Hemifacial spasm (HFS) is a debilitating disease characterized by involuntary tonic and clonic contractions of muscles innervated by the facial nerve. Botulinum toxin A (BTX-A) is the first-line option and the most effective medical treatment for HFS. The objective of this study was to evaluate the effect of BTX-A therapy on the physical and mental health of HFS patients.
MethodsParticipants included 65 HFS patients and 65 matched healthy controls in the study. Cornell Medical Index (CMI) self-assessment questionnaire was used to detect the psychological health of all participants. Local injection of BTX-A was applied, and the Cohen hierarchical criteria were employed to stratify the degree of spasticity, further evaluating the efficacy of BTX-A before and two months after treatment in HFS patients. The HFS patients at two months post-treatment were re-evaluated by CMI self-assessment questionnaire, and the evaluated factors of these patients were compared with those of patients before treatment.
ResultsThe scores of somatization, depression, anxiety, inadaptation, sensitivity, anger, tension, M-R, and total scores in the HFS group were significantly higher than those in the control group (all P<0.05). Two months post-treatment, among 65 HFS patients who received with BTX-A treatment, 42 (64.6%) were completely relieved, 16 (24.6%) were significantly relieved, 7 (10.8%) were partially relieved, and 0 (0%) cases were invalid, and the total effective rate was 89.2%. Two months after BTX-A treatment, the scores of somatization, tension, anxiety, depression, sensitivity, M-R and total scores of patients with HFS were lower than those before treatment (all P<0.05).
ConclusionsPatients with HFS are often accompanied by somatization, anger, inadaptation, sensitivity, anxiety, depression, and tension. BTX-A can not only alleviate the symptoms of HFS, but also improve the somatization, tension, anxiety, depression, and sensitivity.
El espasmo hemifacial (EH) es una enfermedad debilitante que se caracteriza por contracciones tónico-clónicas involuntarias de los músculos inervados por el nervio facial. La toxina botulínica A (TBA) es el tratamiento de primera línea y el más efectivo para el EH. El objetivo de nuestro estudio es analizar el efecto del tratamiento con TBA en la salud física y mental de los pacientes con EH.
MétodosIncluimos 65 pacientes con EH y 65 controles sanos en el estudio. Utilizamos un cuestionario de autoevaluación, el índice médico de Cornell (IMC), para determinar la salud psicológica de todos los participantes y realizar una comparación de los factores más relevantes. Inyectamos TBA localmente y aplicamos los criterios jerárquicos de Cohen para determinar el grado de espasticidad, evaluando la eficacia de la TBA a los dos meses de haber administrado el tratamiento. Igualmente, volvimos a usar el IMC en los pacientes con EH y comparamos los resultados con los obtenidos antes de administrar el tratamiento en este mismo grupo.
ResultadosLas puntuaciones en síntomas corporales, depresión, ansiedad, inadaptación, sensibilidad, irritabilidad, tensión, síntomas psicológicos (ítems M-R) y puntuación total en el grupo de pacientes eran significativamente superiores que las del grupo control (p < 0,05). A los dos meses del tratamiento, 42 pacientes presentaron una resolución total de los síntomas (64,6%), 16 una mejoría importante (24,6%) y siete una mejoría parcial (10,8%); en ningún caso se reportó un efecto nulo del tratamiento, por lo que la tasa de efectividad fue del 89,2%. Dos meses después del tratamiento con TBA, las puntuaciones en síntomas somáticos, tensión, ansiedad, depresión, sensibilidad, síntomas psicológicos (ítems M-R) y puntuación total de los pacientes con EH fueron significativamente menores a las obtenidas antes del tratamiento (p < 0,05).
ConclusionesLa EH a menudo también se manifiesta acompañada de síntomas corporales, irritabilidad, inadaptabilidad, sensibilidad, ansiedad, depresión y tensión. El tratamiento con TBA no solo puede aliviar los síntomas de la EH, sino también mejorar los síntomas corporales, tensión, ansiedad, depresión y sensibilidad.
Hemifacial spasm (HFS) is a chronic neurological disease, characterized by unconscious clonus and forced contraction of facial expression muscles innervated by the facial nerve.1 The generally considered inducement of HFS is an ectatic or aberrant blood vessel, which compresses the entry or exit zone of the nerve. HFS is more common in women than men, with a ratio of 1.5:1, and the average age of disease onset is about 55 years.2 Intermittent contractions cause sudden and involuntary eye closure, probably leading to social embarrassment. Compared to the average population, depression and anxiety are more prevalent among people with HFS, of which symptoms also adversely affect their quality of life.
HFS not only brings physical discomfort to patients but also causes severe social and psychological dysfunction.3 In recent years, several lines of studies have revealed that most HFS patients experience fear and tension, thus initiating emotional problems like anxiety and depression.4,5 These emotional problems would further increase the incidence of HFS, causing a vicious cycle.6,7 A case of psychological HFS with depression was first reported in 2001. Clinical findings showed that some HFS patients had subjective depressive symptoms during the consultation.8
Botulinum toxin A (BTX-A) is a promising biotoxin that functions on the presynaptic area of the neuromuscular junction and impedes the release of acetylcholine, which ultimately brings about functional reversible paralysis of the associated muscles.9 Regarding the symptomatic treatment of HFS, it has been proved that BTX injection is effective.10 Repeated injections have demonstrated a better ameliorative outcome on patients’ quality of life.9,11 BTX-A is regarded as the most popular treatment method for HFS. However, the alleviation of anxiety and depression in HFS patients after injection of BTX-A is still inadequately understood. Therefore, this study attempted to investigate the effect of BTX-A on the physical and mental health of patients with HFS. 65 patients with HFS were treated with BTX-A, and the Cornell Medical Index (CMI) self-assessment questionnaire was used for self-determination of health levels before and 2 months after BTX-A treatment.
Subjects and methodsParticipantsA total of 65 patients with HFS, who were first diagnosed in our hospital from April 2018 to December 2019, were selected for the HFS group. The group included 27 males and 38 females with an average age of (54.71±11.80) years. Accordingly, 65 healthy people were recruited as the control group during the same period. This group included 31 males and 34 females with an average age of (55.20±12.05) years. There was no significant difference in gender and age between the two groups (χ2=0.498, P=0.480; t=−0.235, P=0.814). The participants were diagnosed with HFS using the Consensus of Chinese Experts on Diagnosis and Treatment of Hemifacial Spasm.12 Except for patients with other related diseases that affect physical and mental health, all patients signed an informed consent form for BTX-A injection and participation in this study. All participants voluntarily took part in the self-assessment of the scale, except those who could not communicate or complete the assessment. Figure 1 shows the workflow of this study.
CMI self-assessmentThe factors such as somatization, fatigue and habit, previous health status and medical history, depression, anxiety, maladjustment, sensitivity, anger, tension, M-R value, and CMI total scores were statistically analyzed. Before BTX-A treatment, the CMI self-assessment questionnaire was used to conduct a preliminary evaluation of the participants and the factors of HFS group were compared with those of the control group. At two months after-treatment, patients in the HFS group were re-evaluated using the same CMI self-assessment questionnaire, and the factors were compared between before and after treatment.
Local injection of BTX-AAll patients were injected with BTX-A developed by Lanzhou Institute of Biological Products (Hengli, 100U/bottle) and diluted with sterile saline to 40U/ml before use. The injection of BTX-A was performed using a 1ml disposable sterile insulin syringe (Shurui) produced by BD Medical Instruments (Shanghai) Co., Ltd. Orbicularis oculi muscle, zygomatic muscle, orbicularis oris muscle, and mental muscle of the affected side were selected for injection according to the site and degree of spasm of the patient, as shown in Fig. 2. Each point was 2—4 U.
Schematic diagram of injection site and dosage of botulinum toxin A. Take the spasm of the left hemifacial muscle, including orbicularis oculi muscle, zygomatic muscle, orbicularis oris muscle, and mental muscle, as an example. Round: Required injection site; Square:Alternative injection site.
According to the Cohen et al.’s grading standard, the degree of spasticity is graded as follows: 0: normal; I: external stimulation to increase blinking; II: mild, no external stimulation, slight tremor of facial muscles and eyelids, no dysfunction; III: moderate, obvious spasticity accompanied by mild dysfunction; IV: severe, severe spasticity and dysfunction, affecting work and life).13 Curative effect is evaluated using the following criteria: (1) After treatment, the spasticity of patients was reduced from grade II–IV to grade as complete remission. (2) The spasticity was reduced from grade II–III to grade I or from grade IV to grade I–II as significant relief. (3) The spasticity was reduced from grade III to grade II or from grade IV to grade III as partial relief. (4) No change in the grade of spasticity was considered invalid. Total effective rate=(number of complete remission cases+ number of obvious remission cases)/total number of treatment cases.
Statistical analysesThe measurement data conforming to the normal distribution were represented as x¯±s. Student's t-tests were employed to evaluate differences between outcomes for each variable. Independent sample t-test was used to compare the means between the two groups, and the comparison before and after BTX-A treatment was determined by paired t-test. The measurement data inconsistent with the normal distribution were determined by Wilcox test. The counting data were expressed as a percentage (%), and the difference between the groups was measured by the χ2 test. Spearman correlation coefficients were calculated for the correlation. P<0.05 indicated statistical significance between or among compared groups. SPSS version 20 software package (SPSS, Chicago, IL, USA) was used for analysis.
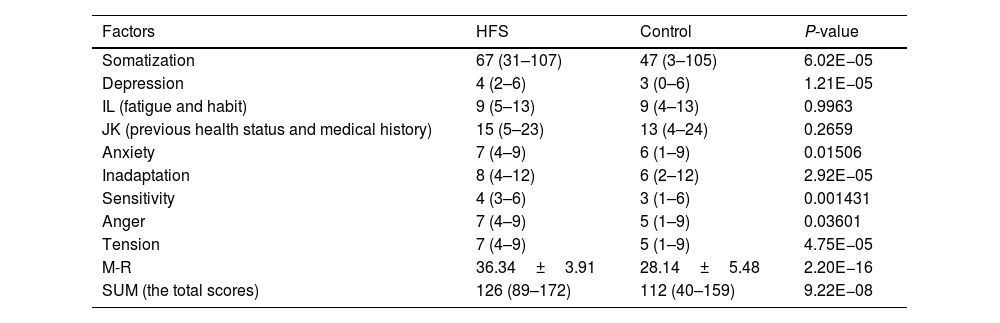
ResultsComparison of various factors of CMI self-assessment questionnaire between HFS group and control groupThe scores of somatization, depression, anxiety, inadaptation, sensitivity, anger, tension, and M-R and total scores in the HFS group were significantly higher than those in the control group (all P<0.05); There was no significant difference in scores of fatigue, habit, previous health status and medical history (all P>0.05); see Table 1 and Fig. 3.
Comparison of various factors of CMI self-assessment questionnaire between the HFS group and the control group (X¯±s).
| Factors | HFS | Control | P-value |
|---|---|---|---|
| Somatization | 67 (31–107) | 47 (3–105) | 6.02E−05 |
| Depression | 4 (2–6) | 3 (0–6) | 1.21E−05 |
| IL (fatigue and habit) | 9 (5–13) | 9 (4–13) | 0.9963 |
| JK (previous health status and medical history) | 15 (5–23) | 13 (4–24) | 0.2659 |
| Anxiety | 7 (4–9) | 6 (1–9) | 0.01506 |
| Inadaptation | 8 (4–12) | 6 (2–12) | 2.92E−05 |
| Sensitivity | 4 (3–6) | 3 (1–6) | 0.001431 |
| Anger | 7 (4–9) | 5 (1–9) | 0.03601 |
| Tension | 7 (4–9) | 5 (1–9) | 4.75E−05 |
| M-R | 36.34±3.91 | 28.14±5.48 | 2.20E−16 |
| SUM (the total scores) | 126 (89–172) | 112 (40–159) | 9.22E−08 |
CMI: Cornell Medical Index; HFS: hemifacial spasm.
Cohen grades before treatment in the HFS group were as follows: 8 cases of grade II, 43 cases of grade III, and 14 cases of grade IV. The average effect onset time of BTX-A injection was five days, and the effect was significant in about three weeks. After two months, 42 (64.6%) of the 65 patients had complete relief, 16 (24.6%) had significant relief, 7 (10.8%) had partial relief, and 0 (0%) were invalid. The total effective rate was 89.2%. See Table 2.
The therapeutic effect of BTX-A on HFS.
| Grades | Numbers (n) | Complete relief (n) | Significant relief (n) | Partial relief (n) | Invalid (n) | Valid (n) |
|---|---|---|---|---|---|---|
| II | 8 | 7 | 1 | 0 | 0 | 8 |
| III | 43 | 27 | 12 | 4 | 0 | 39 |
| IV | 14 | 8 | 3 | 3 | 0 | 11 |
| Summation | 65 | 42 (64.6%) | 16 (24.6%) | 7 (10.8%) | 0 (0%) | 58 (89.2%) |
BTX-A: botulinum toxin A; HFS: hemifacial spasm.
Before BTX-A treatment, the somatization, previous health status and medical history, depression, anxiety, inadaptation, sensitivity, anger, tension, M-R, and total scores of patients in the HFS group were significantly correlated with Cohen's grades (all P<0.05). See Fig. 4.
Comparison of various factors of CMI self-assessment questionnaire in HFS patients before and after BTX-A treatmentThe scores of somatization, tension, anxiety, depression, and sensitivity in patients with HFS at two months post-treatment with BTX-A were significantly lower than those before treatment (all P<0.05). There was no significant difference in scores of fatigue and habits, previous health status and medical history, inadaptation, and anger after BTX-A treatment compared with those before treatment (all P>0.05). See Table 3 and Fig. 5.
Comparison of various factors of CMI self-assessment questionnaire in HFS patients before and after BTX-A treatment (X¯±s).
| Factors | Before treatment | After treatment | P-value |
|---|---|---|---|
| Somatization | 67 (31–107) | 55 (20–100) | 3.69E−02 |
| Depression | 4 (2–6) | 3 (1–5) | 1.84E−04 |
| IL (fatigue and habit) | 9 (5–13) | 8 (5–13) | 0.4124 |
| JK (previous health status and medical history) | 15 (5–23) | 14 (6–23) | 0.2774 |
| Anxiety | 7 (4–9) | 5 (3–8) | 8.31E−04 |
| Inadaptation | 8 (4–12) | 8 (3–12) | 5.37E−01 |
| Sensitivity | 4 (3–6) | 3 (2–5) | 3.21E−04 |
| Anger | 7 (4–9) | 6 (4–8) | 1.09E−01 |
| Tension | 7 (4–9) | 5 (3–8) | 2.49E−06 |
| M-R | 36.34±3.91 | 31.54±3.98 | 2.03E−08 |
| SUM (the total scores) | 126 (89–172) | 113 (67–158) | 1.27E−03 |
CMI: Cornell Medical Index; HFS: hemifacial spasm; BTX-A: botulinum toxin A.
Although HFS is not fatal, it can have a relatively significant effect on patients’ quality of life. Patients cannot control their HFS. If it is not effectively managed for a long time, HFS can change the patient's appearance and cause emotional problems such as anxiety and depression.14 The etiology of HFS is not completely clear, and compression of the facial nerve byabnormal venous or arterial loops is considered to be the most common cause.15,16 Treatments of HFS mainly include oral drugs, local injection of BTX-A,11,17 and microvascular decompression.18,19 Studies have shown that oral medications have poor therapeutic effects and large side effects. Microvascular decompression surgery can result in cerebrospinal fluid leakage and cranial nerve damage and postoperative symptoms of some patients cannot be fully improved. Local injection of BTX-A has been the preferred treatment for many patients with positive efficacy and low side effects.20 This study showed that the average scores of depression, anxiety, inadaptation, sensitivity, anger, tension, and somatization of HFS patients are significantly higher than those of healthy people. And the somatization, previous health status and medical history, depression, anxiety, inadaptation, anger, tension, M-R, and total scores were significantly correlated with Cohen grades in the HFS group. In addition, we found that BTX-A has significant benefits for HFS patients. It leads to a decrease in the scores of somatization, tension, anxiety, depression, and sensitivity after treatment.
Patients with HFS tend to focus only on physical discomfort and rarely mention psychological and spiritual issues. The CMI self-assessment questionnaire is currently a widely used checklist for mental illness and mental disorders. In this study, we used the CMI self-assessment questionnaire to conduct self-evaluation among all research subjects and compared various factors. Our results showed that average scores of somatization, interpersonal sensitivity, depression, anxiety, and phobia in patients with HFS were higher than those of normal volunteers, indicating that patients had the obvious tendency of somatization, interpersonal sensitivity, depression, anxiety, and inadaptation at patient's first clinical visit. One of the possible reasons we assumed is that HFS affects the patient's self-image, influences the patient's satisfaction of life, and promotes the development of related mental and psychological problems. Second, previous studies have found that the brains of patients with HFS were involved in activating the hemifacial motor cortex and increasing the excitability of the gray matter nuclei, while the function of inhibiting the hemifacial motor cortex facial motor cortex was weakened.21 It was suggested that the central nervous system was involved in the pathogenesis of HFS and it may also be involved in the mental and psychological changes of HFS patients. Evaluation results of Chengyun Wang et al. found that average scores of interpersonal sensitivity, phobia, anxiety, depression, and somatization of HFS patients were significantly higher than those of healthy people,20 in line with our reports. In addition, because female patients, especially young female patients, pay more attention to their appearance, their HFS’mental and psychological impact on them may be more prominent. It is known that most HFS patients are middle-aged and elderly, and many female patients are in menopause, which may aggravate their mental and psychological problems. This study is only an overall analysis of patients with HFS and failed to discuss the mental and psychological problems between males and females. It is expected that a more detailed analysis can be conducted in subsequent studies.
BTX-A, produced by gram-positive anaerobic Clostridium bacteria, prevents the release of acetylcholine from nerve endings, causing muscle relaxation and paralysis.22 In line with the study by Batisti et al. and Xing et al, our research reveals that BTX-A has a considerable treatment effect and low side effects in the therapy of HFS.11,23 The ability of BTX-A to relieve anxiety and depression in patients with HFS was further supported in this study. BTX-A alleviates somatization, tension, anxiety, depression, sensitivity, and other psychiatric problems, which may be due to the relief of HFS symptoms, reducing it's negative impact on patients. In the future, more studies on the mechanisms of BTX-A involvement in HFS should be conducted.
In summary, patients with HFS are frequently coupled with somatization, interpersonal sensitivity, depression, anxiety, anger, tension, and inadaptation. BTX-A exhibits potent efficacy and safety with low side effects in the treatment of HFS, alleviating somatization, tension, anxiety, depression, and sensitivity. BTX-A plays an important role in improving HFS patients’ quality of life and promoting their physical and mental health.
Availability of data and materialsThe analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Ethics approvalThis study was approved by the Ethics Committee of Huai’an First People's Hospital. All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participateThe written informed consent to participate was provided by all participants.
FundingThis work was supported by Science and Technology Development Fund of Nanjing Medical University, China (No.NMUB2020146).
Conflict of interestNone.
None.