Progression of Parkinson's disease (PD) is associated with significantly decreased mobility, increased risk of falls, and such non-motor symptoms as cognitive and psychotic disorders. The main phenotypic manifestations of advanced PD (APD) are motor fluctuations, severe dyskinesia, axial motor symptoms resistant to levodopa, and non-motor symptoms such as cognitive impairment.1 Conventional treatment, which fails to control symptoms satisfactorily in these patients, is usually administered to patients on H&Y stages 4 or 5. Approximately 50% of patients with PD display alterations characteristic of APD 5 years after clinical diagnosis; such alterations include motor fluctuations, non-motor symptoms, and treatment-induced dyskinesia.2 Treatment with continuous infusion of apomorphine (CIA) has been found to effectively improve motor symptoms and overall function in patients with APD.3–5 However, the literature provides little information on the effectiveness of CIA according to the degree of functional disability in these patients. With this in mind, we conducted an observational study to determine the effectiveness and tolerability of CIA in patients with APD and different degrees of functional disability. We believe that our partial results are worth publishing.
Patients and methodsWe included consecutive patients with PD, diagnosed according to the Queen Square Brain Bank diagnostic criteria,6 who were attended in the Parkinson's and movement disorders unit at Consorci Sanitari de Terrassa (Barcelona, Spain); all patients were in advanced stages of the disease. The included patients had motor and non-motor fluctuations and experienced disabling ‘off’ periods. These patients were offered initiation of treatment with continuous subcutaneous infusion of apomorphine in line with the indications listed on the summary of product characteristics.7 All patients signed informed consent forms before inclusion. Our study was approved by the hospital's Clinical Research Ethics Committee.
Assessment of functional disability and patient classificationParticipants were categorised in 2 groups: a) patients with APD and moderate functional disability, defined as rates of 60% to 80% on the Schwab & England Activities of Daily Living Scale (S&E) and H&Y stages 2 to 3 (group A); and b) patients with APD and severe functional impairment, defined as S&E rates <60% and H&Y stages >3 (group B).
ProcedureWe tested CIA in all patients for 3 to 4 days. The initial infusion rate of apomorphine (Apomorfina Archimedes® 10mg/mL, infusion solution; Archimedes Pharma Ibérica S.L., Madrid, Spain), at a concentration of 5mg/mL, was 0.20mL/h. Infusion speed was adjusted according to clinical response in increments of 0.20mL/h until either symptom control was achieved or patients displayed side effects. An infusion time of 12hours/day was recommended for all patients; doses were subsequently adjusted during follow-up visits. Antiparkinsonian drugs administered before inclusion in the study were maintained during the initial phase and progressively adjusted or discontinued during follow-up according to each patient's clinical response. Previous treatments included levodopa, dopamine agonists (pramipexole and rotigotine), and pen-injected apomorphine (APO-go PEN® 10mg/mL; Britannia Pharmaceuticals Ltd., Newbury, UK); the latter was used as a rescue treatment. Patients were assessed at inclusion (before starting CIA) and during follow up (at 1, 3, 6, and 12 months).
Outcome variables1) Patient diary of motor fluctuations, 2) Abnormal Involuntary Movement Scale (AIMS) for dyskinesia, 3) Schwab & England Activities of Daily Living Scale, and 4) Hospital Anxiety and Depression Scale (HADS).
Statistical analysisWe initially conducted a descriptive analysis. Continuous variables were expressed as means±SD and qualitative variables as frequencies and percentages. Given the small sample size, we used the non-parametric Friedman test followed by the Wilcoxon signed rank test for paired comparisons in order to analyse progression of the follow-up variables at different stages of the disease. The percentages of change between baseline status and status at 12 months were compared using the Mann–Whitney U test. We used R statistical software for analysis. Statistical significance was set at P<.05.
ResultsTwelve patients with APD were included in our study; 6 of them had moderate functional disability (group A) and the remaining 6 had severe functional disability (group B). Clinical and demographic characteristics of our sample are summarised in Table 1. Before CIA was started, all patients were receiving levodopa dosed at 728±100mg in group A and 925±100mg in group B. All patients in group A and one patient in group B also received pramipexole, and 4 patients in group B received rotigotine (as patches). Use of dopamine agonists progressively decreased during follow-up; these drugs were finally discontinued in all patients in group A at 6 months. Only one patient in group B continued to receive rotigotine dosed at 4mg.
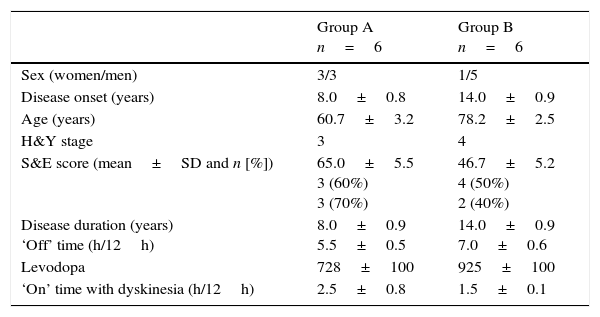
Baseline demographic and clinical characteristics of patients with APD by degree of functional disability: moderate (Group A) and severe (Group B).
| Group A n=6 | Group B n=6 | |
|---|---|---|
| Sex (women/men) | 3/3 | 1/5 |
| Disease onset (years) | 8.0±0.8 | 14.0±0.9 |
| Age (years) | 60.7±3.2 | 78.2±2.5 |
| H&Y stage | 3 | 4 |
| S&E score (mean±SD and n [%]) | 65.0±5.5 3 (60%) 3 (70%) | 46.7±5.2 4 (50%) 2 (40%) |
| Disease duration (years) ‘Off’ time (h/12h) | 8.0±0.9 5.5±0.5 | 14.0±0.9 7.0±0.6 |
| Levodopa | 728±100 | 925±100 |
| ‘On’ time with dyskinesia (h/12h) | 2.5±0.8 | 1.5±0.1 |
Levodopa doses were not changed significantly during the study period (12 months); however, the number and volume of rescue apomorphine injections did decrease progressively in both groups.
Apomorphine infusion speed at the beginning of the follow-up period was 0.4±0.2mL/h in group A and 0.4±0.2mL/h in group B. Infusion speed was increased progressively in both groups until reaching 1.2±0.2mL/h in group A and 0.9±0.1mL/h in group B by the end of the study period.
Regarding clinical variables, motor fluctuations in patients in group A had decreased by 63.9% at the last follow-up consultation. These fluctuations were reported to last approximately 2hours of every 12hours in the last follow-up visit, compared to 5.5hours in the baseline consultation (Table 2). During this period, dyskinesia decreased by 85.7% according to the AIMS; significant improvements were also observed in S&E rates (28.6%) (Table 2).
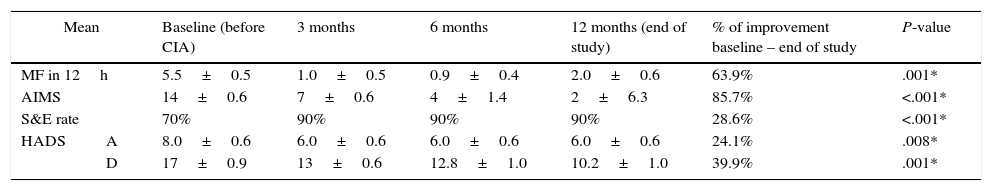
Follow-up results for motor symptoms, non-motor symptoms, and emotional status in patients with moderate functional disability (group A).
| Mean | Baseline (before CIA) | 3 months | 6 months | 12 months (end of study) | % of improvement baseline – end of study | P-value | |
|---|---|---|---|---|---|---|---|
| MF in 12h | 5.5±0.5 | 1.0±0.5 | 0.9±0.4 | 2.0±0.6 | 63.9% | .001* | |
| AIMS | 14±0.6 | 7±0.6 | 4±1.4 | 2±6.3 | 85.7% | <.001* | |
| S&E rate | 70% | 90% | 90% | 90% | 28.6% | <.001* | |
| HADS | A | 8.0±0.6 | 6.0±0.6 | 6.0±0.6 | 6.0±0.6 | 24.1% | .008* |
| D | 17±0.9 | 13±0.6 | 12.8±1.0 | 10.2±1.0 | 39.9% | .001* | |
Analysis performed using the Friedman test.
AIMS: Abnormal Involuntary Movement Scale; CIA: continuous infusion of apomorphine; MF: motor fluctuations; HADS: Hospital Anxiety (A) and Depression (D) Scale; S&E: Schwab & England Activities of Daily Living Scale.
Patients in group B also experienced improvements for all variables, although these were less marked. Motor fluctuations had decreased by 56.4% at 12 months of follow-up (from 7.0±0.6h/12h to 3.0±0.6h/12h); dyskinesia decreased by 74.0%, and S&E rates improved by 16.7%, with a mean independence rate of 70% at the end of the study period (Table 3).
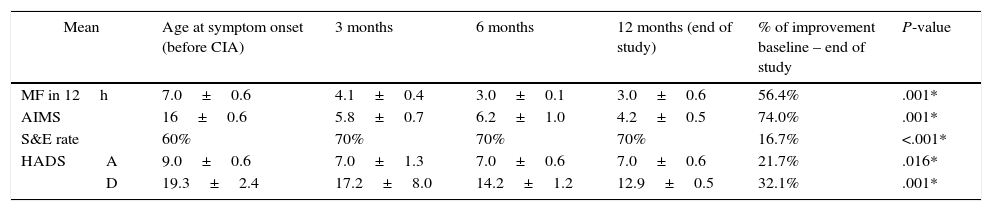
Follow-up results for motor symptoms, non-motor symptoms, and emotional status in patients with severe functional disability (group B).
| Mean | Age at symptom onset (before CIA) | 3 months | 6 months | 12 months (end of study) | % of improvement baseline – end of study | P-value | |
|---|---|---|---|---|---|---|---|
| MF in 12h | 7.0±0.6 | 4.1±0.4 | 3.0±0.1 | 3.0±0.6 | 56.4% | .001* | |
| AIMS | 16±0.6 | 5.8±0.7 | 6.2±1.0 | 4.2±0.5 | 74.0% | .001* | |
| S&E rate | 60% | 70% | 70% | 70% | 16.7% | <.001* | |
| HADS | A | 9.0±0.6 | 7.0±1.3 | 7.0±0.6 | 7.0±0.6 | 21.7% | .016* |
| D | 19.3±2.4 | 17.2±8.0 | 14.2±1.2 | 12.9±0.5 | 32.1% | .001* | |
Analysis performed using the Friedman test.
AIMS: Abnormal Involuntary Movement Scale; CIA: continuous infusion of apomorphine; MF: motor fluctuations; HADS: Hospital Anxiety (A) and Depression (D) Scale; S&E: Schwab & England Activities of Daily Living Scale.
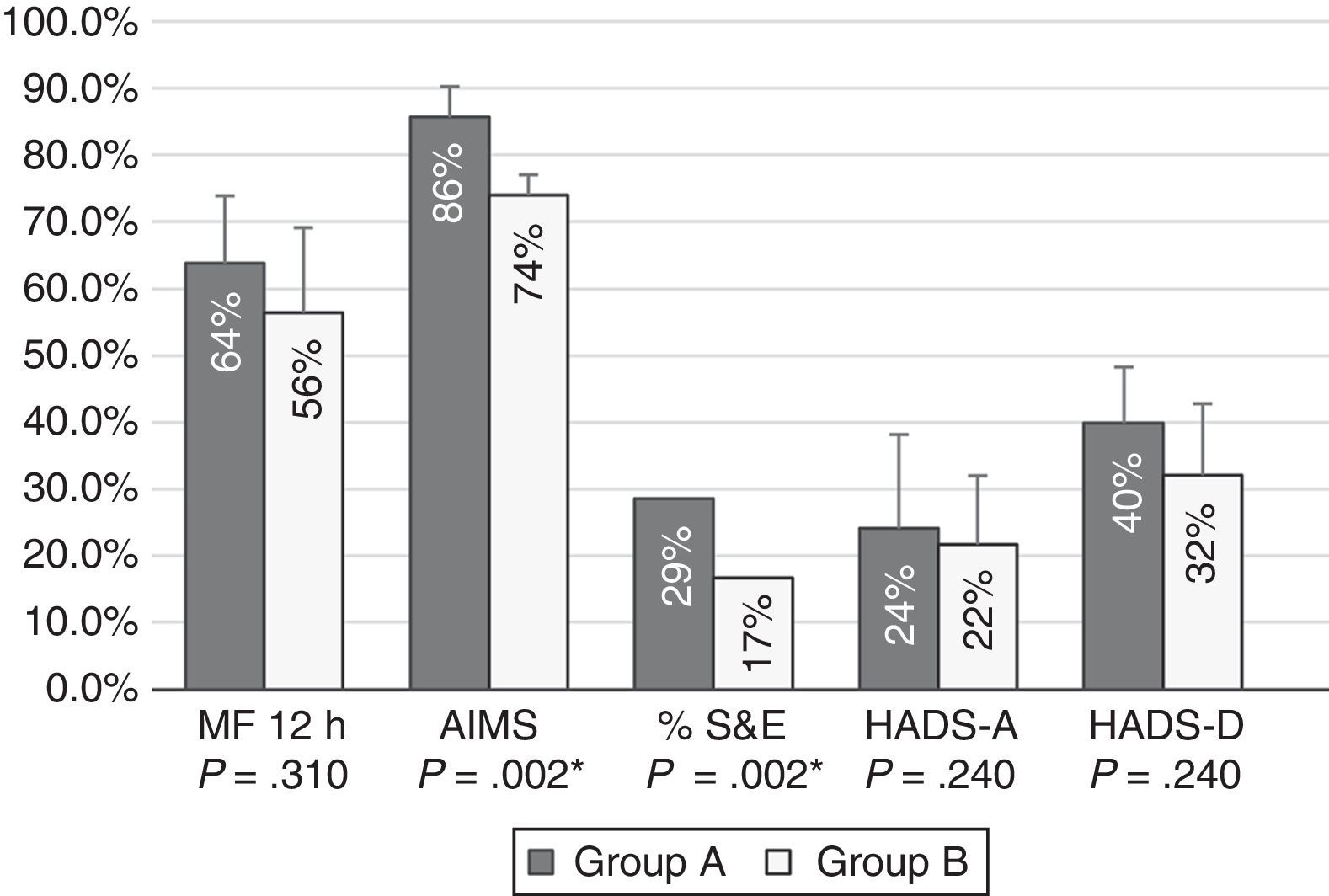
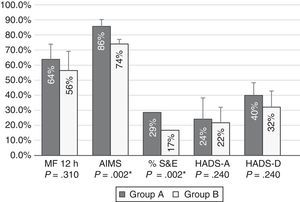
Patients with moderate functional disability achieved higher percentages of improvement after 12 months of treatment with CIA than patients with severe functional disability for all tests; differences were statistically significant for AIMS scores and S&E rates (Fig. 1).
Percentages of improvement in the outcome variables in patients with moderate (group A) and severe (group B) functional disability. Patients with moderate functional disability displayed more marked improvements in all areas; differences were statistically significant for motor fluctuations (MF), Schwab & England Activities of Daily Living Scale (S&E) rates, Abnormal Involuntary Movement Scale (AIMS) scores, and Hospital Anxiety and Depression Scale (HADS-A and HADS-B).
Regarding tolerance to treatment, 4 patients in group A had nausea, which was effectively controlled with 10mg domperidone every 8hours, with no need to reduce apomorphine doses. However, domperidone only achieved partial relief in 3 patients from group B who also experienced nausea; in these cases, symptoms were resolved by reducing the dose of CIA. None of the patients reported hallucinations, although 2 patients from group B experienced hypersexuality and delusion of jealousy; these symptoms improved when the dose was reduced, with no need for antipsychotics. Lastly, 4 patients developed small subcutaneous nodules8 (fewer than 3 palpable nodules in the infusion area) and displayed few or no skin marks. Nodules were painless, measured 1cm in diameter, showed no signs of local infection, and were largely inconsequential for patients and carers. Only 2 of these patients required treatment (anti-inflammatory ointments and ice) at the affected area.
DiscussionIn the past few years, several studies have proved the effectiveness of CIA for improving motor and non-motor symptoms and quality of life in patients with APD.9–11 However, the published studies addressing the effectiveness of CIA in APD display heterogeneous criteria for when to start treatment in terms of time since diagnosis or symptom progression. The patients included in these studies have severely impaired functions3–5; this seems to be linked to high rates of side effects or of loss of efficacy which lead to a short mean treatment duration (around 26 months) and treatment discontinuation.12
In our study, we observed that patients with APD and moderate functional disability experienced greater overall improvement than did those with severe functional disability during a one-year follow-up period (Fig. 1). This was true for scales measuring functional independence, motor fluctuations, dyskinesia, and emotional state.
At the beginning of the study period, patients with severe functional disability showed poorer executive function, greater gait instability, and more marked overall cognitive impairment leading to a higher level of dependence. We hypothesise that this greater impairment at baseline in group B prevented patients from displaying the more marked functional improvements as seen in group A. Some researchers have recently suggested that CIA should be considered for patients in earlier stages of the disease than is generally contemplated in current clinical practice.13–15
In our study, levodopa doses did not change significantly during the follow-up period; some dopamine agonists, however, were successfully discontinued. At the end of the follow-up period, patients with moderate functional disability were receiving higher apomorphine doses to control symptoms. One potential explanation is that, as a general rule, patients with more severe functional disability did not tolerate higher doses due to side effects, which were usually mild but nonetheless forced clinicians to administer lower doses. Lower doses are very probably responsible for the more discreet improvements in this group.
This study has the limitations inherent to a single-centre observational study with a small sample size; its results, however, offer preliminary data on a topic that should be thoroughly addressed. Our results suggest new treatment options for patients with APD and moderate functional disability who are resistant to conventional treatment, and complement the results of other studies addressing treatment alternatives for APD. Greater overall impairment may reduce the benefits of treatment with CIA in APD. These findings suggest that CIA may be more beneficial for eligible patients with APC who have moderate functional and cognitive impairment and are not severely dependent. This idea should be explored further in studies with larger sample sizes and longer follow-up times to deliver more robust conclusions.
FundingThis study was financed by Joan Costa Roma Foundation. Consorci Sanitari de Terrassa, Terrassa, Barcelona, Spain.
Please cite this article as: Salazar G, Martín J, Fragoso M, Font MA. Apomorfina en bomba de perfusión continua en pacientes con enfermedad de Parkinson avanzada con diferente grado de afectación funcional. Neurología. 2017;32:407–410.