Posterior circulation stroke accounts for 15% to 20% of ischaemic strokes, but is associated with poor functional and vital prognosis in over 60% of cases. Due to its clinical and radiological peculiarities, diagnosis and management are more complex than in anterior circulation stroke. This study analyses and characterises patients with vertebrobasilar strokes treated with mechanical thrombectomy in our region.
MethodsWe conducted a descriptive, retrospective analysis of patients with vertebrobasilar stroke and treated with mechanical thrombectomy at our centre, a reference centre for cerebrovascular emergencies for the region of Aragon. We recorded baseline characteristics, risk factors, signs and symptoms at onset, radiological assessment scale scores, procedure-related variables, management times, and functional prognosis at 3 months.
ResultsWe selected 37 patients (39.5% women; mean age [standard deviation], 68.34 [14.1] years). Cardioembolic stroke (42.1%) was the most common aetiology, followed by atherothrombosis (28.9%). The top of the basilar artery was the most common site of obstruction (55.3%). The most frequent clinical features were somnolence (76.3%), motor deficits (71.1%), and nausea (55.3%). Successful reperfusion (mTICI ≥ 2b) was achieved in 81.1% of patients. Functional outcome at 90 days was poor (mRS < 3) in 59% of patients.
ConclusionsPosterior circulation stroke is associated with high rates of morbidity and mortality. Its subacute, nonspecific clinical course prolongs management times and hinders early detection. Mechanical thrombectomy is a safe and effective procedure, although further studies are needed to establish the optimal patient profile.
Los ictus de circulación posterior suponen un 15-20% del total de ictus isquémicos, pero asocian en más del 60% de casos mal pronóstico funcional y vital. Por sus características clínicas y radiológicas su manejo y diagnóstico es más complejo que en los ictus de circulación anterior. El objetivo de nuestro trabajo es analizar los pacientes con ictus del sistema vertebro-basilar tratados mediante trombectomía mecánica en nuestra comunidad y definir sus características.
MétodoAnálisis retrospectivo-descriptivo de los pacientes con ictus del sistema vertebro-basilar tratados mediante trombectomía mecánica en nuestro centro (hospital de referencia para la patología cerebrovascular urgente en Aragón). Se definen características basales, factores de riesgo, signos y síntomas de presentación, escalas radiológicas, variables procedimiento y tiempos de atención y pronóstico funcional a 3 meses.
Resultadosse incluyeron 37 pacientes (39,5% mujeres) con una edad media de 68,34 ± 14,1 años. La primera causa etiológica fue la cardioembólica (42,1%) seguida de la aterotrombótica (28,9%). La localización más frecuente de la obstrucción fue el top de la basilar (55,3%). Los síntomas más frecuentes fueron somnolencia (76,3%), déficit motor (71,1%) y las náuseas (55,3%). Se logró reperfusión exitosa (mTICI ≥ 2b) en el 81,1% de pacientes. El pronóstico funcional a 90 días fue desfavorable (mRS < 3) en el 59% de los casos.
Conclusioneslos ictus de circulación posterior se asocian a una importante morbimortalidad. Su presentación clínica subaguda y larvada dilata los tiempos de atención y dificulta una identificación precoz. La trombectomía mecánica es un procedimiento seguro y eficaz, si bien son necesarios más estudios que esclarezcan la selección de pacientes.
Acute stroke or cerebrovascular disease is one of the main challenges for public healthcare worldwide. It represents the second leading cause of death in the Western world and the first leading cause of permanent disability worldwide.1 Its estimated incidence in the Spanish setting amounts to 187.4 cases per 100 000 population; this figure is expected to increase by 35% between 2015 and 2035 due to population ageing.2,3
Posterior circulation stroke represents 15% to 20% of all ischaemic strokes, but more than 60% of the cases are associated with a poor functional and vital prognosis.4–7 Specifically, basilar artery occlusion is considered the most devastating subtype. It accounts for less than 3% of all strokes and 5% to 10% of large-vessel occlusions, but leads to highly significant morbidity and mortality rates, with severe sequelae in more than 70% of cases.4,7–9
These posterior circulation or vertebrobasilar strokes (VBS) present several differential characteristics influencing their management. Due to their clinical and radiological peculiarities, diagnosing these cases represents a greater challenge. The form of presentation differs substantially from the typical symptoms of anterior circulation stroke, and the most widely used diagnostic scales and techniques are less sensitive for their detection. Furthermore, uncertainties remain regarding the ideal management and the indications for endovascular reperfusion therapies. Unlike in cases of anterior circulation stroke, no randomised studies have been performed to date that show the effectiveness and safety of mechanical thrombectomy (MT) in VBS. The latest indications are patients within the first 24 hours from onset, with moderate-severe symptoms (National Institutes of Health Stroke Scale [NIHSS] > 10) and potentially salvageable tissue.
The aim of our study is to analyse patients with VBS treated with MT in the Spanish region of Aragon, describing their baseline characteristics, clinical presentation of stroke, and radiological characteristics, as well as procedural parameters associated with this entity, and their functional prognosis.
Material and methodsWe conducted a descriptive, retrospective study based on a prospective database of patients with VBS treated with MT at Hospital Universitario Miguel Servet (the reference hospital for acute cerebrovascular disease in Aragon, and the only centre in the region equipped to perform MT).
We selected procedures performed between February 2015 and June 2021 and included in our study those patients diagnosed with acute ischaemic stroke due to occlusion of the vertebrobasilar system as shown by CT angiography, brain MRI, or digital subtraction angiography. We excluded patients with transient posterior circulation stroke, isolated posterior cerebral artery occlusion, and simultaneous anterior and posterior circulation occlusion. The decision to start endovascular treatment was jointly taken by the neurologist from the on-call neurovascular service and the neurointerventionist, based on each patient’s clinical and radiological characteristics.
The demographic variables gathered were: age, sex, previous functional status (using the modified Rankin Scale [mRS]), and cerebrovascular risk factors (hypertension, dyslipidaemia, diabetes mellitus, atrial fibrillation, alcohol and tobacco use, previous cardiac disease, peripheral arterial disease, and previous stroke). Clinical and healthcare variables included the level of consciousness upon arrival (using the Glasgow Coma Scale), assessment of the neurological deficit at the time of code stroke activation (using the NIHSS), and the need for intubation.
From the emergency department reports, we retrospectively gathered data on the clinical manifestations at the time of code stroke activation, grouped into the following signs and symptoms: somnolence, nausea/vomiting, vertiginous syndrome, ataxia, headache, motor deficit (and laterality), sensory deficit, dysarthria, speech alterations, oculomotor alterations, pupil alterations, anopsia, and facial palsy affecting both the upper and lower halves of the face. We also included the reference hospital, fibrinolysis administration, onset-to-door time (interval between symptom onset and arrival at the hospital), door-to-needle time (in those receiving intravenous fibrinolysis), door-to-puncture time, and onset-to-reperfusion time.
Regarding MT, we gathered the duration of the procedure, the number of passes, the degree of reperfusion achieved (using the modified TICI scale), and the type of anaesthesia used. We analysed the presence of the hyperdense basilar artery sign and used the Posterior Circulation Alberta Stroke Program Early CT Score (PC-ASPECTS), Basilar Artery on Computed Tomography Angiography score (BATMAN), and Posterior Circulation Computed Tomography Angiography vascular score (PC-CTA). Scores on these scales were calculated by a single radiologist, blinded to the remaining variables. Occlusion location was divided into 4 categories: tandem occlusion of the vertebral artery and posterior cerebral artery (VA-PCA), vertebral and basilar artery tandem occlusion (VBA), and vertebrobasilar and top of the basilar artery tandem occlusion. Outcome variables included neurological deficit at 24 hours (NIHSS), death during hospitalisation, onset of symptomatic intracerebral haemorrhage (SIH) within the first 36 hours (ECASS-III score), and functional status at 90 days (according to the mRS score).
In the descriptive statistical analysis, we used the Kolmogorov-Smirnov test to study the normality of quantitative variables. Dichotomous variables were compared using the chi-square test or the Fisher exact test, and were expressed as frequencies for each category. Normally distributed continuous variables were compared using the t test and are expressed as mean (standard deviation). Non–normally distributed continuous variables were compared with the Mann-Whitney U test, and are expressed as median and quartiles 1 and 3 (Q1–Q3). The threshold for statistical significance was set at P < .05.
Statistical analysis was performed using the tableone package from the R-Studio software (version 4.1.2.).10,11 Figures and tables were created using Microsoft Word 365 and the ggplot2, ggpubr, and waffle R packages.12–14
The study was approved by the research ethics committee of the region of Aragon (CEICA) with reference number CI-PI21/089.
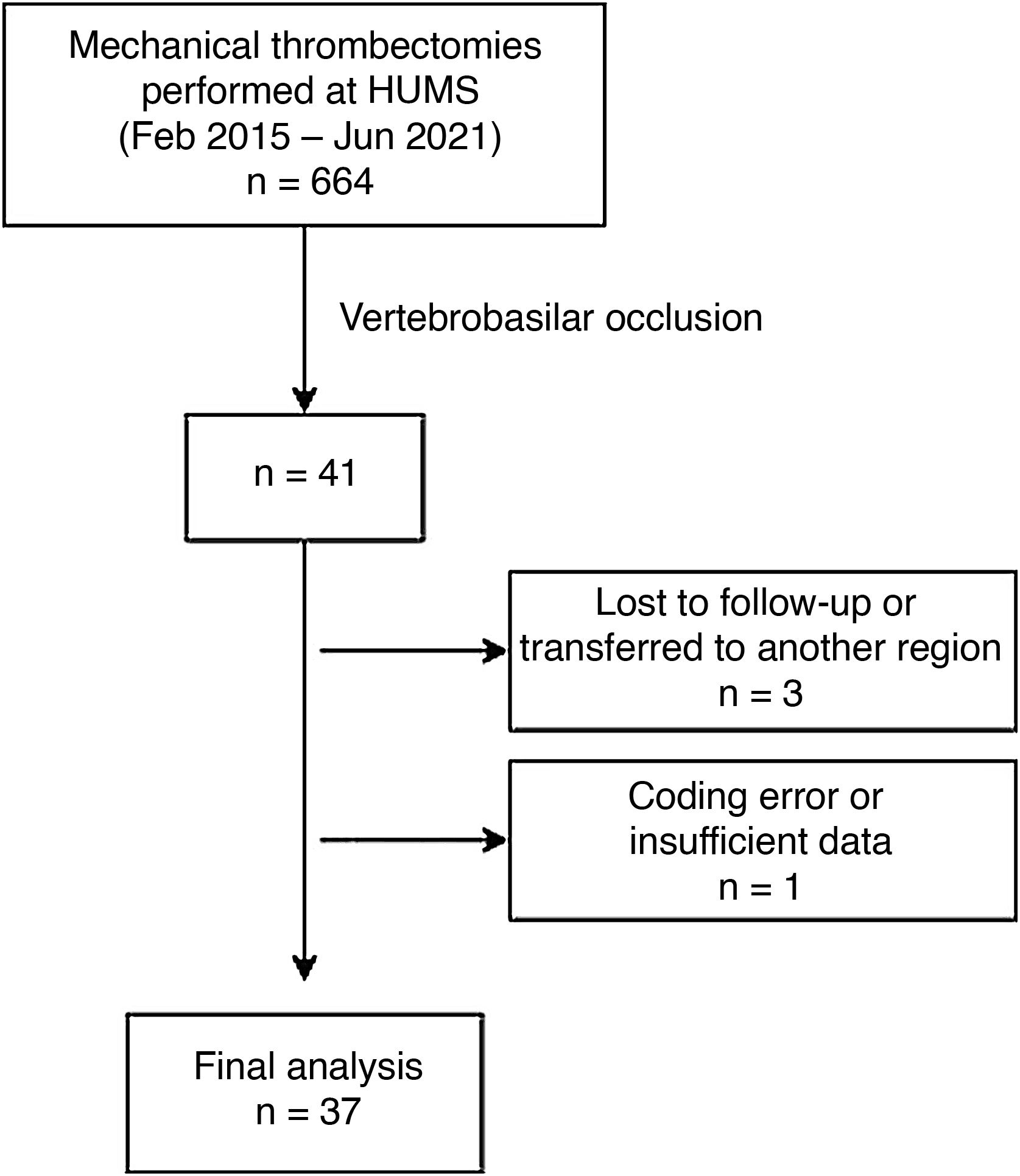
ResultsThe registry included 664 patients treated with MT between February 2015 and June 2021. Of these, we selected 41 (6.17%) of them with vertebrobasilar occlusion. We excluded 3 patients lost to follow-up due to transfer to another region or private centre. Another patient was excluded due to a coding error and lack of data (Fig. 1).
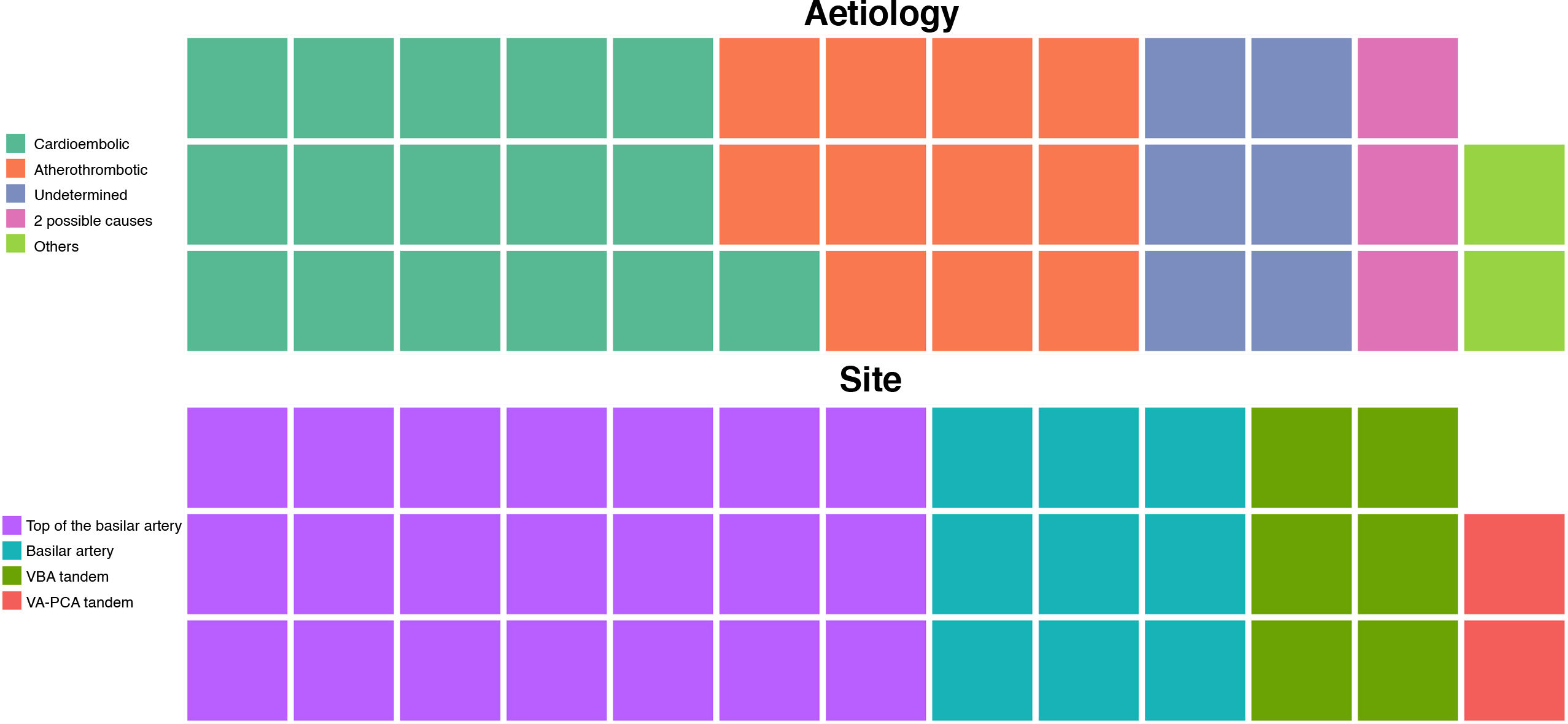
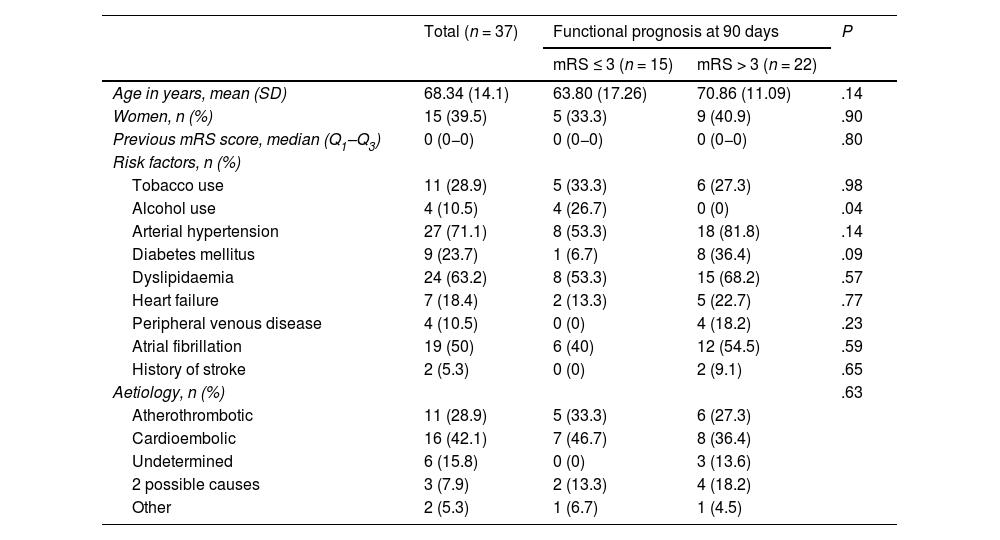
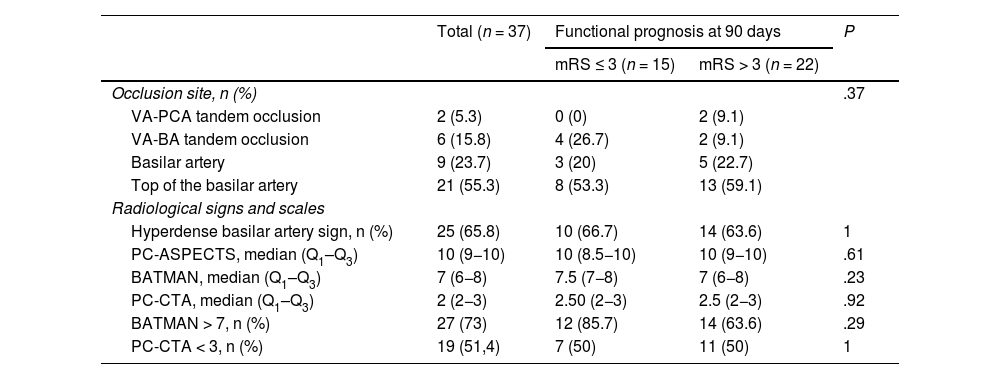
The mean age of patients with vertebrobasilar occlusion and treated with MT was 68.34 (14.1) years; 39.5% (15) were women. The most frequent cerebrovascular risk factors were arterial hypertension (27; 71.1%), dyslipidaemia (24; 63.2%), and atrial fibrillation (19; 50%) (Table 1). By aetiology (Fig. 2), the most frequent cause of stroke was cardioembolic (16; 42.1%), followed by atherothrombotic (11; 28.9%). The most frequent site of occlusion was the top of the basilar artery (21; 55.3%), followed by the trunk of the basilar artery (9; 23.7%) (Table 2).
Baseline characteristics of patients with vertebrobasilar stroke treated with mechanical thrombectomy.
| Total (n = 37) | Functional prognosis at 90 days | P | ||
|---|---|---|---|---|
| mRS ≤ 3 (n = 15) | mRS > 3 (n = 22) | |||
| Age in years, mean (SD) | 68.34 (14.1) | 63.80 (17.26) | 70.86 (11.09) | .14 |
| Women, n (%) | 15 (39.5) | 5 (33.3) | 9 (40.9) | .90 |
| Previous mRS score, median (Q1–Q3) | 0 (0−0) | 0 (0−0) | 0 (0−0) | .80 |
| Risk factors, n (%) | ||||
| Tobacco use | 11 (28.9) | 5 (33.3) | 6 (27.3) | .98 |
| Alcohol use | 4 (10.5) | 4 (26.7) | 0 (0) | .04 |
| Arterial hypertension | 27 (71.1) | 8 (53.3) | 18 (81.8) | .14 |
| Diabetes mellitus | 9 (23.7) | 1 (6.7) | 8 (36.4) | .09 |
| Dyslipidaemia | 24 (63.2) | 8 (53.3) | 15 (68.2) | .57 |
| Heart failure | 7 (18.4) | 2 (13.3) | 5 (22.7) | .77 |
| Peripheral venous disease | 4 (10.5) | 0 (0) | 4 (18.2) | .23 |
| Atrial fibrillation | 19 (50) | 6 (40) | 12 (54.5) | .59 |
| History of stroke | 2 (5.3) | 0 (0) | 2 (9.1) | .65 |
| Aetiology, n (%) | .63 | |||
| Atherothrombotic | 11 (28.9) | 5 (33.3) | 6 (27.3) | |
| Cardioembolic | 16 (42.1) | 7 (46.7) | 8 (36.4) | |
| Undetermined | 6 (15.8) | 0 (0) | 3 (13.6) | |
| 2 possible causes | 3 (7.9) | 2 (13.3) | 4 (18.2) | |
| Other | 2 (5.3) | 1 (6.7) | 1 (4.5) | |
mRS: modified Rankin Scale; Q1–Q3: quartiles 1 and 3; SD: standard deviation.
Radiological variables.
| Total (n = 37) | Functional prognosis at 90 days | P | ||
|---|---|---|---|---|
| mRS ≤ 3 (n = 15) | mRS > 3 (n = 22) | |||
| Occlusion site, n (%) | .37 | |||
| VA-PCA tandem occlusion | 2 (5.3) | 0 (0) | 2 (9.1) | |
| VA-BA tandem occlusion | 6 (15.8) | 4 (26.7) | 2 (9.1) | |
| Basilar artery | 9 (23.7) | 3 (20) | 5 (22.7) | |
| Top of the basilar artery | 21 (55.3) | 8 (53.3) | 13 (59.1) | |
| Radiological signs and scales | ||||
| Hyperdense basilar artery sign, n (%) | 25 (65.8) | 10 (66.7) | 14 (63.6) | 1 |
| PC-ASPECTS, median (Q1–Q3) | 10 (9−10) | 10 (8.5−10) | 10 (9−10) | .61 |
| BATMAN, median (Q1–Q3) | 7 (6−8) | 7.5 (7−8) | 7 (6−8) | .23 |
| PC-CTA, median (Q1–Q3) | 2 (2−3) | 2.50 (2−3) | 2.5 (2−3) | .92 |
| BATMAN > 7, n (%) | 27 (73) | 12 (85.7) | 14 (63.6) | .29 |
| PC-CTA < 3, n (%) | 19 (51,4) | 7 (50) | 11 (50) | 1 |
BA: basilar artery; BATMAN: Basilar Artery on Computed Tomography Angiography; PC-ASPECTS: Posterior Circulation Alberta Stroke Program Early CT Score; PC-CTA: Posterior Circulation Computed Tomography Angiography vascular score; PCA: posterior cerebral artery; Q1-Q3: quartiles 1 and 3; VA: vertebral artery.
Hyperdense basilar artery sign was observed in 25 patients (65.8%). We observed no statistically significant differences in this sign based on the functional prognosis at 90 days. Median (Q1–Q3) scores on the PC-ASPECTS, BATMAN, and PC-CTA scales were 10 (8.5−10), 7.5 (7−8), and 2.5 (2−3), respectively (Table 2).
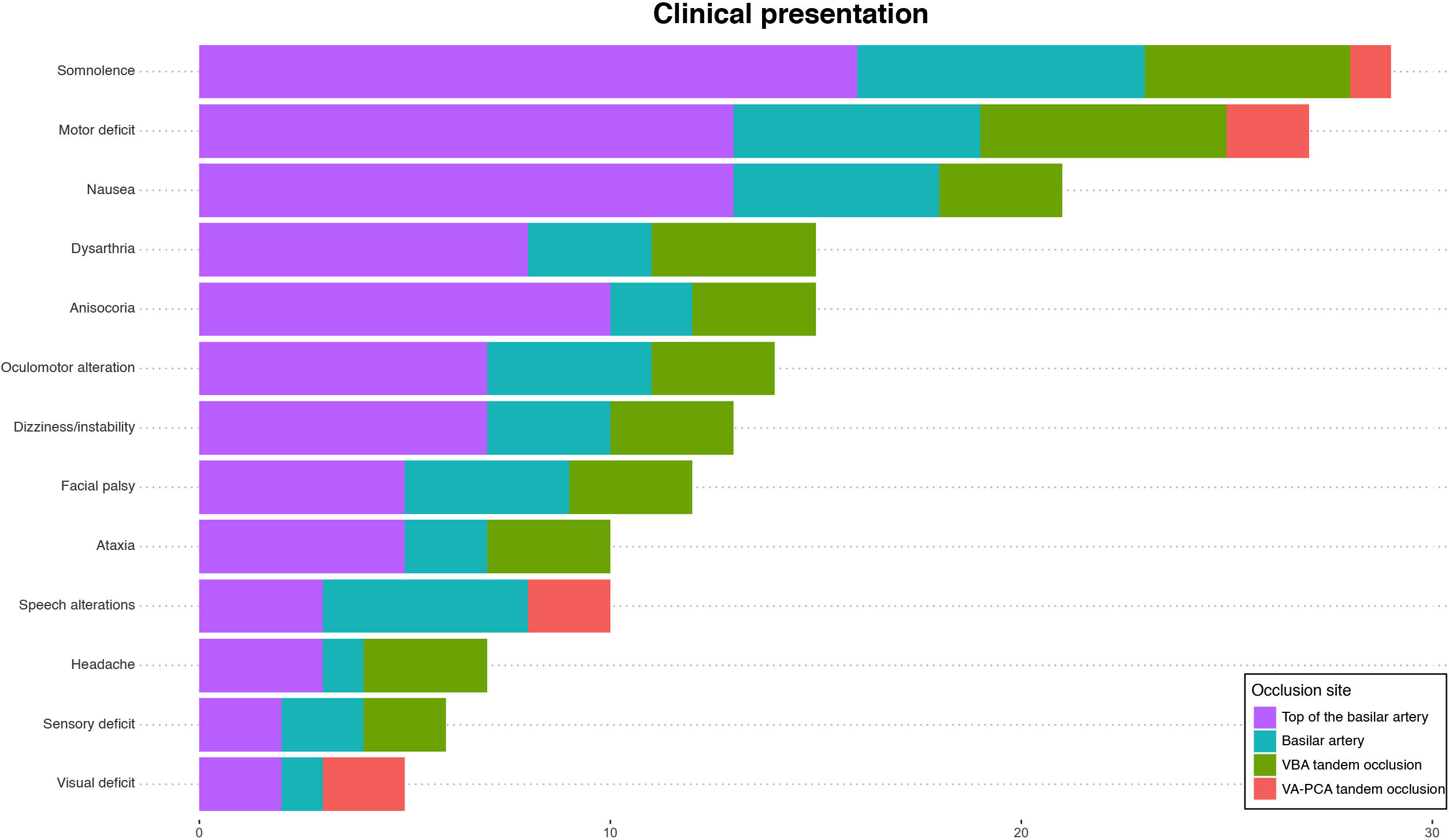
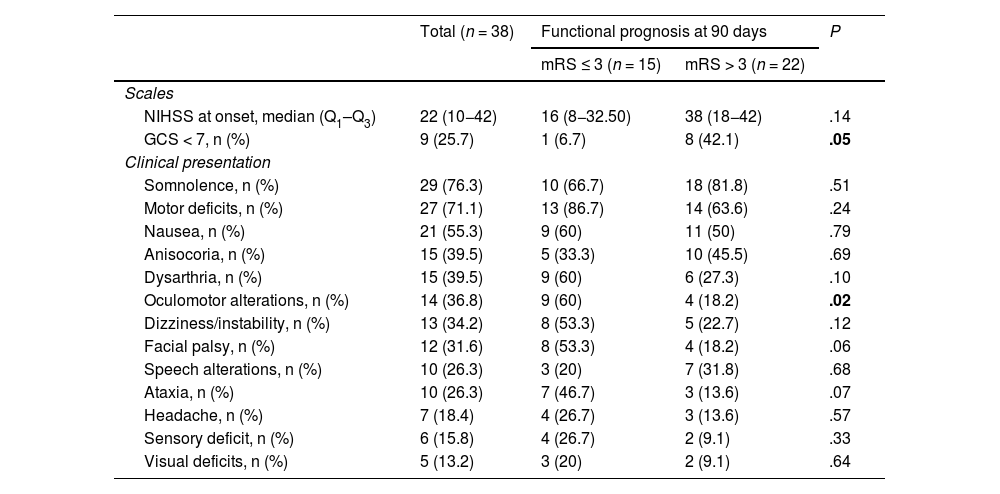
Regarding clinical presentation at onset (Table 3), the median NIHSS score was 22 (10−42). The most frequent symptom was somnolence (29; 76.3%), followed by motor deficits (27; 71.1%) and nausea (21; 55.3%). Regarding the laterality of deficits, 15 patients (55.6%) showed symptoms on the right side whereas symptoms were bilateral in 4 cases (14.8%). We identified significant differences in the proportion of patients with a decreased level of consciousness (Glasgow Coma Scale > 7) as a function of good functional prognosis at 90 days (6.7% vs 42.1%; P = .05). We also observed significant differences in median NIHSS score at 24 hours between patients with good and poor functional prognosis (5 vs 42; P = .05) (Table 4). Fig. 3 shows the frequency of the different signs and symptoms as a function of the occlusion site.
Clinical presentation of vertebrobasilar strokes treated with mechanical thrombectomy.
| Total (n = 38) | Functional prognosis at 90 days | P | ||
|---|---|---|---|---|
| mRS ≤ 3 (n = 15) | mRS > 3 (n = 22) | |||
| Scales | ||||
| NIHSS at onset, median (Q1–Q3) | 22 (10−42) | 16 (8−32.50) | 38 (18−42) | .14 |
| GCS < 7, n (%) | 9 (25.7) | 1 (6.7) | 8 (42.1) | .05 |
| Clinical presentation | ||||
| Somnolence, n (%) | 29 (76.3) | 10 (66.7) | 18 (81.8) | .51 |
| Motor deficits, n (%) | 27 (71.1) | 13 (86.7) | 14 (63.6) | .24 |
| Nausea, n (%) | 21 (55.3) | 9 (60) | 11 (50) | .79 |
| Anisocoria, n (%) | 15 (39.5) | 5 (33.3) | 10 (45.5) | .69 |
| Dysarthria, n (%) | 15 (39.5) | 9 (60) | 6 (27.3) | .10 |
| Oculomotor alterations, n (%) | 14 (36.8) | 9 (60) | 4 (18.2) | .02 |
| Dizziness/instability, n (%) | 13 (34.2) | 8 (53.3) | 5 (22.7) | .12 |
| Facial palsy, n (%) | 12 (31.6) | 8 (53.3) | 4 (18.2) | .06 |
| Speech alterations, n (%) | 10 (26.3) | 3 (20) | 7 (31.8) | .68 |
| Ataxia, n (%) | 10 (26.3) | 7 (46.7) | 3 (13.6) | .07 |
| Headache, n (%) | 7 (18.4) | 4 (26.7) | 3 (13.6) | .57 |
| Sensory deficit, n (%) | 6 (15.8) | 4 (26.7) | 2 (9.1) | .33 |
| Visual deficits, n (%) | 5 (13.2) | 3 (20) | 2 (9.1) | .64 |
GCS: Glasgow Coma Scale; NIHSS: National Institutes of Health Stroke Scale.
Statistically significant values (P < .05) are shown in bold.
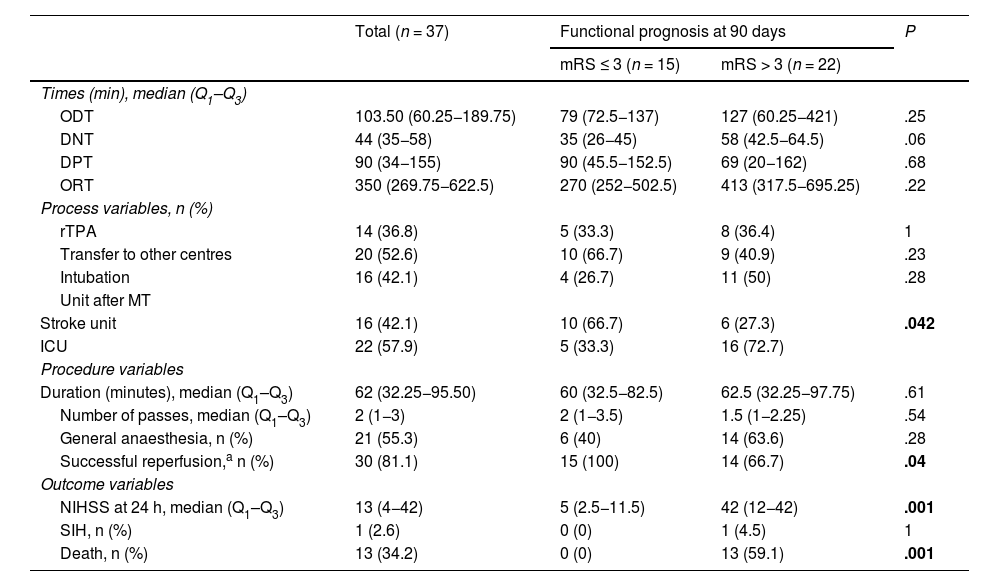
Process, procedure, and outcome variables.
| Total (n = 37) | Functional prognosis at 90 days | P | ||
|---|---|---|---|---|
| mRS ≤ 3 (n = 15) | mRS > 3 (n = 22) | |||
| Times (min), median (Q1–Q3) | ||||
| ODT | 103.50 (60.25−189.75) | 79 (72.5−137) | 127 (60.25−421) | .25 |
| DNT | 44 (35−58) | 35 (26−45) | 58 (42.5−64.5) | .06 |
| DPT | 90 (34−155) | 90 (45.5−152.5) | 69 (20−162) | .68 |
| ORT | 350 (269.75−622.5) | 270 (252−502.5) | 413 (317.5−695.25) | .22 |
| Process variables, n (%) | ||||
| rTPA | 14 (36.8) | 5 (33.3) | 8 (36.4) | 1 |
| Transfer to other centres | 20 (52.6) | 10 (66.7) | 9 (40.9) | .23 |
| Intubation | 16 (42.1) | 4 (26.7) | 11 (50) | .28 |
| Unit after MT | ||||
| Stroke unit | 16 (42.1) | 10 (66.7) | 6 (27.3) | .042 |
| ICU | 22 (57.9) | 5 (33.3) | 16 (72.7) | |
| Procedure variables | ||||
| Duration (minutes), median (Q1–Q3) | 62 (32.25−95.50) | 60 (32.5−82.5) | 62.5 (32.25−97.75) | .61 |
| Number of passes, median (Q1–Q3) | 2 (1−3) | 2 (1−3.5) | 1.5 (1−2.25) | .54 |
| General anaesthesia, n (%) | 21 (55.3) | 6 (40) | 14 (63.6) | .28 |
| Successful reperfusion,a n (%) | 30 (81.1) | 15 (100) | 14 (66.7) | .04 |
| Outcome variables | ||||
| NIHSS at 24 h, median (Q1–Q3) | 13 (4−42) | 5 (2.5−11.5) | 42 (12−42) | .001 |
| SIH, n (%) | 1 (2.6) | 0 (0) | 1 (4.5) | 1 |
| Death, n (%) | 13 (34.2) | 0 (0) | 13 (59.1) | .001 |
DNT: door-to-needle time; DPT: door-to-puncture time; ICU: intensive care unit; mRS: modified Rankin Scale; MT: mechanical thrombectomy; NIHSS: National Institutes of Health Stroke Scale; ODT: onset-to-door time; ORT: onset-to-reperfusion time; Q1-Q3: quartiles 1 and 3; rTPA: fibrinolysis using recombinant tissue plasminogen activator; SIH: symptomatic intracerebral haemorrhage.
Statistically significant values (P < .05) are shown in bold.
Of 37 procedures performed, 24 (63%) were primary MT and 14 (37%) rescue MT. Median (Q1–Q3) procedure duration was 62 minutes (32.25−95.50), with reperfusion being successful (mTICI grade ≥ 2b) in 30 cases (81.1%). After analysing management times, we observed a median time of 103.50 minutes (60.25–89.75) from symptom onset to the first contact with a hospital centre, as well as a median time of 350 minutes (269.75−622.5) from symptom onset to vascular reperfusion (Table 4).
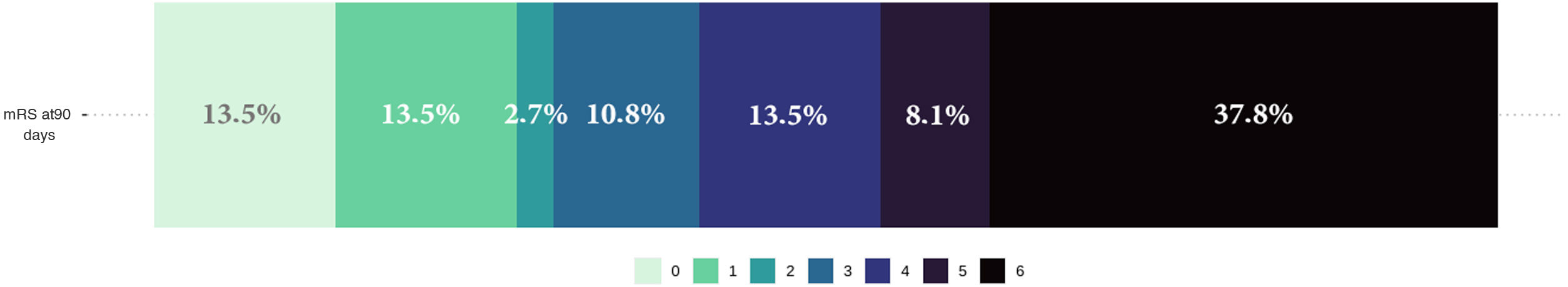
Lastly, functional prognosis at 90 days was poor (mRS > 3) in 22 (59%) patients, and 13 (34.2%) patients died during hospitalisation. Fig. 4 shows the distribution of frequencies in the mRS score at 90 days.
DiscussionPosterior circulation strokes present a series of characteristics differentiating them from anterior circulation stroke, which will determine their diagnosis, management, and study. Our study analysed patients attended in the region of Aragon who underwent endovascular treatment. Our aim was to provide an overall description of baseline, clinical, and radiological characteristics and, of course, data related to the procedure and outcomes.
Firstly, we observe that the most frequent site of occlusion was the distal area and the top of the basilar artery (50%); this is consistent with the results of other published series.15,16 Regarding risk factors and aetiology, VBS has traditionally been thought to have a different profile to that of anterior circulation strokes, with cardioembolic aetiology in up to 40% of cases.16 However, more recent studies have reported no significant differences between VBS and anterior circulation stroke in terms of aetiology or risk factors. Large-artery atherosclerosis is the leading cause of VBS (32%-37%), followed by cardioembolic aetiology (18%) and arterial dissection (10%).17–19 The latter cause should be always considered in younger patients, in whom the most frequent site of dissection is the V2 and V3 segments.4,6,20 In our series, the high rate of dyslipidaemia (63%) differs from that reported in other registries, as well as the higher number of patients with cardioembolic disease (50%), probably because only patients treated with MT were analysed.
Secondly, our data suggests that VBS represents a clinical challenge due to the form of presentation. Unlike in anterior circulation stroke, in which the classic clinical symptoms are easily recognisable, the course of VBS is more subacute and fluctuating. This type of stroke usually manifests with non-specific symptoms, such as headache, somnolence, or instability, which may be overlooked by typical screening systems.4,6,18,21,22 Similarly, more subtle signs such as Horner syndrome, oculomotor alterations, or lower cranial nerve involvement, may go unnoticed in an initial assessment by a physician not specialising in neurology. All these factors lead to a delay in diagnosis and longer management times.21,23,24
In our series, the most frequently observed symptoms were somnolence, nausea, and motor alterations (76%, 71%, and 55%, respectively). Sensory or visual alterations were less frequent (13% and 15%), probably because they go unnoticed in the initial assessment. The difficulties in the examination of patients with decreased level of consciousness also play a secondary role. A surprising finding was the identification of 10 patients (26.3%) presenting speech alterations. When analysed in detail, all these patients presented occlusions in the distal basilar artery or top of the basilar artery. In those undergoing brain MRI scans (40%), the affected area extended beyond the posterior circulation: the left thalamic nucleus was affected in all cases. Lesions to such sites have been related to language impairment.25–27
Regarding management times, a previous analysis of our registry showed that posterior circulation strokes presented significantly longer door-to-puncture and onset-to-reperfusion times than anterior circulation strokes.28 The present study found a tendency toward significance in the association between door-to-needle time and functional prognosis. We also observed significant differences in onset-to-door time, onset-to-reperfusion time, and procedure time depending on the percentage of successful reperfusions. All of these findings underscore the relevance of early identification in starting treatment as soon as possible.
Another significant aspect is the limitations of the NIHSS in VBS. The NIHSS is highly sensitive to anterior circulation strokes, especially those affecting the left hemisphere. The typical symptoms of posterior circulation strokes, such as bulbar signs, gait alteration, truncal ataxia, and vertical gaze palsy are not considered in the NIHSS.7,29 As a result of this, patients with VBS tend to obtain lower scores than patients with anterior circulation strokes. Observational studies have shown that 75% of patients with VBS obtain NIHSS scores between 0 and 5 at onset30,31; however, if we focus exclusively on those who received endovascular treatment, the median NIHSS score amounted to 20–22, as in our registry.32,33
The following point to address regarding VBS is the radiological characteristics of these strokes. Head CT scan is the first test performed in the acute management of ischaemic stroke. Unfortunately, due to bone artefacts at the base of the skull and the poor resolution of the parenchyma in the infratentorial area, the sensitivity of CT in detecting acute ischaemic changes is very limited, even lower than 40% in some series.6,34,35 CT angiography presented high sensitivity and specificity in detecting vascular occlusions, and a good correlation with cerebral angiography36; therefore, it is essential in therapeutic decision making. Finally, brain MRI is considered the reference technique for detecting acute ischaemic changes in the posterior fossa37; however, its reduced availability and long duration limit is usefulness in the emergency department.
Therefore, several scales and signs have been proposed to support diagnosis and predict outcomes. The first is the hyperdense basilar artery sign in the baseline CT scan. In an appropriate clinical context, acute thrombosis of the basilar artery may be diagnosed, with a sensitivity of up to 71% (65% in our series).38 Another useful tool is the PC-ASPECTS scale, a version of the ASPECTS scale adapted to VBS.39 It has been used in clinical trials for patient selection and to characterise the extension of ischaemia. It may be assessed at the time of the CT scan, but it has been reported to be twice as sensitive in detecting early ischaemic changes when assessed together with CT angiography and CT perfusion studies.40 Several studies have also reported data on mortality and functional prognosis.39–42 Our series showed a median score of 10 (8.5−10), in line with other series of patients eligible for MT. In our series, we found no significant associations between the different scales and functional prognosis or the percentage of successful reperfusions, possibly due to our sample size.
Lastly, endovascular treatment in VBS has been subject to debate in recent years. Unlike in anterior circulation strokes, in which the efficacy and safety of MT are supported by numerous studies,43 the available evidence in VBS is more limited. Several studies have shown the safety and high rates of revascularisation achieved with MT, although no randomised studies were conducted until recently.9,41,44,45
The results of the BAOCHE46 and ATTENTION47 studies were recently published, showing the effectiveness of endovascular treatment versus medical treatment. The BAOCHE study included patients with stroke who received treatment 6 to 24 hours after onset, with a median NIHSS score of 20. Patients receiving endovascular treatment achieved better functional outcomes at 3 months (mRS of 0−3: 46% vs 24%) and lower mortality rates (31% vs 42%). The study reported a higher number of cerebral haemorrhages, but the difference was not statistically significant. The ATTENTION study reported similar results. It included patients attended within the first 12 hours after stroke with an NIHSS score ≥ 10 (median of 22). Patients treated with endovascular treatment presented better functional prognosis (mRS: 0-3; 46% vs 23%) and lower mortality rates (36.7% vs 55.3%). Regarding the selection of patients based on neuroimaging criteria, we should underscore that the median PC-ASPECTS score was higher than 8 in both studies. Overall, these results support the use of MT in patients with VBS, severe symptoms, and salvageable tissue in neuroimaging studies, although uncertainties remain regarding the selection or management of patients with scores below 10.32,33 Another limitation is the inclusion of Chinese patients, in whom the percentage of intracranial stenosis is higher; this was the case in both studies. Further studies with more diverse patient samples are needed.48
The main limitation of this study is its retrospective, observational design. Despite including all the patients treated in our hospital, the sample size is small, which constitutes a limitation for comparative analysis. Analysing a long time interval may have resulted in differences in the selection of patients and the techniques used. However, selection criteria for posterior fossa strokes have not undergone significant changes in the past decade, and all patients were managed according to the currently applicable stroke action plan for the region of Aragon, which has remained substantially the same. Lastly, the retrospective review of clinical records and the possibility of coding errors constitute limitations for the retrieval of information.
In conclusion, VBS currently represents a challenge for neurovascular neurology. Despite its lower prevalence, it causes significant morbidity and mortality. Its clinical presentation is more latent, with a non-specific, subacute course that results in longer management times and hinders early identification. MT is a safe procedure with high reperfusion rates, and has been shown to improve functional prognosis and mortality rates. However, further studies are needed to define the selection of the ideal patient.
FundingThis study has received no specific funding from any public, commercial, or non-profit organisation.
I would like to thank Dr Tejada for his support, guidance, and dedication to publishing this study.
I also would like to thank Dr de Selby and Dr Sancho for guiding me through the labyrinth of life.