Descriptions on impact of SARS-CoV-2 infection in patients with cardiac amyloidosis (CA) are lacking. Our aim was to describe the prognosis of those patients.
MethodsRetrospective observational study of unvaccinated patients with CA who developed SARS-CoV-2 infection enrolled in eleven centres (March 2020 to May 2021). Descriptive analysis of basal characteristics, hospitalization, mortality, and severe clinical course was performed. Comparisons to a population-based control group were made.
ResultsForty-one patients were identified. Most patients had wild-type transthyretin CA (61%) and were on NYHA Class II–III (80.5%). CA patients were commonly hospitalized (73.2%) and those were more symptomatic than outpatients (p=0.035). The 24.4% of CA patients died as consequence of SARS-CoV-2 infection. Patients with CA had an increased risk of hospitalization [OR 6.23 (3.05–12.74), p<0.001] and mortality [OR 2.18 (1.01–4.68), p=0.047] when compared to control population after adjustment by age and sex. After a medium follow-time of 311 days, 41.5% of the CA cohort died.
ConclusionsSARS-CoV-2 infection is associated with high mortality and hospitalization rates in patients with CA, which exceed that expected by their sex and advanced age.
El impacto de la infección por SARS-CoV-2 en pacientes con amiloidosis cardíaca (AC) es desconocido. El principal objetivo de este estudio es describir el pronóstico de estos pacientes.
MétodosEstudio observacional retrospectivo de pacientes con AC no vacunados que desarrollaron infección por SARS-CoV-2 identificados en 11 centros (marzo 2020/mayo 2021). Se realiza un análisis descriptivo de características basales, hospitalización, mortalidad y curso clínico grave, y se comparan los resultados con una cohorte poblacional.
ResultadosCuarenta y un pacientes fueron identificados. La mayoría eran AC por transtirretina wild-type (61%) y estaban en clase NYHA II-III (80,5%). La mayoría de los pacientes fueron hospitalizados (73,2%), los cuales tenían peor clase funcional que los ambulatorios (p=0,035). El 24,4% de los pacientes fallecieron como consecuencia de la infección. Los pacientes con AC tenían un mayor riesgo de hospitalización (OR: 6,23; 3.05-12.74; p<0,001) y fallecimiento (OR: 2,18; 1,01-4,68; p=0,047) que la cohorte poblacional tras ajuste por sexo y edad. Tras un seguimiento medio de 311 días, el 41,5% de los pacientes fallecieron.
ConclusionesLa infección por SARS-CoV-2 presenta alto riesgo de mortalidad y hospitalización en pacientes con AC, mayor que la esperada por su sexo y edad.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, popularly known as the COVID-19, has a great variability in clinical presentation and outcomes between individuals. It has been described an adverse association between patient's pre-existing comorbidities such as cardiac disease (including heart failure [HF]), with SARS-CoV-2 related mortality.1,2 However, different causes of HF could carry different SARS-CoV-2 infection prognosis.3
Cardiac amyloidosis (CA) is a progressive infiltrative disease caused by the deposit of amyloid fibrils in the heart. Most of CA cases are caused by the deposit of monoclonal immunoglobulin light chains (AL) or transthyretin (ATTR), that could be hereditary (“variant”, ATTRv) or acquired (“wild-type”, ATTRwt).4 Nowadays, it is acknowledged that CA is a more frequent disease than traditionally considered, mostly due to an improved diagnosis of ATTRwt, now the most common form of CA.5,6 The prevalence of ATTRwt increases with age and it has been found in 10%–16% of patients with preserved HF or aortic stenosis.4,7,8 Amyloid deposition in the heart causes an increase in left ventricular mass, diastolic dysfunction with evolution to restrictive filling and systolic dysfunction in an advanced phase. Moreover, amyloidosis is a systemic disease so deposits are not limited to the heart and could happen in other organs.
CA patients with SARS-CoV-2 infection are likely to have worse outcomes9 and an excess of mortality was described in amyloid patients in early pandemic years.10 The relative rarity of CA mandates a multicentre study on a large scale to adequately examine outcomes associated with COVID-19. Thus, the primary aim of the registry was to describe the short and intermediate term natural history of SARS-CoV-2 infection in patients with CA in early pandemic and to compare it to a control group.
Materials and methodsRegistry design and patientsInclusion criteria were prior diagnosis of CA and demonstration of SARS-CoV-2 infection by antigen or polymerase chain reaction tests. Participating centres were selected based on an established cardiomyopathy clinic and/or inherited cardiac disease unit. There was a total of 11 centres from Spain (n=9), Italy (n=1) and Russia (n=1) reporting CA cases. Patients were consecutively enrolled over a 14-month period, from early in the SARS-CoV-2 pandemic in March 2020, to the last patient included on 12th May 2021. The full spectrum of SARS-CoV-2 cases, from mild to severe, including outpatient and hospitalized individuals, was enrolled. Last follow-up data was also collected. Because of the retrospective design of the registry, only unvaccinated patients against SARS-CoV-2 were included to maintain homogeneity into the cohort.
The study was approved by each local Ethics Committee and informed consent was obtained prior to data collection. All diagnostic or therapeutic procedures were left to the discretion of the attending physician. A dedicated on-line software was developed for the study (Dilemma Solutions SL) to capture data in the desired format.
Patient populationThe participating sites identified patients with CA and SARS-CoV-2 infection through regular query of the electronic medical records using available local cardiomyopathy registries. This was supplemented by direct communication to and from providers at the time of SARS-CoV-2 infection diagnosis, and review of SARS-CoV-2 history at the time of scheduled and unscheduled clinical encounters. Contribution from participants centres is summarized in supplementary online Table 1.
A control group consisting of all consecutive individuals with 40 years-old or older diagnosed with SARS-CoV-2 infection in the Region of Murcia, Spain from 8th March 2020 till 12th of May 2021 was obtained from the Subdirección General de Tecnologías de la Información of the Servicio Murciano de Salud.11 Baseline demographics and vital status, but not comorbidities and medications, were available for all control patients.
Clinical variables and outcomesRecorded variables included the type of amyloidosis, demographics, cardiovascular risk factors (hypertension, diabetes, obesity, smoking), comorbidities [coronary artery disease, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), stroke], baseline cardiac related variables [rhythm, maximal left ventricular wall thickness (LVWT), left ventricular ejection fraction (LVEF) and type of HF,12 New York Heart Association (NYHA) Class, pulmonary hypertension, cardiac medications], SARS-CoV-2 related characteristics (care setting, symptoms, admission tests, diagnosis of pneumonia, medications) and outcome. The following endpoints were assessed in the study:
- •
SARS-CoV-2 related mortality: death caused by or precipitated by SARS-CoV-2 infection (occurring as direct consequence of the infection or death in less than 30 days since diagnosis).
- •
Severe clinical course: defined as SARS-CoV-2 related mortality or need for intensive care unit (ICU) admission.
Incident CA related outcomes, including new onset of atrial fibrillation, ventricular arrhythmias, cardiogenic shock, and stroke, were collected. Vital status at last follow-up was also recorded.
Statistical analysisContinuous variables were tested for normality using Shapiro–Walk test and were reported as mean±standard deviation (SD) if their distribution was normal and as median±interquartile range (IQR) if it was not normal. Among-group comparisons were made using a t-student or non-parametric test where appropriate. Categorical variables were reported as counts and percentages. Among-group comparisons 2×2 were made using a chi-square test or Fisher's exact test if any expected cell count was less than five. For comparison between CA and control group, multivariable logistic regression was performed, including age and sex as potential confounding variables. A two-sided p-value of <0.05 was considered as statistically significant. All analyses were performed using Stata software version 14.2 (StataCorp LCC, College Station, TX, USA).
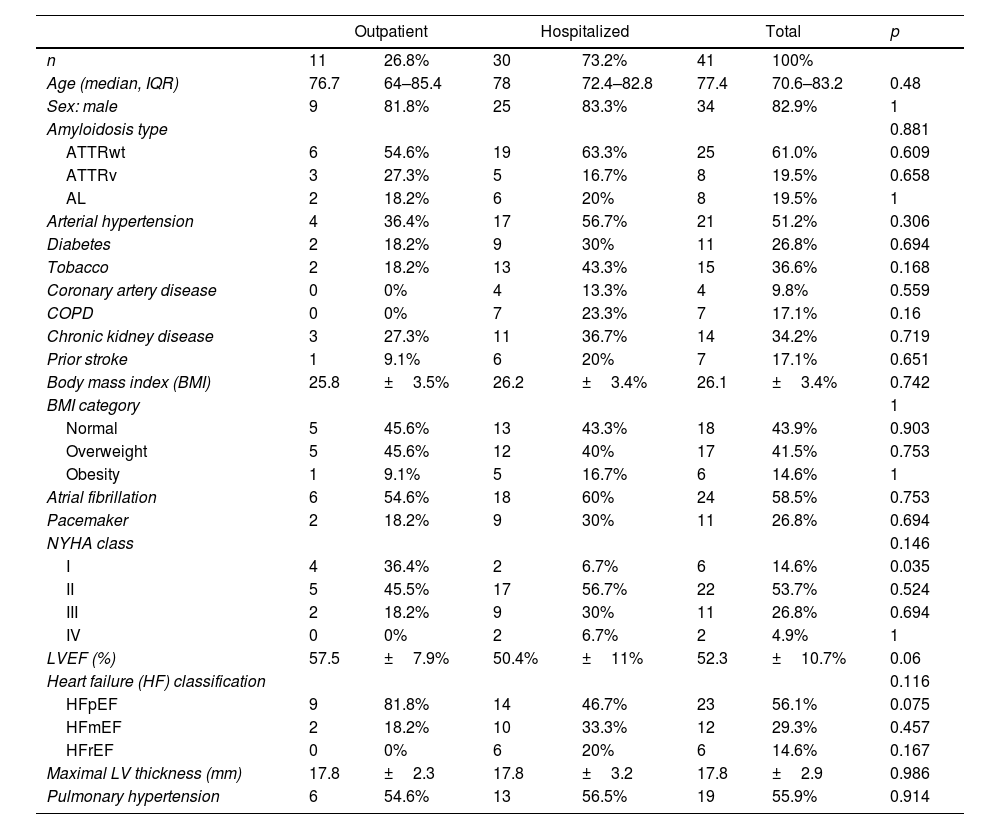
ResultsBaseline characteristics of the CA cohort with SARS-CoV-2 infectionA total of 41 patients [aged 77.4 (IQR 70.6–83.2) years old, 34 (82.9%) males, 7 (17.1%) females] with prior CA experiencing SARS-CoV-2 infection were enrolled (Table 1). Twenty-five (61%) had ATTRwt, 8 (19.5%) had ATTRv and 8 (19.5%) had AL. Patients with ATTRwt were older when compared to other CA patients (80.4 vs. 68.5 years-old, p <0.001). Within the ATTRv cohort, the most common variant found in the TTR gene was p.Val142Ile (3, 37.5%); the other variants in this cohort were p.Ser43Asn, p.Val50Met, p.Ser97Tyr, p.Glu109Gln and p.Glu112Lys. Comorbidities and cardiovascular risk factors associated were common: 56.1% had overweight or obesity, 51.2% patients had prior arterial hypertension, 36.6% were smokers and 26.8% were diabetic. In total, 70.7% patients had any cardiovascular risk factor, and 56.1% had other comorbidities.
Baseline characteristics of outpatient and hospitalized CA patients with SARS-CoV-2 infection.
| Outpatient | Hospitalized | Total | p | ||||
|---|---|---|---|---|---|---|---|
| n | 11 | 26.8% | 30 | 73.2% | 41 | 100% | |
| Age (median, IQR) | 76.7 | 64–85.4 | 78 | 72.4–82.8 | 77.4 | 70.6–83.2 | 0.48 |
| Sex: male | 9 | 81.8% | 25 | 83.3% | 34 | 82.9% | 1 |
| Amyloidosis type | 0.881 | ||||||
| ATTRwt | 6 | 54.6% | 19 | 63.3% | 25 | 61.0% | 0.609 |
| ATTRv | 3 | 27.3% | 5 | 16.7% | 8 | 19.5% | 0.658 |
| AL | 2 | 18.2% | 6 | 20% | 8 | 19.5% | 1 |
| Arterial hypertension | 4 | 36.4% | 17 | 56.7% | 21 | 51.2% | 0.306 |
| Diabetes | 2 | 18.2% | 9 | 30% | 11 | 26.8% | 0.694 |
| Tobacco | 2 | 18.2% | 13 | 43.3% | 15 | 36.6% | 0.168 |
| Coronary artery disease | 0 | 0% | 4 | 13.3% | 4 | 9.8% | 0.559 |
| COPD | 0 | 0% | 7 | 23.3% | 7 | 17.1% | 0.16 |
| Chronic kidney disease | 3 | 27.3% | 11 | 36.7% | 14 | 34.2% | 0.719 |
| Prior stroke | 1 | 9.1% | 6 | 20% | 7 | 17.1% | 0.651 |
| Body mass index (BMI) | 25.8 | ±3.5% | 26.2 | ±3.4% | 26.1 | ±3.4% | 0.742 |
| BMI category | 1 | ||||||
| Normal | 5 | 45.6% | 13 | 43.3% | 18 | 43.9% | 0.903 |
| Overweight | 5 | 45.6% | 12 | 40% | 17 | 41.5% | 0.753 |
| Obesity | 1 | 9.1% | 5 | 16.7% | 6 | 14.6% | 1 |
| Atrial fibrillation | 6 | 54.6% | 18 | 60% | 24 | 58.5% | 0.753 |
| Pacemaker | 2 | 18.2% | 9 | 30% | 11 | 26.8% | 0.694 |
| NYHA class | 0.146 | ||||||
| I | 4 | 36.4% | 2 | 6.7% | 6 | 14.6% | 0.035 |
| II | 5 | 45.5% | 17 | 56.7% | 22 | 53.7% | 0.524 |
| III | 2 | 18.2% | 9 | 30% | 11 | 26.8% | 0.694 |
| IV | 0 | 0% | 2 | 6.7% | 2 | 4.9% | 1 |
| LVEF (%) | 57.5 | ±7.9% | 50.4% | ±11% | 52.3 | ±10.7% | 0.06 |
| Heart failure (HF) classification | 0.116 | ||||||
| HFpEF | 9 | 81.8% | 14 | 46.7% | 23 | 56.1% | 0.075 |
| HFmEF | 2 | 18.2% | 10 | 33.3% | 12 | 29.3% | 0.457 |
| HFrEF | 0 | 0% | 6 | 20% | 6 | 14.6% | 0.167 |
| Maximal LV thickness (mm) | 17.8 | ±2.3 | 17.8 | ±3.2 | 17.8 | ±2.9 | 0.986 |
| Pulmonary hypertension | 6 | 54.6% | 13 | 56.5% | 19 | 55.9% | 0.914 |
Key baseline cardiac characteristics included LVEF of 52.3±10.7% and a maximal LVWT of 17.8±2.9mm. There was a high rate of patients with atrial fibrillation (58.5%) and pacemaker (26.8%). Prior to SARS-CoV-2 infection, most patients (53.7%) exhibited NYHA class II functional status, 31.7% were in class III/IV and only 14.6% were in class I.
Cardiac medications prior to infection included 80.5% patients on a loop diuretic, 53.7% on anticoagulation, 34.2% patients on beta-blockers and 24.4% on angiotensin converting enzyme inhibitor/angiotensin II receptor blocker.
Finally, there were not significant differences in basal characteristics between men and women (supplementary online Table 2).
OutcomesMost CA patients with SARS-CoV-2 were hospitalized (73.2%), the rest were outpatients with 2.4% of patients needing emergency department evaluation without requiring hospital admission (Table 1). Of those hospitalized, 12 (40%) developed a severe clinical course (defined as requiring ICU care and/or death). Hospitalized patients had a median age of 78 years-old, high prevalence of cardiovascular risk factors (73.3%) and other comorbidities (63.3%), and significantly more HF symptoms (NYHA Class II or more in 93.3% hospitalized vs. 63.6% outpatients, p=0.035). They had significantly more SARS-CoV-2 related pneumonia (70% vs. 0%, p <0.001) and acute heart failure (56.7% vs. 9.1%, p=0.011) than patients managed on an outpatient basis. There were not statistically significant differences in the remaining characteristics between hospitalized and non-hospitalized patients (Table 1).
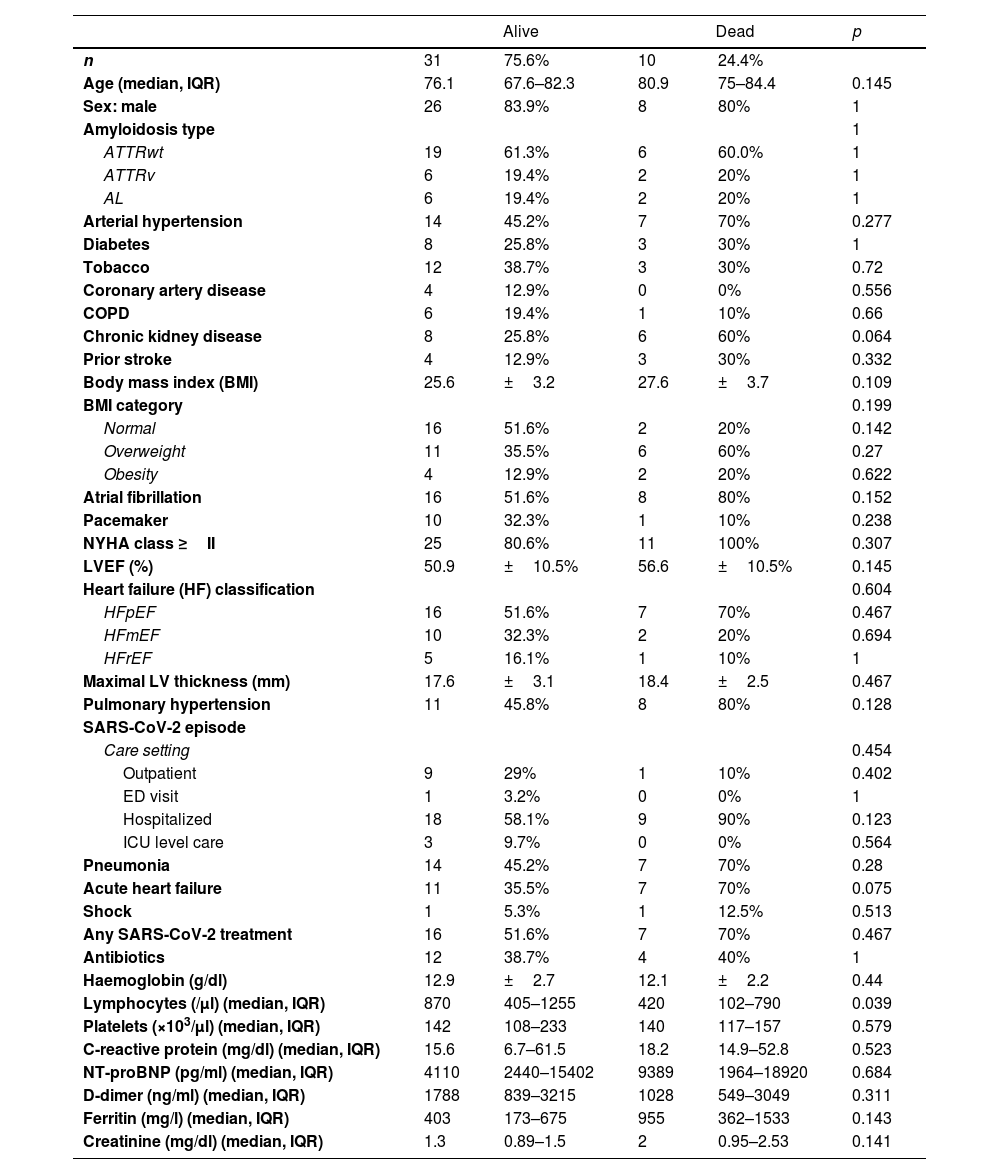
There were ten CA patients with SARS-CoV-2 related mortality (24.4%) (Table 2). Five deaths were due to SARS-CoV-2 pneumonia, two refractory HF, one respiratory failure, one sudden cardiac death and one died at home after palliative care was decided (supplementary online Table 3). Median age of the deceased patients was 80.9 years old, and they had high rate of cardiovascular risk factors (90%) and comorbidities (70%). Atrial fibrillation (80%) and preserved LVEF (70%) were common, and all of them were in NYHA Class II or higher. Those who died were commonly hospitalized (90%) and most of them developed pneumonia (70%) and acute heart failure (70%). Among those patients, 70% received any SARS-CoV-2 treatment and they had significantly less lymphocytes than survivors (p=0.039). There were not significant differences regarding other basal characteristics or laboratory parameters (Table 2).
Characteristics of alive and dead patients with CA and SARS-CoV-2 infection.
| Alive | Dead | p | |||
|---|---|---|---|---|---|
| n | 31 | 75.6% | 10 | 24.4% | |
| Age (median, IQR) | 76.1 | 67.6–82.3 | 80.9 | 75–84.4 | 0.145 |
| Sex: male | 26 | 83.9% | 8 | 80% | 1 |
| Amyloidosis type | 1 | ||||
| ATTRwt | 19 | 61.3% | 6 | 60.0% | 1 |
| ATTRv | 6 | 19.4% | 2 | 20% | 1 |
| AL | 6 | 19.4% | 2 | 20% | 1 |
| Arterial hypertension | 14 | 45.2% | 7 | 70% | 0.277 |
| Diabetes | 8 | 25.8% | 3 | 30% | 1 |
| Tobacco | 12 | 38.7% | 3 | 30% | 0.72 |
| Coronary artery disease | 4 | 12.9% | 0 | 0% | 0.556 |
| COPD | 6 | 19.4% | 1 | 10% | 0.66 |
| Chronic kidney disease | 8 | 25.8% | 6 | 60% | 0.064 |
| Prior stroke | 4 | 12.9% | 3 | 30% | 0.332 |
| Body mass index (BMI) | 25.6 | ±3.2 | 27.6 | ±3.7 | 0.109 |
| BMI category | 0.199 | ||||
| Normal | 16 | 51.6% | 2 | 20% | 0.142 |
| Overweight | 11 | 35.5% | 6 | 60% | 0.27 |
| Obesity | 4 | 12.9% | 2 | 20% | 0.622 |
| Atrial fibrillation | 16 | 51.6% | 8 | 80% | 0.152 |
| Pacemaker | 10 | 32.3% | 1 | 10% | 0.238 |
| NYHA class ≥II | 25 | 80.6% | 11 | 100% | 0.307 |
| LVEF (%) | 50.9 | ±10.5% | 56.6 | ±10.5% | 0.145 |
| Heart failure (HF) classification | 0.604 | ||||
| HFpEF | 16 | 51.6% | 7 | 70% | 0.467 |
| HFmEF | 10 | 32.3% | 2 | 20% | 0.694 |
| HFrEF | 5 | 16.1% | 1 | 10% | 1 |
| Maximal LV thickness (mm) | 17.6 | ±3.1 | 18.4 | ±2.5 | 0.467 |
| Pulmonary hypertension | 11 | 45.8% | 8 | 80% | 0.128 |
| SARS-CoV-2 episode | |||||
| Care setting | 0.454 | ||||
| Outpatient | 9 | 29% | 1 | 10% | 0.402 |
| ED visit | 1 | 3.2% | 0 | 0% | 1 |
| Hospitalized | 18 | 58.1% | 9 | 90% | 0.123 |
| ICU level care | 3 | 9.7% | 0 | 0% | 0.564 |
| Pneumonia | 14 | 45.2% | 7 | 70% | 0.28 |
| Acute heart failure | 11 | 35.5% | 7 | 70% | 0.075 |
| Shock | 1 | 5.3% | 1 | 12.5% | 0.513 |
| Any SARS-CoV-2 treatment | 16 | 51.6% | 7 | 70% | 0.467 |
| Antibiotics | 12 | 38.7% | 4 | 40% | 1 |
| Haemoglobin (g/dl) | 12.9 | ±2.7 | 12.1 | ±2.2 | 0.44 |
| Lymphocytes (/μl) (median, IQR) | 870 | 405–1255 | 420 | 102–790 | 0.039 |
| Platelets (×103/μl) (median, IQR) | 142 | 108–233 | 140 | 117–157 | 0.579 |
| C-reactive protein (mg/dl) (median, IQR) | 15.6 | 6.7–61.5 | 18.2 | 14.9–52.8 | 0.523 |
| NT-proBNP (pg/ml) (median, IQR) | 4110 | 2440–15402 | 9389 | 1964–18920 | 0.684 |
| D-dimer (ng/ml) (median, IQR) | 1788 | 839–3215 | 1028 | 549–3049 | 0.311 |
| Ferritin (mg/l) (median, IQR) | 403 | 173–675 | 955 | 362–1533 | 0.143 |
| Creatinine (mg/dl) (median, IQR) | 1.3 | 0.89–1.5 | 2 | 0.95–2.53 | 0.141 |
A total of thirteen patients had a severe clinical course (31.7%) (supplementary online Table 4). Of three ICU admissions, two were AL patients with 62.1 and 69.5 years-old, and one 76 years-old ATTRwt patient. Patients with a severe clinical course had high rates of CKD (53.9%) and overweight/obesity (76.9%). Acute heart failure was significantly more common in those (69.2% vs. 32.1%, p=0.043). Finally, ferritin values were significantly higher in this group (p=0.035). No significant differences were found regarding other basal characteristics, prior medications, or laboratory parameters.
Non-fatal CA related complications coincident with SARS-CoV-2 infection included atrial fibrillation in 2 patients (7.4%) and stroke in 1 patient (4.6%) of the cohort. There were not ventricular arrythmias in CA patients.
Mean follow-up time of discharged patients was 311 days (range 42–588 days). At the end of follow-up, there were 7 additional deaths apparently not related to SARS-CoV-2 infection (supplementary online Table 3). Of those, 6 patients were males, 4 of them with ATTRwt, >80 years old and LVEF <50%; one of which was followed in an outpatient basis and suffered sudden cardiac death at home 42 days after the infection. Other three patients died of sepsis, refractory HF, and subdural haematoma, respectively, which happened several months after the SARS-CoV-2 infection. Other 2 male patients had AL and died of cancer related complications. Finally, the remaining death was an elderly female patient with ATTRwt who died of severe acute anaemia months later. Overall, 41.5% of our cohort had died at the end of follow-up.
Comparison of SARS-CoV-2 infection in CA vs. control populationWe compared mortality among CA patients to a contemporary cohort of 49,773 consecutive patients of the same age intervals diagnosed with SARS-CoV-2 infection in the general population.
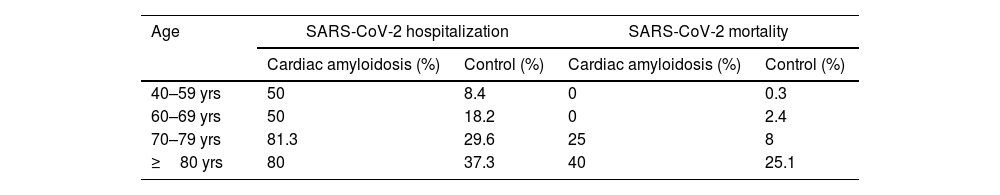
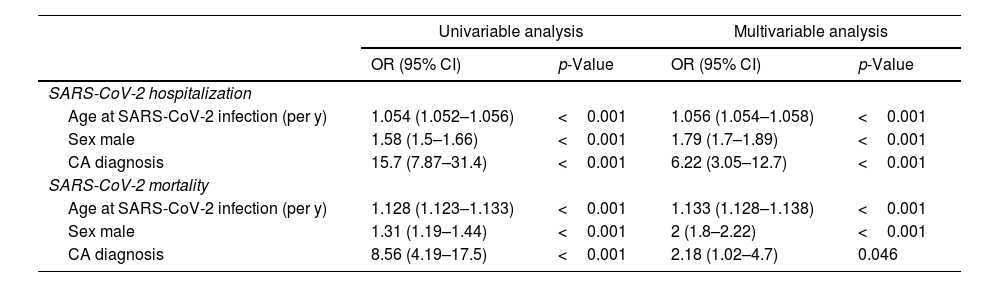
CA patients had a higher frequency of men (82.9% vs. 46.9%, p <0.001) and were older (mean age 75.7 vs. 57.7 years-old, p <0.001) compared to the control cohort. Age and male sex were associated in univariable analysis with SARS-CoV-2 hospitalization and mortality (Tables 3 and 4). In a multivariable model adjusted by sex and age, hospital admissions were significantly more common in the CA group than the control cohort [OR 6.23 (3.05–12.74), p <0.001]. CA patients also had significantly higher SARS-CoV-2 related adjusted mortality than control cohort [OR 2.18 (1.01–4.68), p=0.047] (Tables 3 and 4). However, when considering only hospitalized patients, age [OR 1.096 (1.09–1.102) per year, p <0.001] and male sex [OR 1.55 (1.36–1.78), p <0.001] were independent predictors of mortality, but not CA diagnosis [OR 0.98 (0.43-2.21), p=0.959].
Mortality and hospitalization in cardiac amyloidosis and control groups according to age intervals.
| Age | SARS-CoV-2 hospitalization | SARS-CoV-2 mortality | ||
|---|---|---|---|---|
| Cardiac amyloidosis (%) | Control (%) | Cardiac amyloidosis (%) | Control (%) | |
| 40–59 yrs | 50 | 8.4 | 0 | 0.3 |
| 60–69 yrs | 50 | 18.2 | 0 | 2.4 |
| 70–79 yrs | 81.3 | 29.6 | 25 | 8 |
| ≥80 yrs | 80 | 37.3 | 40 | 25.1 |
Comparison of CA patients with control population (univariable and multivariable analysis).
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| SARS-CoV-2 hospitalization | ||||
| Age at SARS-CoV-2 infection (per y) | 1.054 (1.052–1.056) | <0.001 | 1.056 (1.054–1.058) | <0.001 |
| Sex male | 1.58 (1.5–1.66) | <0.001 | 1.79 (1.7–1.89) | <0.001 |
| CA diagnosis | 15.7 (7.87–31.4) | <0.001 | 6.22 (3.05–12.7) | <0.001 |
| SARS-CoV-2 mortality | ||||
| Age at SARS-CoV-2 infection (per y) | 1.128 (1.123–1.133) | <0.001 | 1.133 (1.128–1.138) | <0.001 |
| Sex male | 1.31 (1.19–1.44) | <0.001 | 2 (1.8–2.22) | <0.001 |
| CA diagnosis | 8.56 (4.19–17.5) | <0.001 | 2.18 (1.02–4.7) | 0.046 |
In this multicentre study, we have examined the impact of SARS-CoV-2 infection in early pandemic on patients with CA. A vast majority of patients were hospitalized and there were high rates of severe clinical course, with almost one quarter of CA patients dying because of COVID-19 in the first month after diagnosis. This finding could reflect an older population, which was expected due to the epidemiology of CA, specially ATTRwt, which was the most common CA in our cohort.4,5,13 However, when compared to a control cohort, risks of hospitalization and mortality were 6.2 and 2.2 respectively higher in CA patients after adjustment by age and sex (Table 4). Although there were not differences in mortality when considering only hospitalized patients, it is known that hospitalized patients from general population had high rates of comorbidities that put them at high risk of mortality like CA group.1,2 CA patients were anticipated to be at high risk of complications and death from SARS-CoV-2 infection9 and a recently published study showed that in early pandemic years there were an excess of mortality in amyloidosis patients, likely reflecting high COVID-19 related mortality in this group.10 These patients have limited cardiac reserve and elevated pulmonary vascular resistances, so SARS-CoV-2 infection would be poorly tolerated, with an increased risk for lower cardiac output and pulmonary oedema which can further worsen respiratory failure.9
It is worth to comment that, despite high hospitalization and mortality rates, only 7.3% patients were admitted to ICU. This is likely to reflect a conservative approach in early pandemic when managing elderly patients with ATTRwt due to the perception that poor outcomes were inevitable for those specially in a time of shortage of resources.9
In our cohort, 50% of AL patients had the combined endpoint of ICU or death and 25% died as consequence of COVID-19. In this sense, it is known that AL patients have poorer prognosis than other CA patients.6,14 Despite the anticipated worse clinical evolution, AL patients are younger and therefore it is more likely that those are considered for ICU admission in the case of severe clinical course. There was prior concern regarding the vulnerability of AL patients with SARS-CoV-2 infection, which commonly have multisystemic involvement and usually need to receive chemotherapeutic agents with or without autologous stem cell transplant, whose immunosuppression probably increases infection risk.15 A recent small observational study that included 13 AL patients with COVID-19, also showed that those patients have high mortality (23%), all of them with cardiac involvement, which was an independent predictor of mortality in this study.14 Of note, this study also included vaccinated patients and there were no deceased patients in this group.14 Our data supports that AL patients are at high risk and strict monitorization of those should be carried out.
We have also found that higher NYHA Classes are associated with hospitalization, and that presence of acute heart failure predicts severe clinical course. Patients with CA usually have preserved or mildly depressed LVEF and they could have advanced HF because of severe diastolic dysfunction.5,6 Thus, ICU or death only happened in symptomatic patients before SARS-CoV-2 infection (NYHA Class II or higher), likely reflecting advanced disease.
CA patients with lymphopenia are at high risk of SARS-CoV-2 infection related death. Presence of lymphopenia was independent of the type of amyloidosis. Those findings are in accordance with prior literature that found that lymphopenia on admission was associated with poorer outcome in those patients.16 Although its mechanism is still unclear, it has been hypothesized that either the expression of ACE2 receptor in lymphocytes or the increase in proinflammatory cytokines could lead to lymphopenia in the context of SARS-CoV-2 infection.16,17 Finally, higher levels of ferritin were also associated with ICU admission or death, which is in accordance prior literature reflecting a more severe inflammatory syndrome and that is also associated with lymphopenia.17,18
In our CA cohort, we did not find significant differences regarding to cardiovascular risk factors or comorbidities. This is likely related to limitations on sample size. In fact, like studies conducted in general population,1,2 there was a trend showing that advanced age (≥80 years-old) and BMI could be related to SARS-CoV-2 mortality.
Finally, there was a high rate of mortality not-related to SARS-CoV-2 infection over a median follow-up of over 10 months, which is in accordance with the poor prognosis of CA patients.6
The present study is in keeping with the published literature which has suggested that patients with cardiovascular comorbidities and heart conditions are more likely to develop an adverse course SARS-CoV-2 infection.1 We have expanded upon prior findings by describing that CA patients had significant worse prognosis than other patients. It has been described that vaccinated amyloidosis patients are still at risk of complications but with low death rates in this group.10,14 Thus, careful preventive measures should be carried in CA patients to prevent infection, including use of vaccination, booster vaccine doses, masks, and avoiding exposure to other infected patients. Unvaccinated CA patients with COVID-19 disease are at high risk of death (≈25%) so careful monitoring in a dedicated setting and specific treatment should be initiated at the minimum sign of respiratory or haemodynamic decompensation.
There are inherent limitations to this type of studies based on multicentre registries. The magnitude of the pandemic and the isolation and lockdown measurements to avoid spreading of the SARS-CoV-2, had led to heterogenicity of clinical data, without a unified protocol of examinations. Access to blood tests for outpatients and clinical evaluations of very severe cases were difficult to obtain. Completeness of the data collection was based on the interview of patients/relatives and the review of available medical records. Importantly, because of the rarity of the CA, there are obvious limitations on sample size that reduced the power of our analysis. Besides, there might be a selection bias towards the most severe cases (those were more likely to be identified by their doctors/investigators, while mildly symptomatic patients could be underdiagnosed), although this limitation also applies for the control group. We did not have information on comorbidities of the control cohort so we could not adjust for those in the comparison analysis, which could limit interpretation of our results.
Finally, we cannot rule out that new SARS-CoV-2 strains or infection in vaccinated CA patients could have different prognosis, so our results should not be extrapolated to those cohorts.
ConclusionsCA patients with SARS-CoV-2 infection are at high risk of hospitalization and mortality, which exceed that expected by their sex and advanced age. Preventive measures should be implemented to reduce their risk of infection and close monitoring of those infected must be undertaken to reduce the risk of death.
Ethical considerationsThe study was approved by each local Ethics Committee and informed consent was obtained prior to data collection.
Author's contributionsJ.M. Larrañaga-Moreira, A.I. Rodriguez-Serrano, J.R. Gimeno-Blanes and R. Barriales-Villa conceived and designed the study. C. Peña-Gil. and L. Monserrat participated in the design of the study, built, and maintained the database. J.M. Larrañaga-Moreira collected clinical data, performed the statistical analysis, and drafted the manuscript. A.I. Rodriguez-Serrano, F. Hernández-Terciado, A. Lalario, C. Gómez-González, L. Tojal-Sierra, T. Ripoll-Vera, E. Zorio, E. Villacorta, O. Chumakova, D. Vaqueriza-Cubillo, M. Gallego-Delgado, J.C. Sánchez-Martínez, F. Domínguez, M. Merlo, M.A. Espinosa-Castro, G. Sinagra and P. García-Pavia collected clinical data. A.I. Rodriguez-Serrano, E. Zorio, E. Villacorta, F. Dominguez, M. Merlo, J.R. Gimeno-Blanes and R. Barriales-Villa revised the manuscript critically. All authors approved the final version to be published.
FundingThe project was funded by a grand of the Instituto de Salud Carlos III (ISCIII) through grants COV20 00420 and PI20/1379.
Conflict of interestCarlos Peña, and Lorenzo Monserrat work for Dilemma Solutions SL company and made substantial contributions to the study. They participated in development of the eCRF, in the revision, and in the approval of the manuscript. The rest of the authors declared no conflict of interest.
José M. Larrañaga-Moreiraa*, Ana I. Rodriguez-Serranob,c, Fernando Domínguezc,d,e, Andrea Lalariof, Esther Zorioe,g, Roberto Barriales-Villaa,e, Fernando Hernández-Terciadod, Marco Merlof, Cristina Gómez-Gonzálezh, Lucas Tojal-Sierrai, Tomás Ripoll-Veraj, Eduardo Villacortae,k, Olga Chumakoval, David Vaqueriza-Cubillom, María A. Espinosa-Castroe,h, María Gallego-Delgadoe,k, José Carlos Sánchez-Martínezg, Gianfranco Sinagraf, Pablo García-Paviac,d,e,n, Carlos Peña-Gilo, Lorenzo Monserrato, Juan R. Gimeno-Blanesb,c,e
aUnidad de Cardiopatías Familiares. Instituto de Investigación Biomédica de A Coruña (INIBIC), Hospital Universitario de A Coruña (HUAC), Servizo Galego de Saúde (SERGAS), Universidade da Coruña (UDC), A Coruña, España; bHospital Universitario Virgen de Arrixaca, Murcia. Spain; cEuropean Reference Networks for rare, low prevalence and complex diseases of the heart (ERN GUARD-Heart), Amsterdam, The Netherlands; dUnidad Cardiopatias Familiares, Hospital Universitario Puerta Hierro Majadahonda, IDIPHISA, Madrid, Spain; eCenter for Biomedical Network Research on Cardiovascular Diseases (CIBERCV), Madrid, Spain; fCardiovascular Department ‘Ospedali Riuniti’ and University of Trieste, Trieste, Italy; gUnidad Cardiopatías Familiares, Hospital Universitario y Politécnico La Fe, Valencia, Spain. Instituto de Investigación Sanitaria La Fe, Valencia, Spain; hUnidad de Cardiopatías Familiares, Hospital General Universitario Gregorio Marañón, Madrid, Spain; iHospital Universitario de Araba (Txagorritxu), Vitoria, Spain; jUnidad Cardiopatias Familiares, Hospital Universitario Son Llàtzer, Mallorca, Spain; kInherited Cardiovascular Disease Unit. Cardiology Department. Complejo Asistencial Universitario de Salamanca; Biomedical Research Institute of Salamanca (IBSAL); Gerencia Regional de Salud de Castilla y León (SACYL). Medicine department. University of Salamanca, Salamanca, Spain; lMunicipal Clinical Hospital #17, Moscow, Russia; mHospital Universitario Infanta Leonor, Madrid, Spain; nCentro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain; oDilemma Solutions SL, A Coruña, Spain.