ 2000;322:517-519
7. Schiolar T, Lipczak H, Pedersen BL et al. Forekomsten af utilsigtede haendesler pa sygehuse. En retrospektiv gennemgang af journaler. Ugeskrift for Laeger 2001;163:5370-8.
8. Davis P, Lay-Yee R, Schug S et al. Adverse events regional feasibility study: indicative findings. NZ Med J 2001;114:203-5.
9. Michel P, Quenon JL, de Sarasqueta AM, Scemama O. Comparison of three methods for estimating rates of adverse events and rates of preventable adverse events in acute care hospitals. BMJ. 2004;328:199-202.
10. Baker GR, Norton PG, Flintoft V et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170:1678-86.
11. Thomas EJ, Studdert DM, Runciman WB et al. A comparison of iatrogenic injury studies in Australia and the USA. I: Context, methods, casemix, population, patient and hospital characteristics. Int J Qual Health Care. 2000;12:371-8.
12. Runciman WB, Webb RK, Helps SC et al. A comparison of iatrogenic injury studies in Australia and the USA. II: Reviewer behaviour and quality of care. Int J Qual Health Care. 2000;12:379-88.
13. Davis P, Lay-Yee R, Briant R et al. Preventable in-hospital medical injury under the "no fault" system in New Zealand. Qual Saf Health Care 2003;12:251256.
14. Bates DW, Leape LL, Petrycki S. Incidence and preventability of adverse drug events in hospitalised adults. J Gen Intern Med 1993;8:289-94.
15. Neale G, Woloshynowych M, Vincent C. Exploring the causes of adverse events in British NHS hospital practice. J Roy Soc Med 2001;94:322-330.
16. Woloshynowych M, Neale G, Vincent C. Case record review of adverse events: a new approach, Qual Saf Health Care 2003;12(6):411-5.
17. Chapman EJ, Hewish M, Logan S et al. Detection of critical incidents in hospital practice: a preliminary feasibility study. Clinical Governance Bulletin, 2003;4:8-9.
18. Olsen C and Neale G - personal communication.
Management of patient safety in French hospitals
Philippe Michel, MD, PhD
Medical director, CCECQA, France
In France, implementation of patient safety activities greatly developed after the "contaminated blood crisis" in the mid-eighties, when a large number of patients contracted HIV after transfusion of unsafe blood; healthcare professionals and politicians (including the prime minister and the minister of health) were accused; some of them spent some months in jail and others had their political career ruined. Undoubtedly, individual professionals have committed faults but this crisis highlighted the lack of safety culture and of barriers protecting the individuals and the system from errors at all levels, national and local.
This crisis was one of the main reasons why patient safety activities initially were product-oriented, and politically-driven by the mean of numerous laws.
The risk management of healthcare products
A national safety surveillance program was launched and progressively included different healthcare products. The first one concerned the use of drugs (1984) and of blood products; now, medical devices, biochemical reagents and human products are concerned too.
For all risks, voluntary health professionals are trained as technical referees in each private and public hospital. They are in charge of organising the different safety tasks, surveillance, coordination and implementation of regulation and good practices guidelines. These professionals (doctors or nurses) are not full-time and have clinical activities. At the regional level, supportive units coordinate these activities. In 1999, the AFSSAPS national agency was created (Agence Française de Sécurité Sanitaire des Produits de Santé) for evaluating and controlling the security, quality and efficacy of the healthcare products. It provides alert dissemination, guideline production and surveillance.
The surveillance is based on bottom-up reporting and top-down alerts. The type of events to be reported, and the process of reporting, may differ slightly according to the risk. We here describe the organisation of medical device surveillance as an example. The witness of an adverse event related to a medical device has to inform either the local correspondent, when available, or directly the national Agency (AFSSAPS). A major objective of the local correspondent is to act as a filter and to select among the declarations of events those to be transmitted without delay or quarterly to the Agency, and those to be registered only locally. Within 48 hours the national Agency: a) returns an acknowledgement with instructions how to proceed with the involved device; b) communicates the declaration to the chairman of the ad hoc committee; c) informs the manufacturer of the device. In 2002 for example, 2675 adverse events related to a medical device were declared. The chairman decides on the degree of severity (major event requiring an immediate investigation, event requiring an investigation, event to be entered into a follow-up program). In the first two cases, an investigation using the failure mode and effects analysis method is undertaken by one or several members of the committee together with the manufacturer and the professional who declared the event. Conservative measures can be undertaken to prevent the re-occurrence of an event either with the involved and/or with similar devices and to facilitate the investigation.
Organisation and control of activities at risk
Hospitals' organisation for controlling risks as nosocomial infections or sterilization has also been regulated. Specifically for the nosocomial infection control, budgets have been provided to the hospitals in order to set up a structure with dedicated professionals in medium and large-sized hospitals. Five inter-regional structures and a national committee have been implemented to coordinate the activities.
Good practice procedures are now defined in addition to the legal requirements and, during the last decade, mandatory quality assurance programs were launched in all biology and sterilization units and for transfusion and catering activities in healthcare organizations.
Finally, the technical conditions for performing some activities as anaesthesia, ambulatory surgery or perinatal care, have been precisely described by the Ministry of health, which is also in charge of controlling the compliance of hospitals with these requirements.
Strengths and weaknesses of this compartmentalised and highly regulated organisation
The main force of this organization is a very effective alert system, with top-down and bottom-up "red flags". An example of top-down effectiveness was the quickness of the alert concerning diathermy interactions with deep brain systems after the notification of two deaths due to severe brain damage in the area where the lead electrodes were implanted. The FDA notified the alert on December 19, 2002 whereas the French Agency did so six months before, on June 18, 2001. There are of course reverse examples, as the notification of alert concerning a loosened bronchoscope Olympus biopsy port and microbial contamination of the port on November 30, 2001 by FDA and on March 13, 2002 by French agency.
However, the limitations are numerous:
An extremely regulatory approach, although it is well known that an appropriate quality assurance system should not be limited to this approach. For example, the director of the French national transfusion Agency, Dr. Herve stated in 1999 that "transfusion was historically characterized by its ambivalence: it was the first medical discipline which integrated Quality Assurance concepts, it was also the first which proved unable to respond adequately in the face of uncontrolled risks".
A compartmentalization of the vigilance activities according to products, without bridges between each other, within the hospitals, at the regional and at the national levels;
The absence of information system on other iatrogenic injuries
An lack of a proactive approach
A limited knowledge of organizational defects and other latent causes of adverse events in hospitals
Toward an integrated management of patient safety
in hospitals
Since 2000, a huge effort has been made to enlarge the scope of patient safety and to improve its effectiveness.
Two laws were published in 2002 with the main following consequences:
Guidelines will be published for each risky practice in medicine with a precise description of who, where and when it should be practised. The National agency for Evaluation and Accreditation (ANAES) will be in charge of these guidelines.
The surveillance system will no longer be restricted to the adverse events related to healthcare products and to nosocomial infections, and will cover all types of adverse events. The national agency InVS is currently developing the principles of the reporting which will be soon experimented. In addition, a national survey on all adverse events is being carried out in 71 hospitals, 298 wards and 7500 patients. Its aims at assessing the incidence of adverse event and at realising a root cause analysis of the most frequent and prominent events in order to find out the frequency of the main contributory factors related to their occurrence.
The patient must be told the occurrence of an adverse event within 15 days after its identification.
Patients may receive financial compensation even if malpractice is not proven.
Finally, the 2004-2008 national public health program for France is currently being examined by the French National Deputy Assembly. It will include, for the first time, quality and safety indicators. The five first safety indicators, defined and tested in 2004, are in the field of nosocomial infections.
Guidelines for the implementation of risk management are launched. The Ministry of Health edited guidelines for the organization of the risk management activities in hospitals, and the French evaluation and accreditation Agency (ANAES) released guidelines on risk management activities. These guidelines will be soon completed by a manual on the tools from the industrial settings useful for health care organisations. In the future, the implementation of these guidelines will be evaluated: the second accreditation manual provides a renewed section on risk management and patient safety. Its will be used for accrediting hospitals from 2005.
Information and training of healthcare professionals are upmost important for the development of a safety culture. A working group from the Ministry of Health is responsible for proposing changes in the initial education of healthcare professionals, while regional structures are developing programs for continuous education.
Last but not least, three domains of improvement have been prioritised, drug management in hospitals, nosocomial infections and theatre functioning on criteria such as frequency, severity and cost of errors and adverse events, and numerous national, regional and local initiatives have been implemented.
Conclusion
In France, patient safety management was first developed as parallel efforts in reducing risks related to different products and risky activities. Parallel organisations were implemented in hospitals that were mostly effective. However the compartmentalisation of patient safety activities undoubtedly hampered the development of hospital's risk management policies, safety culture and identification of root causes. Efforts have now being devoted to reorienting the hospital organization of patient safety activities and to setting up a national reporting system.
Proyecto IDEA: Identificación de efectos adversos
Jesús Mª Aranaz por el Grupo de Estudio del Proyecto IDEA*
Dpto. Salud Pública. Universidad Miguel Hernández d'Elx. Servicio de Medicina Preventiva. Hospital Universitari Sant Joan d'Alacant.
Los antecedentes
La creciente complejidad de los sistemas sanitarios y del entorno de la práctica clínica en el tercer milenio suponen un nuevo escenario para el ejercicio de las ciencias de la salud, pues como dijera Chantler, "El ejercicio de la medicina en el pasado solía ser simple, poco efectivo y relativamente seguro; en la actualidad se ha transformado en complejo, efectivo, pero potencialmente peligroso"1.
Los riesgos de la asistencia sanitaria eran considerados en la mitad del siglo pasado, como "el precio a pagar por los modernos métodos de diagnóstico y terapia"2, y aceptados como "las enfermedades del progreso de la medicina"3. En un trabajo clásico4 pero de actualidad, por esa razón recientemente reeditado, Schimmel habló de los peligros de la hospitalización, cuando encontró episodios desfavorables en un 20% de los pacientes hospitalizados5 en un estudio prospectivo.
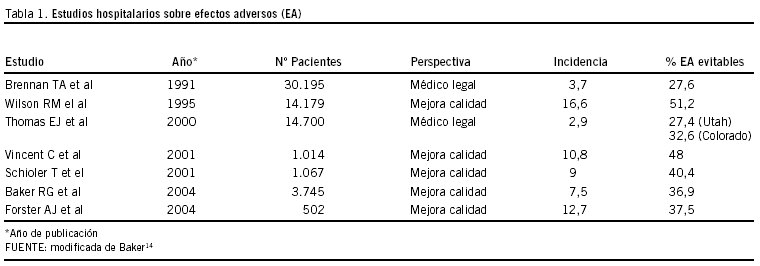
El último cuarto de siglo pasado fue especialmente prolífico en la presentación de resultados consecuencia de estudios importantes, unos de corte poblacional y otros multicéntricos6. Los estudios realizados mediante revisión retrospectiva de las historias clínicas en Hospitales de Estados Unidos7-9 y Australia10 compartían un mínimo común denominador al definir efecto adverso, como daño no intencionado provocado por un acto médico más que por el proceso nosológico en sí11i. Sin embargo, la motivación es diferente entre los estudios de EE UU y el resto, lo que tal vez condicione en alguna medida los resultados, que presentamos en la tabla 1, que incluye últimas aportaciones12,13.
Las motivaciones que han impulsado la realización de los diferentes estudios pueden haber sido distintas, ya sea por la búsqueda de respuestas al exceso de demandas a profesionales en EE.UU, origen de los estudios de Nueva York7 y de Utah y Colorado8, o bien inferir políticas nacionales para mejorar la seguridad de la atención sanitaria del país conociendo los problemas de los efectos adversos, incluyendo los llamados errores médicos, su gravedad y su importancia, en el caso australiano9. Pero estos estudios retrospectivos de análisis de casos clínicos realizados en los Estados Unidos y Australia, a los que han seguido otros, como el emprendido en el Sistema Nacional de Salud NHS británico15,16ii, iii, constituyen los cimientos y el impulso de iniciativas que reduzcan el daño a los pacientes y hagan más eficientes los recursos hospitalarios disponibles.
Como consecuencia de los estudios de Nueva York, Utah y Colorado, el Institute of Medicine inició en 1998 un proyecto denominado: "Quality of Health Care in America" con el objetivo de desarrollar una estrategia que diera lugar a una mejora significativa en la calidad de la sanidad en los EE.UU a lo largo de la siguiente década. Dentro de este amplio proyecto, se inscribe en una fase inicial, el libro: "To Err is Human: building a Safer Health System"17 que examina los errores médicos en los EE.UU, y es una llamada a la acción para hacer los cuidados sanitarios más seguros para los pacientes. Pero este tan conocido informe contenía dos mensajes distintos. Por un lado incidía en la importancia de comprender las causas que provocaban los errores producidos en el Sistema de Salud, y estimulaba al desarrollo de tecnologías, como la informática, que contribuyesen a la disminución de la tasa de errores. Pero el segundo mensaje resulta controvertido y aterrador, al declarar que en los hospitales de EE UU "Los efectos adversos previsibles son una causa primordial de muerte" y que entre 44.000 y 98.000 pacientes mueren en los hospitales cada año como resultado de errores médicos. Sin embargo esta conclusión no está en absoluto ni fundamentada, ni contrastada porque ninguno de los dos estudios era metodológicamente apropiado para mantener esa hipótesis18.
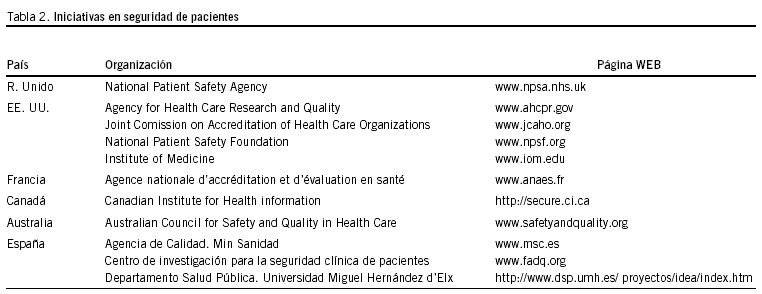
Numerosas han sido las iniciativas internacionales como consecuencia del mencionado informe. En la tabla 2 presentamos un resumen que incluye las páginas web. A pesar de ello, algunos autores han cuestionado la efectividad de las mismas, para propiciar el necesario sereno debate en el seno de los profesionales e incluso en la sociedad19.
Conocer la epidemiología de los efectos adversos y su naturaleza, tanto sea individual como agrupada, permite desarrollar estrategias y mecanismos de prevención para evitarlos. La mejora de la calidad asistencial que de ello se deriva constituye el objetivo principal de la Gestión de Riesgos Sanitarios. Su concepto y aplicación en nuestro medio dentro del sector sanitario, todavía está en mantillas, pero con toda seguridad que tras el impulso profesional, como ha ocurrido en otros países de la Unión Europea, alcanzará rápidamente un buen nivel de desarrollo.
El origen del Proyecto IDEA
El origen del Proyecto IDEA lo podemos fijar en 2001, cuando obtuvimos financiación de la Dirección General de la Agencia para la Calidad, Evaluación y Modernización de los Servicios Asistenciales de la Consellería de Sanitat i Consum de la Generalitat Valenciana (Convocatoria 2001 DOGV núm 4.131 de 20/11/2001. Expediente 014/2001), para desarrollar un proyecto de investigación que estimara la Incidencia de acontecimientos adversos en un servicio de cirugía general y de aparato digestivo. Lo desarrollamos en el Hospital General Universitario de Alicante, siguiendo la metodología del Harvard Medical Practice study, y encontramos un 7,4% de EA. Vimos que se relacionaban positivamente con la edad, con los factores de riesgo intrínsecos del sujeto y con la duración de la estancia hospitalaria, alcanzando en todos los casos significación estadística20.
En el curso de la investigación mencionada, pudimos establecer contacto con los grupos de investigación que habían llevado a cabo los estudios de Nueva York, Utah y Colorado y Reino Unido.
Todo ello nos animó para emprender una nueva investigación que fue financiada por el FIS en la convocatoria de 2002, para desarrollar en tres años (PROYECTO FIS PI021076). Es un estudio de cohortes multicéntrico (13 servicios medico-quirúrgicos de 8 hospitales de 5 Comunidades Autónomas) de que persigue los siguientes objetivos:
Identificar y definir los Efectos Adversos que se derivan de la asistencia hospitalaria.
Estimar la incidencia de Efectos Adversos en distintos servicios de siete hospitales durante el año 2003.
Analizar las características del paciente y las de la asistencia que se asocian a la aparición de Efecto Adverso.
Estimar el impacto de la asistencia en los Efectos Adversos distinguiendo los evitables de los que no lo son.
Pero además, siguiendo las recomendaciones de la Organización Mundial de la Salud del Informe del Consejo Europeo sobre la seguridad del paciente (diciembre de 2001), pretendíamos animar el debate sobre los efectos adversos entre los profesionales, para intentar cambiar una cultura punitiva por una cultura de identificación de oportunidades para la mejora de la calidad.
El proyecto IDEA aparece con vocación de observatorio, con estructura de red, y como foro de discusión científica para facilitar la investigación y la mejora de la calidad de la asistencia.
Los resultados del Proyecto IDEA
Presentaremos los resultados alcanzados siguiendo las líneas programáticas del proyecto, que utiliza como vehículo de difusión la página web del Departamento de Salud Pública de la Universidad Miguel Hernández (UMH) (http://www.dsp.umh.es/proyectos/idea/index.htm). Tienen enlace a esta web, la Sociedad Española de Calidad Asistencial y el Hospital Universitari Sant Joan d'Alacant. Es también una información disponible en INTRANET del hospital y a la que pueden acceder todos los profesionales del Área de Salud 16 de Alicante.
1.- Observatorio: incluye una sección donde, a sugerencia de los investigadores del proyecto, se van incorporando aquellos artículos científicos sobre efectos adversos, de libre disposición.
Está previsto en los próximos meses incorporar una sección de artículos comentados, para aquellos que no son de libre disposición.
Incluye también una sección donde se pueden consultar los artículos propios resultado de las investigaciones en curso, en particular las del proyecto FIS.
2.- Estructura de Red: al grupo inicial de investigación se han unido 33 investigadores de 6 servicios de 5 hospitales.
Está disponible una guía para el cribado de efectos adversos basada en el formulario del Harvard Medical Practice study, un formulario modular para caracterizar el efecto adverso, basado en estudios del Reino Unido21, y un manual de procedimiento que contiene los detalles del estudio.
Se ha elaborado una Base de Datos con un "entorno amigable", que se facilita a los investigadores, y que en breve estará disponible en la Web previa cumplimentación del formulario de solicitud y aceptación por equipo de dirección del proyecto, bajo clave de acceso.
En el momento de redactar este manuscrito estamos elaborando el módulo de explotación de datos, para que también cada equipo pueda disponer del análisis de sus datos, independientemente de que puedan exportarse a otras aplicaciones de análisis estadístico sin ninguna dificultad.
3.- Foro de discusión. Para animar el debate, se han realizado dos seminarios Internacionales: uno a través de la Escuela Valenciana de Estudios para la salud, celebrado en mayo de 2003 en Valencia, y el segundo a través de la Universidad Internacional Menéndez Pelayo, celebrado en noviembre de 2003 en Alicante. Toda la información tanto en texto como en imágenes de los dos seminarios, puede consultarse en la web del Proyecto IDEA.
4.- Red de investigación: En el proyecto IDEA se han anidado los siguientes proyectos de investigación:
Evaluación test diagnóstico: Guía Cribado. Proyecto de Tesis Doctoral presentado en el Dpto de Cirugía de la UMH.
Opinión de cirujanos valencianos sobre los EA en la práctica clínica. Proyecto de Tesis Doctoral presentado en el Dpto de Cirugía de la UMH.
Impacto de los EA de Obstetricia en el neonato. Proyecto de Tesis Doctoral en fase de elaboración en el Dpto de Ginecología y Obstetricia de la Universidad de las Palmas de Gran Canaria.
¿Son más frecuentes los EA en el enfermo pluripatológico? Proyecto de Tesis Doctoral en fase de elaboración en el Dpto de Medicina Interna de la Universidad Complutense de Madrid.
¿Hay asociación entre EA y exitus hospitalario? Proyecto de Tesis Doctoral en fase de elaboración en el Dpto de Salud Pública de la UMH.
Efectos adversos en Medicina Interna según GRDs Proyecto de Tesis Doctoral en fase de elaboración en el Dpto de Anatomía de la UMH.
Estimación de la incidencia de efectos adversos en un servicio de Urgencias Hospitalario. Proyecto de Tesis Doctoral en fase de elaboración en el Dpto de Salud Pública de la UMH.
5.- Producción científica:
Aranaz JMª, Álvarez EE. Acontecimientos adversos de la asistencia sanitaria: entre la mala práxis y la mejora de la calidad. Gestión Clínica y Sanitaria 2001; 3(2): 58. Resumen y comentario de: Thomas EJ, Studdert DM, Burstin HR, Orav EJ, Williams EJ et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care, 2001; 38(3): 261-71.
Aranaz JMª, Gea MT, Marín G. Acontecimientos adversos en un servicio de cirugía general y de aparato digestivo de un hospital universitario. Cirugía Española 2003; 73(2): 103-8.
Aibar C, Aranaz JMª. ¿Pueden evitarse los sucesos adversos relacionados con la atención hospitalaria? Anales del Sistema Sanitario de Navarra 2003; 26 (2): 195-209.
Aranaz JMª, Aibar C, Gea MT, León MT. Los efectos adversos en la asistencia hospitalaria. Una revisión crítica. Med Clín (Barc) 2004; 123(1): 21-5.
Aranaz JMª, Peribáñez J. Los efectos adversos previos a la hospitalización son tan importantes como los desarrollados en el hospital. Gestión Clínica y Sanitaria, 2004; 0(0): 00 (en prensa). Resumen y comentario de: Forster A J, Asmis T R, Clark H D, Saied G A, Code C C, Caughey S C, Baker K, Watters J, Worthington J, Walraven Cv. Ottawa Hospital Patient Safety Study: incidence and timing of adverse events in patients admitted to a Canadian teaching hospital. Can Med Assoc J 2004; 170 (8): 1235-40.
Aranaz JMª, Massó P. Riesgos de la hospitalización. El inicio de una controversia. Gestión Clínica y Sanitaria 2004; 0(0): 00 (en prensa). Resumen y comentario de: Schimmel EM. The hazards of hospitalization. Qual Saf Health Care 2003; 12: 58-64.
Aranaz JMª, Vitaller J. y Grupo de Estudio del Proyecto IDEA: Identificación de Efectos Adversos. De las complicaciones y efectos adversos a la gestión de los riesgos de la asistencia. Estudios para la salud, nº 00. Generalitat Valenciana. Valencia, 2004. (En prensa).
A modo de conclusión preliminar
Es necesario garantizar la seguridad del paciente en el sistema sanitario, porque es un componente esencial de la calidad asistencial. Pero son los profesionales sanitarios quienes han de liderar el debate, para eliminar toda crispación y tener a la vez la garantía del sereno ejercicio profesional. En muy poco tiempo se ha pasado de la total comprensión hacia los efectos adversos a la criminalización de los mismos22v. Mejorando la comunicación con el paciente y la sociedad, ésta será menos intransigente, sobre todo si comprueba la constante preocupación de los profesionales para investigar los EA y la capacidad de aprendizaje de las situaciones menos favorables.
Tal vez la clave esté en buscar sistemas de notificación no punitivos, confidenciales, independientes de la autoridad con capacidad sancionadora, que permita el análisis de expertos, que sea ágil en las recomendaciones, que sea sensible y esté orientado más hacia el sistema que hacia el individuo23.