Treating systemic inflammation caused by SARS-COV 2 (COVID-19) has become a challenge for the clinician. Corticosteroids have been the turning point in the treatment of this disease. Preliminary data from Recovery clinical trial raises hope by showing that treatment with dexamethasone at doses of 6mg/day shows a reduction on morbidity in patients requiring added oxygen therapy. However, both the start day or what kind of corticosteroid, are still questions to be clarified. Since the pandemic beginning, we have observed large differences in the type of corticosteroid, dose and initiation of treatment.
Our objective is to assess the predictive capacity of the characteristics of patients treated with methylprednisolone pulses to predict hospital discharge.
Materials and methodsWe presented a one-center observational study of a retrospective cohort. We included all patients admitted between 03/06/2020 and 05/15/2020 because of COVID-19. We have a total number of 1469 patients, of whom 322 received pulses of methylprednisolone. Previous analytical, radiographic, previous disease data were analyzed on these patients. The univariant analysis was performed using Chi-squared and the T test of Student according to the qualitative or quantitative nature of the variables respectively. For multivariate analysis, we have used binary logistic regression and ROC curves.
ResultsThe analysis resulted statistically significant in dyspnea, high blood pressure, dyslipidemia, stroke, ischemic heart disease, cognitive impairment, solid tumor, C-reactive protein (CRP), lymphopenia and d-dimer within 5 days of admission. Radiological progression and FIO2 input are factors that are associated with a worst prognosis in COVID-19 that receive pulses of methylprednisolone. Multivariate analysis shows that age, dyspnea and C-reactive protein are markers of hospital discharge with an area below the curve of 0.816.
ConclusionsIn patients with methylprednisolone pulses, the capacity of the predictive model for hospital discharge including variables collected at 5 days was (area under the curve) 0.816.
Tratar la inflamación sistémica producida por el SARS-COV 2 (COVID-19) se ha convertido en un reto para el clínico. Los corticoides han sido el punto de inflexión en el tratamiento de esta enfermedad. Los datos preliminares del ensayo clínico Recovery alentan esperanza mostrando que con el tratamiento con dexametasona a dosis de 6 mg/día hay una disminución de la morbimortalidad en pacientes que requieren oxigenoterapia añadida. Sin embargo, tanto el día de inicio, o qué tipo de corticosteroide, son todavía preguntas por aclarar. Desde el inicio de la pandemia hemos observado grandes diferencias en cuanto al tipo de corticoide, dosis e inicio de tratamiento.
Nuestro objetivo es valorar la capacidad predictiva de las características de los pacientes tratados con bolos de metilprednisolona para predecir el alta hospitalaria.
Materiales y métodosPresentamos un estudio unicéntrico observacional de cohorte retrospectiva. Incluimos a todos los pacientes ingresados entre el 06/03/2020 y el 15/05/2020 por COVID-19. Contamos con un número total de 1469 pacientes, de los cuales 322 recibieron pulsos de metilprednisolona. De estos pacientes se analizaron los datos clínicos, analíticos, radiográficos, enfermedades previas. El análisis univariante se realizó mediante Chi cuadrado y el test t de Student según la naturaleza cualitativa o cuantitativa de las variables respectivamente. Para el análisis multivariante hemos empleado la regresión logística binaria y las curvas ROC.
ResultadosEn el análisis resultó estadísticamente significativo la disnea, hipertensión arterial, dislipemia, accidente cerebrovascular, cardiopatía isquémica, deterioro cognitivo, tumor sólido, la proteína C reactiva (PCR), linfopenia y d-dímero a los 5 días de ingreso. La progresión radiológica y de aporte de FIO2 son factores que se asocian a peor pronóstico en la COVID-19 que reciben pulsos de metilprednisolona. En el análisis multivariante se observa que la edad, disnea y la proteína C reactiva son marcadores de alta hospitalaria con un área bajo la curva de 0,816.
ConclusiónEn pacientes con bolos de metilprednisolona, la capacidad del modelo predictivo del alta hospitalaria incluyendo variables recogidas a los 5 días ha sido (Área Bajo la Curva) de 0.816.
At the end of December 2019, the first cases of severe viral pneumonia of unknown etiology were reported in China and weeks later it was confirmed that it was a new coronavirus, called SARS-CoV-2.1
Coronaviruses receive their name by crown-shaped microscopic morphology on their surface, and there are four main subgroups of coronavirus known as alpha, beta, gamma, and delta. SARS-CoV-2 belongs to the beta group and is one of the seven coronaviruses that infect humans.2 SARS-CoV-2 shares many genetic characteristics with other similar viruses, such as SARS-CoV and MERS-CoV. As they are peer viruses, they also have shared clinical characteristics in terms of their behavior.1,3
Due to this genetic and clinical similarity between SARS-CoV-2, SARS-CoV and MERS-CoV, and the absence of scientific evidence on the benefits of glucocorticoid use in the treatment of viral pneumonia, this therapy was initially not recommended for its management.4–6
In addition, some studies defend that the use of corticosteroids could delay viral clearance and even increase mortality.1,4,7,8
Over time we have gained more knowledge about SARS-COV2, both in pathological behavior and clinical spectrum.
Currently, two main phases have been defined in the pathogenesis of the disease, the first, in which direct damage by the virus predominates, and the second due to the hyper-inflammatory response of the host. These phases are usually overlaid for a few days, in which the patient may develop an intermediate phase that has a pulmonary commitment to or without hypoxia and slightly elevated analytical inflammation markers.9–11
In the second phase of the disease, there is extrapulmonary systemic hyperinflammation in which there is a decrease in T-cell count and a storm of inflammatory cytokines that overshadow the prognosis of the disease.
Cytokine storm is a very serious complication in SARS-CoV-2 infection by in many cases committing the lives of patients.12
Several articles have been published on the beneficial use of glucocorticoids, including the randomized recovery (Randomized Evaluation of COVID-19 therapy), which was initiated in March 2020 to approve potential treatments for COVID-19 in a total of 176 hospitals in the UK, which has shown that the use of dexamethasone at doses of 6mg/day reduces mortality, the need for mechanical ventilation and oxygen therapy.13
The doses of corticosteroids used have been very varied. The study made by Callejas R et al. used pulses of methylprednisolone doses of 2mg/kg/d, 250mg/d or 500mg/d for 3 days in patients meeting cytokine release syndrome (FTA) criteria defined as an elevation of IL6>40pg/ml and/or 2 of the following: ferritin>300μg/l, d-dimer>1mg/l, triglycerides>300mg/dl. This study found that pulses of methylprednisolone could decrease the outcome of intubation or death.14
The poor prognostic risk factors present in the different patients admitted because of SARS-COV-2 pneumonia such as age, diabetes, hypertension and tobacco, together with the variability of response to hospital treatment makes it essential to find which factors are associated with a better pharmacological response.15
The clear benefit of corticosteroids in SARS-COV-2 disease, as well as in viruses counterpart to it, is likely to be based on three fundamental pillars: the dose, when to start and the patient's profile better corticoid responder.
The objective of our study was to analyze the factors that influence the response to methylprednisolone pulses and determine whether there is a mathematical model to predict that response.
MethodsThis is an observational retrospective cohort study in which patients admitted to the La Mancha Centro General Hospital (HGMC) in Alcázar de San Juan with a diagnosis of respiratory infection or SARS-CoV-2 pneumonia were included between 03/06/2020 and 05/15/2020. A total of 1469 patients were collected from these receiving pulses of methylprednisolone 322.
In all patients, the diagnosis of COVID-19 was confirmed by nucleic acid detection amplification test (RT-PCR) or by a rapid antigenic test with nasopharyngeal swabs.
Patients were treated at doses of 250mg/24h/3 days or 500mg/24h/3 days according to the hospital protocol that allowed these doses to be used, all patients have been grouped for statistical analysis. The characteristics have been explored by grouping patients into hospital discharge and deceased.
Demographic characteristics, personal history such as cardiovascular risk factors (arterial hypertension, diabetes, dyslipidemia, tobacco), lung diseases (chronic obstructive pulmonary disease (COPD) asthma), stroke, ischemic heart disease, cognitive impairment, solid tumor, autoimmune diseases, as well as previous treatments were collected.
In addition, we analyzed the clinical characteristics of the disease (such as symptoms, start date, progressive oxygen therapy needs). Analytical data at admission, at five days of stay and discharge, including hemoglobin levels, absolute lymphocytes, creatinine, transaminases, C-reactive protein (CRP), D-dimer, ferritin, lactate dehydrogenase (LDH) and Interleukin 6 (IL6).
We checked for differences in the degree of pulmonary involvement, based on X-ray findings using chest X-rays upon entry and discharge. As a radiological criterion for assessing the degree of pulmonary involvement by COVID-19, we used the scale proposed by Warren et al. called RALE score (Radiographic Assessment of lung), used for radiographic evaluation in pulmonary edema. A score of 0–4 was assigned for each lung depending on the affected lobes, with 0-normal and 4 being an affectation greater than 75%.16
Statistical analysisQualitative variables were analyzed using the Chi-square test and the quantitative variables Student's T test.
To compare which variables were independently associated with the final result of our study (hospital discharge), a binary logistic regression analysis was performed, with those variables that were statistically significant from the univariate analysis previously performed with a p<0.05. Using the ROC curve, we analyzed the sensitivity and specificity of the equation generated by logistic regression. The statistical analysis was performed with the SPSS version 21.0 program.
Results1469 patients were admitted in Hospital General Mancha Centro during the study period. Of these, 322 received pulses of methylprednisolone and were included in our study. Finally, 247 patients (76.7%) were discharged and 75 died (23.3%).
The main clinical and demographic characteristics of the patients included are presented in Table 1. Briefly, the mean age was 68.4±15.2 years (rank 1–99). Patients who died were older (mean [SD] age, 76.5 [11.1] years vs. 65.9 [15.4] years; p<0.001) than patients who survived. In our study, we have not observed significant differences in sex in patients where corticosteroid bolus was used (50.9% were male and 49.1% were female) neither in their mortality rates.
Demographic, baseline comorbidities and clinical characteristics at admission of patients with COVID-19, and differences between patients who survived and dead. COPD: chronic obstructive pulmonary disease.
| Overall (n=322) | Dead (n=75) | Discharged (n=247) | p | |
|---|---|---|---|---|
| Mean age at admission, years (SD; rank) | 68.4 (15.2; 1–99) | 76.5 (11.1; 47–99) | 65.9 (15.4; 1–96) | <0.001 |
| Sex | ||||
| Male, n (%) | 164 (50.9%) | 43 (57.3%) | 121 (49%) | 0.205 |
| Female; n (%) | 158 (49.1%) | 32 (42.7%) | 126 (51%) | |
| Smoker | ||||
| No; n (%) | 138 (61.1%) | 27 (46.6%) | 111 (66.1%) | 0.019 |
| Yes; n (%) | 19 (8.4%) | 5 (8.6%) | 14 (8.3%) | |
| Former; n (%) | 69 (30.5%) | 26 (44.8%) | 43 (25.6%) | |
| No data; n (%) | 96 (29.8%) | 17 (22.7%) | 79 (32%) | – |
| Arterial hypertension; n (%) | 194 (60.2%) | 60 (80%) | 134 (54.3%) | <0.001 |
| Dyslipidemia; n (%) | 107 (33.2%) | 32 (42.7%) | 75 (30.4%) | 0.048 |
| Diabetes; n (%) | 102 (31.7%) | 29 (38.7%) | 73 (29.6%) | 0.137 |
| Obesity; n (%) | 63 (19.6%) | 16 (21.3%) | 47 (19%) | 0.659 |
| COPD; n (%) | 30 (9.3%) | 10 (13.3%) | 20 (8.1%) | 0.172 |
| Asthma; n (%) | 17 (5.3%) | 3 (4%) | 14 (5.7%) | 0.771 |
| Cerebrovascular accident; n (%) | 19 (5.9%) | 8 (10.7%) | 11 (4.5%) | 0.048 |
| Ischemic heart disease; n (%) | 35 (10.9%) | 14 (18.7%) | 21 (8.5%) | 0.013 |
| Venous thromboembolic disease; n (%) | 14 (4.3%) | 4 (5.3%) | 10 (4%) | 0.746 |
| Cognitive impairment; n (%) | 30 (9.3%) | 12 (16%) | 18 (7.3%) | 0.023 |
| Peripheral vascular disease; n (%) | 10 (3.1%) | 1 (1.3%) | 9 (3.6%) | 0.312 |
| Chronic kidney disease; n (%) | 28 (8.7%) | 9 (12%) | 19 (7.7%) | 0.246 |
| Autoimmune disease; n (%) | 15 (4.7%) | 2 (2.7%) | 13 (5.3%) | 0.543 |
| Solid tumor; n (%) | 41 (12.7%) | 15 (20%) | 26 (10.5%) | 0.031 |
| Leukemia; n (%) | 7 (2.2%) | 3 (4%) | 4 (1.6%) | 0.360 |
| Symptoms | ||||
| Cough; n (%) | 193 (59.9%) | 45 (60%) | 148 (59.9%) | 0.990 |
| Fever; n (%) | 199 (61.8%) | 50 (66.7%) | 149 (60.3%) | 0.322 |
| Dyspnea; n (%) | 205 (63.7%) | 61 (81.3%) | 144 (58.3%) | <0.001 |
| Chest pain; n (%) | 22 (6.8%) | 5 (6.7%) | 17 (6.9%) | 0.948 |
| Ageusia; n (%) | 11 (3.4%) | 2 (2.7%) | 9 (3.6%) | 0.999 |
| Anosmia; n (%) | 11 (3.4%) | 0 | 11 (4.5%) | 0.074 |
| Diarrhea; n (%) | 41 (12.7%) | 8 (10.7%) | 33 (13.4%) | 0.540 |
| Vomiting; n (%) | 20 (6.2%) | 6 (8%) | 14 (5.7%) | 0.426 |
| Syncope; n (%) | 6 (1.9%) | 1 (1.35) | 5 (2%) | 0.999 |
The most common comorbidities in our patients were hypertension (60.2%), dyslipidemia (33.2%), diabetes (31.7%), and obesity (19.6%). A significant higher frequency of hypertension (80% vs. 54.3%; p<0.001), dyslipidemia (42.7% vs. 30.4%; p=0.048), cerebrovascular accident (10.7% vs. 4.5%; p=0.048), ischemic heart disease (18.7% vs. 8.5%; p=0.013), venous thromboembolic disease (16% vs. 7.3%; p=0.023) and solid tumors (20% vs. 10.5%; p=0.031) was observed among those patients who died compared to those who survived. The main symptoms were dyspnea, fever and cough (in 60% of patients), although only dyspnea was higher in patients who died compared to survivors (81.3% vs. 58.3%; p<0.001). Less frequently, the patients experienced chest pain, ageusia, anosmia, diarrhea, nausea and vomiting or syncope (Table 1).
Laboratory findings at admission and at 5-days are presented in Table 2. Patients with methylprednisolone pulses who died from COVID-19 infection had a elevation of lymphopenia, creatinine, D-dimer, AST and CRP values in comparison with discharged patients, both on admission and after 5 days (p<0.05). Parameters such as ferritin, hemoglobin, and ALT were not significant in our study.
Laboratory values, expressed as median±interquartile range, at admission and 5 days of patients with COVID-19 overall, and in those who survived or died.
| Overall | Dead | Discharged | p | |
|---|---|---|---|---|
| Admission | ||||
| Radiography at admission | ||||
| Normal (0 points) | 27 (8.6%) | 1 (1.3%) | 26 (10.9%) | <0.001 |
| Mild (1–2 points) | 72 (23%) | 11 (14.7%) | 61 (25.6%) | |
| Moderate (3–6 points) | 151 (48.2%) | 36 (48%) | 115 (48.3%) | |
| Severe (>6 points) | 63 (20.1%) | 27 (36%) | 36 (15.1%) | |
| Hemoglobin, g/dL | 13±2.6 | 13±3 | 13±2.5 | 0.899 |
| Lymphocytes, 103/μL | 0.9±0.7 | 0.6±0.75 | 1±0.6 | <0.001 |
| Creatinine, mg/dL | 0.8±0.4 | 1.1± 0.5 | 0.9±0.4 | <0.001 |
| D-dimer, ng/mL | 1.1±1.3 | 2.1±2.5 | 0.9±1 | <0.001 |
| Ferritin, ng/mL | 584±686 | 576.5±881 | 584±661 | 0.733 |
| AST, U/L | 29±24.2 | 39±33 | 27±23 | 0.003 |
| ALT, U/L | 26±29 | 27±27 | 25±29 | 0.756 |
| C-reactive protein, mg/L | 6.5±12.2 | 16±21.4 | 6±9 | <0.001 |
| 5 days | ||||
| Hemoglobin, g/dL | 12.6±2.8 | 12.3±3 | 12.7±2.9 | 0.904 |
| Lymphocytes, 103/μL | 0.9±07 | 0.55±0.6 | 1±2.4 | 0.007 |
| Creatinine, mg/dL | 0.8±0.3 | 1±0.6 | 0.8±0.4 | 0.022 |
| D-dimer, ng/mL | 1±2.1 | 2.5±5.3 | 0.9±2 | 0.025 |
| Ferritin, ng/mL | 650±1187 | 563±1300 | 669±1206 | 0.999 |
| AST, U/L | 28±24 | 38±62.5 | 27±21 | 0.038 |
| ALT, U/L | 28.5±40 | 41±40.8 | 28±40 | 0.300 |
| C-reactive protein, mg/L | 3.3±6 | 11.1±19.3 | 3.1±5 | 0.034 |
We also see in Table 2, patients with the greatest involvement in chest X-ray during admission had a higher probability to death. However, patients with normal or mild chest X-ray at the onset of the disease were more likely to be hospital discharged (p<0.001). In this sense, patients who needed a progressive increase in oxygen therapy during admission had a higher probability of dying (58.7% vs. 27.1%; p<0.001).
Finally, a binary logistic regression was performed, obtaining a predictive model in which those patients with lower age (OR: 0.933 [95%CI: 0.902–0.965]), less elevated PCR at admission (OR: 0.916 [0.886–0.948]) and not suffer from dyspnea (OR: 0.383 [0.164–0.894]) have a higher rate of hospital discharge (Table 3).
The resulting equation for determining the good response to corticosteroid bowling is Hospital discharge is 8202+(Age*−0.069)+(CRP*−0.087)+(Dyspnea*−0.959).
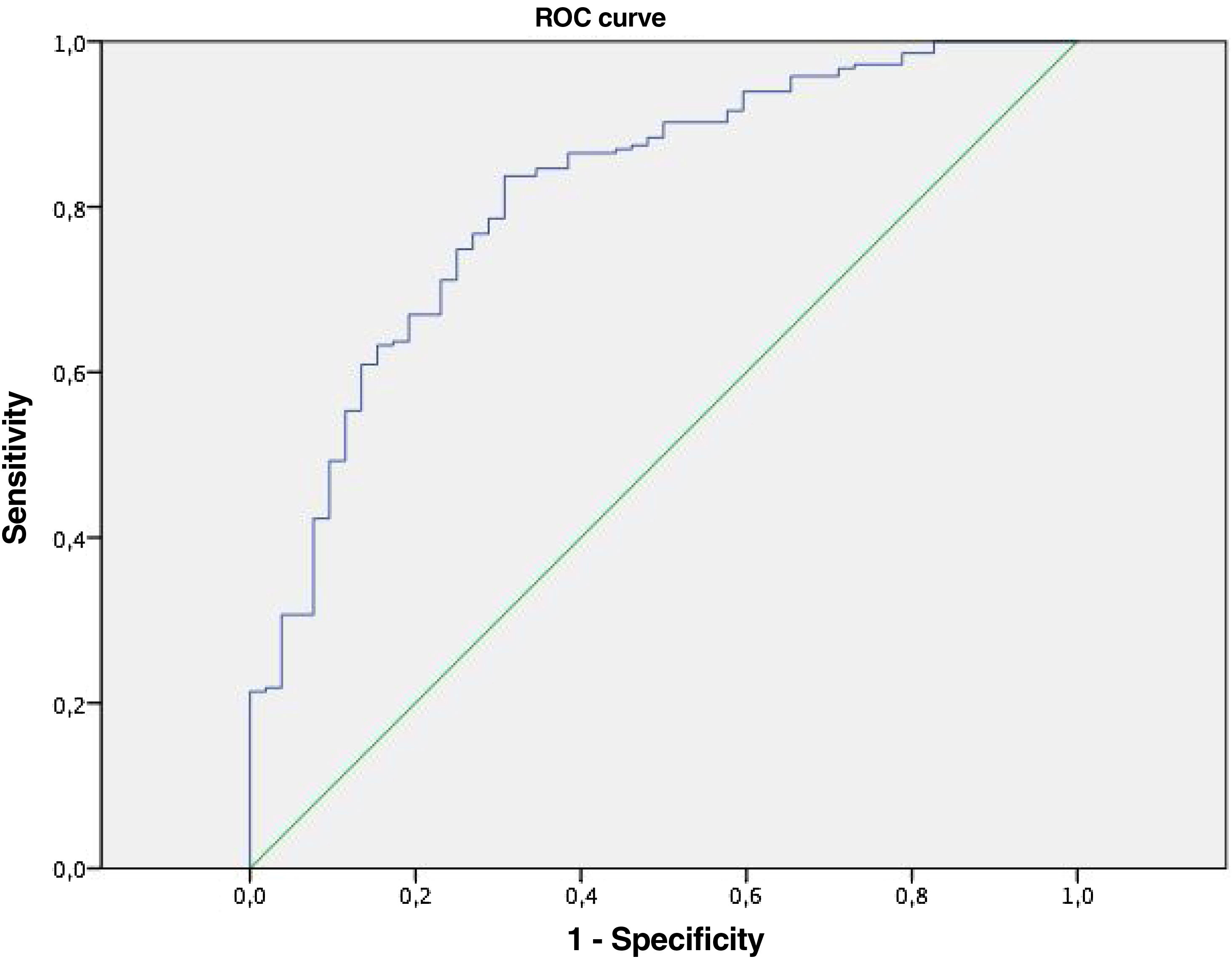
To establish sensitivity and specificity for the use of our equation we make an ROC curve, resulting in an area under the curve of 0.816 (Fig. 1) with a confidence interval (95% CI: 0.751–0.880).
DiscussionThe benefit of corticosteroids in SARS-COV-2 infection could be defined based on three factors: the dose, the day of initiation and the profile of the responding patient to these.
In our study, we try to find the patient profile best responding to pulses of methylprednisolone as a treatment for SARS-CoV-2 infection.
We have observed with high sensitivity and specificity that, at a lower age and lower value of the C-reactive protein (CRP) at admission and not suffer from dyspnea, better response to corticosteroid pulses, understood as a result of hospital discharge.
Ripe old age presents an increased risk of serious illness or even death in COVID-19, this could be related to immunosenescence. Immunosenescence results in a decrease in CD3+ T cell generation and a loss of CD8+ T cells resulting in an inversion of the usual CD4/CD8 ratio. In addition, there is an increase in regulatory T cells and a decrease in B lymphocytes.
Wu et al. in their retrospective cohort study observed how elderly patients had an increased risk of acute respiratory distress syndrome and death and people who survived in their cohort had higher levels of CD8 T cells significantly.17,18
Fan Wang et a establishes in his study a high CD4/CD8 ratio, as immunosenescence has been linked to greater severity in SARS-CoV-2 infection. In addition, it identified the decrease or loss of T CD8+ as an independent factor of gravity by COVID-19.
Therefore, lymphocyte dysregulation could be the point of similarity of poor prognosis between elderly patients and patients severely ill with COVID-19.19
One of the main mechanisms by which corticosteroids suppresses inflammation is to prevent access of lymphocytes and macrophages to the inflammatory site, causing transient lymphopenia after treatment and may contribute to this lymphocyte dysregulation.20,21
In Sterne's meta-analysis22 which includes up to 55 studies, the association between corticosteroids and mortality, OR was 0.69 (95% CI, 0.51–0.93) among 880 patients over 60 years of age, OR was 0.67 (95% CI, 0.48–0.94) among 821 patients 60 years of age or younger (OR ratio, 1.02 [95% CI, 0.63–1.65], p×0.94). According to this meta-analysis, corticosteroid treatment in patients between 60 years of age or younger does not affect mortality as its OR value is close to 1. For patients over 60 years old, the effect of corticosteroid therapy has a protective effect since the value of OR was 0.67. However, these results must be interpreted considering their confidence interval.
In our study the meaning is aimed at young patients, it can largely be because the epidemiological characteristics of our environment tend to an average age of about 66 years which is in line with the results of this study.
In the RECOVERY study (with an average age of 66 years with a 15-year-old ID), shows that the use of dexamethasone for up to 10 days among patients hospitalized by COVID-19 showed a reduction in mortality to 28 days.
Age-related outcomes were divided into three age groups whose results were RR close to one for patients between 70 and 80 years, indicating that there is association. Corticosteroid therapy is a protective factor for patients with COVID under 70 years of age, which is in the same trend as our study with an RR of 0.64.
C-reactive protein (CRP) is a marker that reflects systemic inflammation, as well as being a poor prognosis factor in SARS-COV-2 acute inflammatory response syndrome. We have observed that at lower CRP within 5 days of admission better corticoid response and a greater likelihood of receiving hospital discharge.
Li Yan et al. establish in their study a rapidly used gravity model for the first emergency assessment of the patient with SARS-CoV-2 infection from 3 analytic parameters: CRP, LDH and lymphopenia.23
Other predictive models such as Allenbach et al.24 define as a high-risk group both age and CRP, lymphopenia, IL-6 levels and radiological impact. It also defines as independent factors that are associated with poor prognosis >60 age and elevated CRP levels.
In our study the lymphopenia at admission was not significant but it did show differences within 5 days of admission darkening the prognosis of patients with a higher rate of death. We continue to collect data in this regard to optimize the predictive model.
Dong Ji et al.25 establishes a predictive risk model called CALL (Comorbidity, Age, Lymphocyte and LDH), where it predicts with a ROC area of 0.91 that patients with comorbidity (hypertension, diabetes, cardiovascular disease, liver disease, asthma, chronic lung disease, HIV infections and malignancy for at least 6 months), elderly (>60 years), lymphopenia (<1000) and increased Lactate Dehydrogenase (LDH) (>500U/l) predict a poor evolution of the disease.
Ze Chen et al.26 develop the latest available predictive gravity model, called OURMAPCN score based on baseline data on entry. This predictive model was created from 6415 patients from a Wuhan cohort and validated with two patient cohorts one Italian and one from independent sites in China. Ze Chen et al. tried to facilitate the use of their tool by dichotomizing their 8 variables used. These variables included age >60 and CRP>Upper Mortality Limit (LSM) as severity criteria.
As for autoimmune diseases (EI) and their association with good response to corticosteroid pulses in our study was not significant. Published articles state that patients with autoimmune diseases are at a lower risk of entering an ICU and needing VMNI. This could be because in this study most patients were undergoing treatment based on corticotherapy and classic (non-biological) disease-modifying rheumatologically drugs.12,27
Therefore, most predictive models state that both age and CRP are 2 of the fundamental parameters that change the prognosis of the disease.
In our study, we have used pulses of methylprednisolone and tried to find the main characteristics of the responding patient. In our study age and CRP and not suffer from dyspnea are related to an increased likelihood of hospital discharge.
As a major limitation of our study, the days of symptoms for the initiation of treatment have not been considered. It would be interesting to optimize the predictive model to assess the use of corticosteroids from the 7 days of symptoms, as assessed in the preliminary recovery study in which the response improved. In addition, the integral number of patients studied with pulses of methylprednisolone bolus is somewhat limited, with 322.
These two limitations will try to correct them with our new updated patient cohort pending publication, as well as improve and validate our predictive corticosteroid pulse response model.
Another important limitation of the study is that other simultaneous use treatments such as tocilizumab (anti-interleukin 6) have not been considered.
ConclusionDue to the enormous interpersonal variability of corticoid response during the COVID-19 pandemic, it has become essential to find the characteristics that allow us to know the prognosis and evolution of patients starting with the treatment.
In our study, we reveal an equation with the area under the curve of 0.816 that allows us to determine the response to pulses of methylprednisolone measured as hospital discharge.
C-reactive protein at admission, age and dyspnea are easy parameters to obtain and allow us to know in advance the prognosis after methylprednisolone pulses.
Conflict of interestThe authors declare that they have no conflict of interest.