In recent years, many pharmacological agents for the prevention of delirium have emerged; however, the efficacy of these agents in preventing delirium remains unclear.
ObjectiveTo compare and rank the efficacy of different pharmacological interventions for the prevention of delirium.
DesignA systematic review and network meta-analysis.
MethodsRelevant randomized controlled trials on drug prevention of delirium were extracted from three electronic databases. A network meta-analysis was then conducted to assess the relative efficacy of drug interventions in preventing delirium. The quality of the data was evaluated using the Cochrane Risk of Bias tool.
ResultsA total of 80 randomized controlled trials on drug interventions were included in the final analysis. Treatment with dexmedetomidine can prevent delirium.
ConclusionDexmedetomidine treatment can prevent delirium and reduce patient suffering. Healthcare professionals should be encouraged to use dexmedetomidine for delirium prevention.
En los últimos años, han surgido muchos agentes farmacológicos para la prevención del delirium; sin embargo, la eficacia de estos agentes en la prevención del delirium sigue siendo incierta.
ObjetivoComparar y clasificar la eficacia de diferentes intervenciones farmacológicas para la prevención del delirium.
DiseñoUna revisión sistemática y un metaanálisis en red.
MétodosSe extrajeron ensayos controlados aleatorios relevantes sobre la prevención del delirium mediante medicamentos de tres bases de datos electrónicas. Luego se realizó un metaanálisis en red para evaluar la eficacia relativa de las intervenciones farmacológicas en la prevención del delirium. La calidad de los datos se evaluó utilizando la herramienta de Riesgo de Sesgo de Cochrane.
ResultadosSe incluyeron un total de 80 ensayos controlados aleatorios sobre intervenciones farmacológicas en el análisis final. El tratamiento con dexmedetomidina puede prevenir el delirium.
ConclusiónEl tratamiento con dexmedetomidina puede prevenir el delirium y reducir el sufrimiento del paciente. Se debe alentar a los profesionales de la salud a utilizar dexmedetomidina para la prevención del delirium.
Delirium is an acute disturbance of brain function, primarily characterized by confusion, inattention, emotional agitation, altered sleep–wake cycles and even impulsive and aggressive behaviors. The prevalence of delirium varies by population and environment. Elderly patients and those undergoing more extensive surgeries are at higher risk for delirium.1 The incidence of delirium in patients with acute illnesses is around 23%2 while it can reach up to 45% in patients aged 90 and older.3 Delirium is associated with adverse outcomes such as short- to medium-term mortality during hospitalization or after discharge, prolonged hospital stays, and long-term cognitive decline.4–6 The prevention and treatment of delirium remain significant challenges in clinical practice7–10 and various classes of psychoactive medications (such as antipsychotics, benzodiazepines, opioids, α-2 agonists, and cholinesterase inhibitors) have been studied for their effects on delirium in different patient populations. However, it remains unclear how these medications differ in their efficacy for delirium prevention. Given the adverse outcomes associated with delirium, providing effective pharmacological prevention for patients is crucial.
Network meta-analysis, as a statistical technique, allows for indirect comparisons or the combination of indirect and direct comparisons while analyzing more than two interventions, offering comprehensiveness, flexibility, and practicality.11 Additionally, network meta-analysis can be used to rank drug interventions based on different outcomes and provide evidence-based data to assist in medical decision-making. Therefore, the primary aim of this study is to employ network meta-analysis to compare the efficacy of different drug interventions in preventing delirium by summarizing and analyzing existing evidence.
MethodsThis study adheres to the PRISMA statement12 and the Cochrane Handbook for the Systematic Review of Interventions.
Data sources and searchesTo include studies in this systematic review and network meta-analysis, we searched the Cochrane Central Register of Controlled Trials, Embase, PubMed databases. Additionally, we reviewed the reference lists of all eligible articles, The search strategy is described in Supplementary Table 1.
Inclusion criteriaThe study defined the target trials according to the PICOS (population, interventions, comparators, outcomes, study design) selection criteria.
PopulationParticipants are patients undergoing drug interventions to prevent delirium.
InterventionsTo prevent delirium, drug interventions are employed, including haloperidol, ziprasidone, dexmedetomidine, midazolam, propofol, remimazolam, risperidone, ketamine, lorazepam, morphine, olanzapine, quetiapine, chlorpromazine, and valproic acid. The details of each type of pharmacological intervention are shown in Supplementary Table 2.
ComparisonsAny other types of drug interventions or control groups; the control group is defined as a group that did not receive any treatment (such as standard care) or a placebo (such as saline).
OutcomesThe study must have evaluated the symptoms of delirium and provided existing detailed data. The assessment of delirium requires the use of a complete and specialized scale, such as the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU),13 the 3-Minute Delirium Diagnosis Scale (3D-CAM),14 the 4-Attendance Test (4AT),15 the Intensive Care Delirium Screening Checklist (ICDSC)16 and the Nursing Delirium Screening Scale (Nu-DESC).17
Study designWe only included randomized controlled trials.
Data selection and extractionAll searched literature was imported into Zotero to remove duplicate entries. Two reviewers independently screened all titles and abstracts from the search results. We obtained the full manuscripts of studies that were potentially relevant to the objectives and evaluated them based on the inclusion criteria by two independent reviewers. Any discrepancies were resolved through discussion or adjudication by a third investigator. We applied a data extraction form to facilitate electronic comparison of entries. Extracted data included authors, publication year, participant characteristics, details of interventions and their control groups, as well as outcomes (delirium).
Quality appraisalTwo independent reviewers assessed the quality of individual studies using the Cochrane Risk of Bias tool.18 Any differences were resolved by consensus. Quality assessment items for each study included selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other biases. These items were categorized as low, high, or unclear risk of bias.
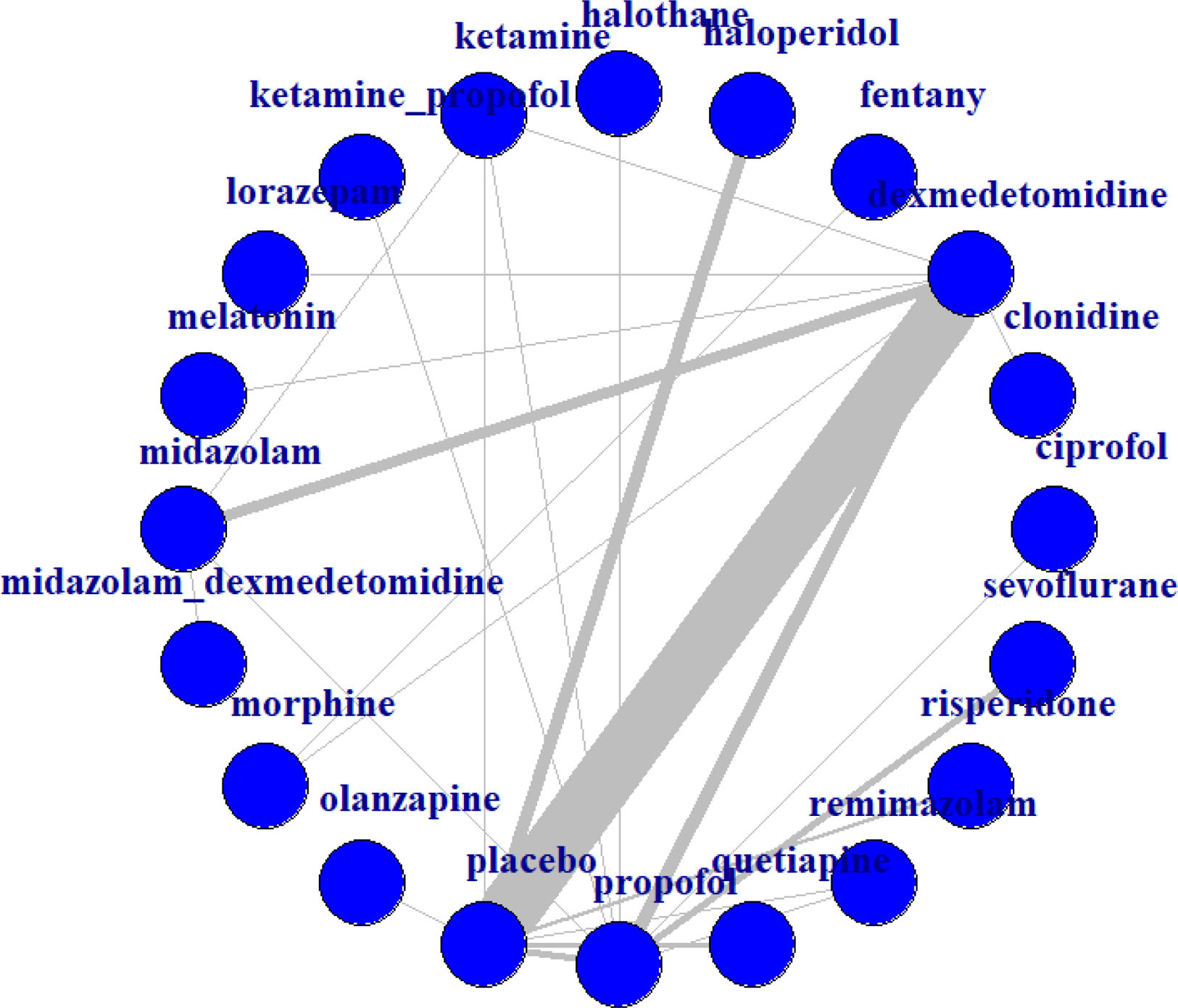
Statistical analysesMethods of analysisAll outcomes were binary variables; therefore, network estimates for all outcome variables are expressed as the standardized mean difference (SMD) with 95% confidence intervals (CIs). Data analysis was performed using R software, version 4.4.1. A random-effects model was adopted. In the network structure diagram, each node represents an intervention, and the lines between nodes indicate direct comparisons between two interventions. The width of each line reflects the number of studies reporting the comparison between the two interventions. The plausibility of the transitivity assumption was assessed based on the characteristics of each individual study. Additionally, indirect evidence was estimated using the entire evidence network. The convergence of the random-effects model was assessed using trace plots, density plots, and Brooks–Gelman–Rubin diagnostic plots. Heterogeneity among studies was evaluated using Cochran's Q statistic and the I2 measure from the network statistical package.19I2 values were interpreted as none (0%), low (25%), moderate (50%), or high (75%).20 The node-splitting method was then used to test the consistency of partial comparison results. Finally, the consistency of the model was evaluated through heterogeneity testing.
Assessment of inconsistencyWe used the node-splitting method and heterogeneity tests to conduct the assessment of inconsistency. In addition, we conducted subgroup analyses based on age and surgical settings, as well as ICU.
Efficacy rankingWe ranked the efficacy of interventions in preventing delirium by creating cumulative probability plots and histograms, providing a more intuitive understanding of the drug efficacy.
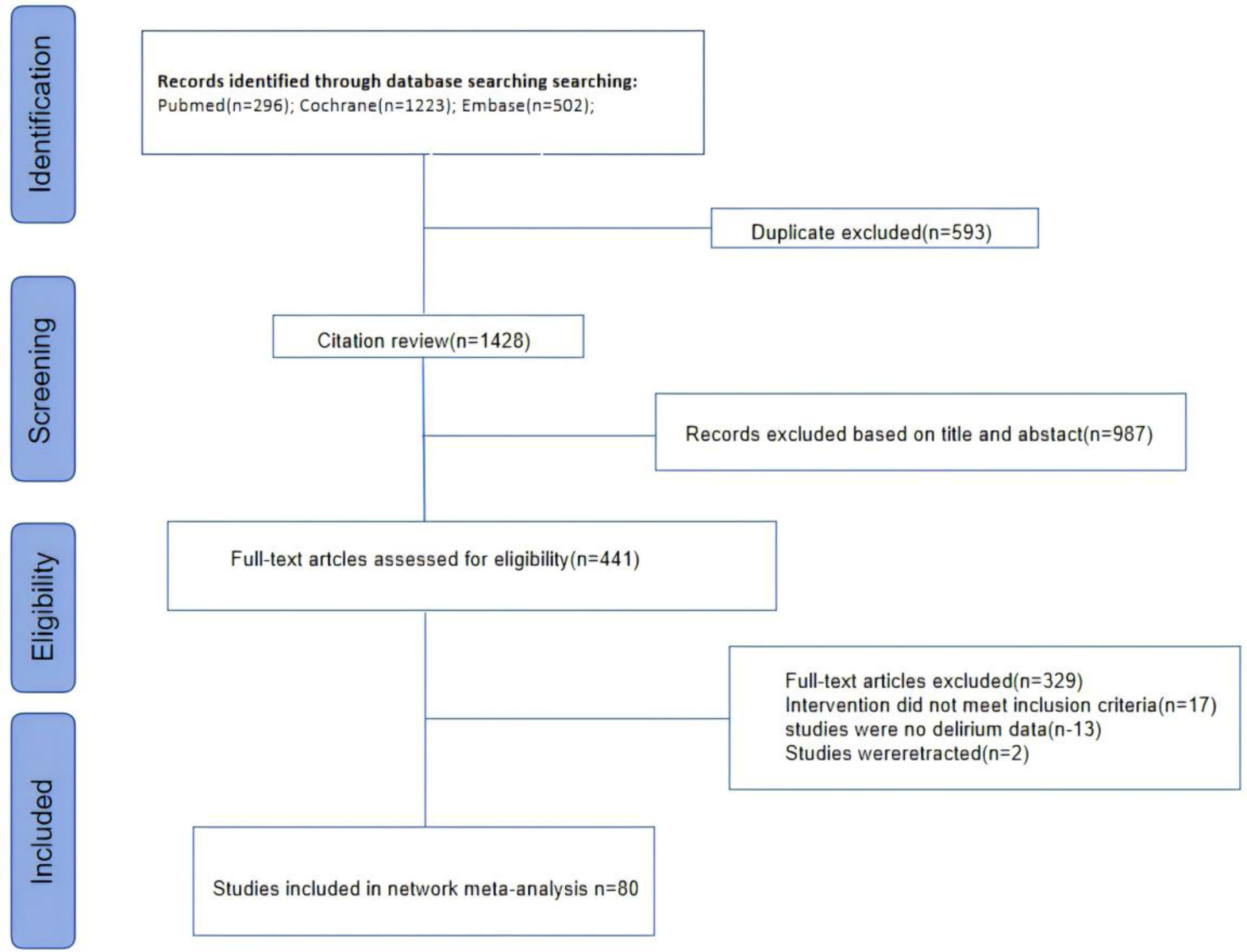
ResultsBaseline characteristics and quality of included studiesThe selection flow diagram for included studies is shown in Fig. 1. A total of 6764 individuals were identified, and ultimately, only 80 randomized controlled trials met the inclusion criteria and were included (Supplementary Table 3).21–100 As shown in Supplementary Table 2, this study included a total of 17,768 participants who received 15 types of drug interventions. The specific interventions included haloperidol, ziprasidone, dexmedetomidine, midazolam, propofol, remimazolam, risperidone, ketamine, lorazepam, morphine, olanzapine, quetiapine, chlorpromazine, and valproic acid. The 80 RCT studies were primarily conducted in China (k=30), followed by the United States (k=10) and South Korea (k=8). There were 32 RCT studies included from other countries. Overall, among the 80 RCT studies, 63 were analyzed in surgical settings, 11 in intensive care units (ICU), and 6 in other environments. All results were related to the prevention of delirium. In terms of age groups, the majority of studies focused on the elderly (k=53), followed by children (k=20) and young adults (k=7).
Methodological quality of the studiesThe quality of the included studies is illustrated in Fig. S1. Overall, the randomized controlled trials included in our network meta-analysis demonstrated acceptable and relatively low risks of bias. All included trials were randomized controlled studies; however, some studies did not sufficiently detail allocation concealment, resulting in an unclear risk of bias. Most studies had an unclear risk of selective reporting bias.
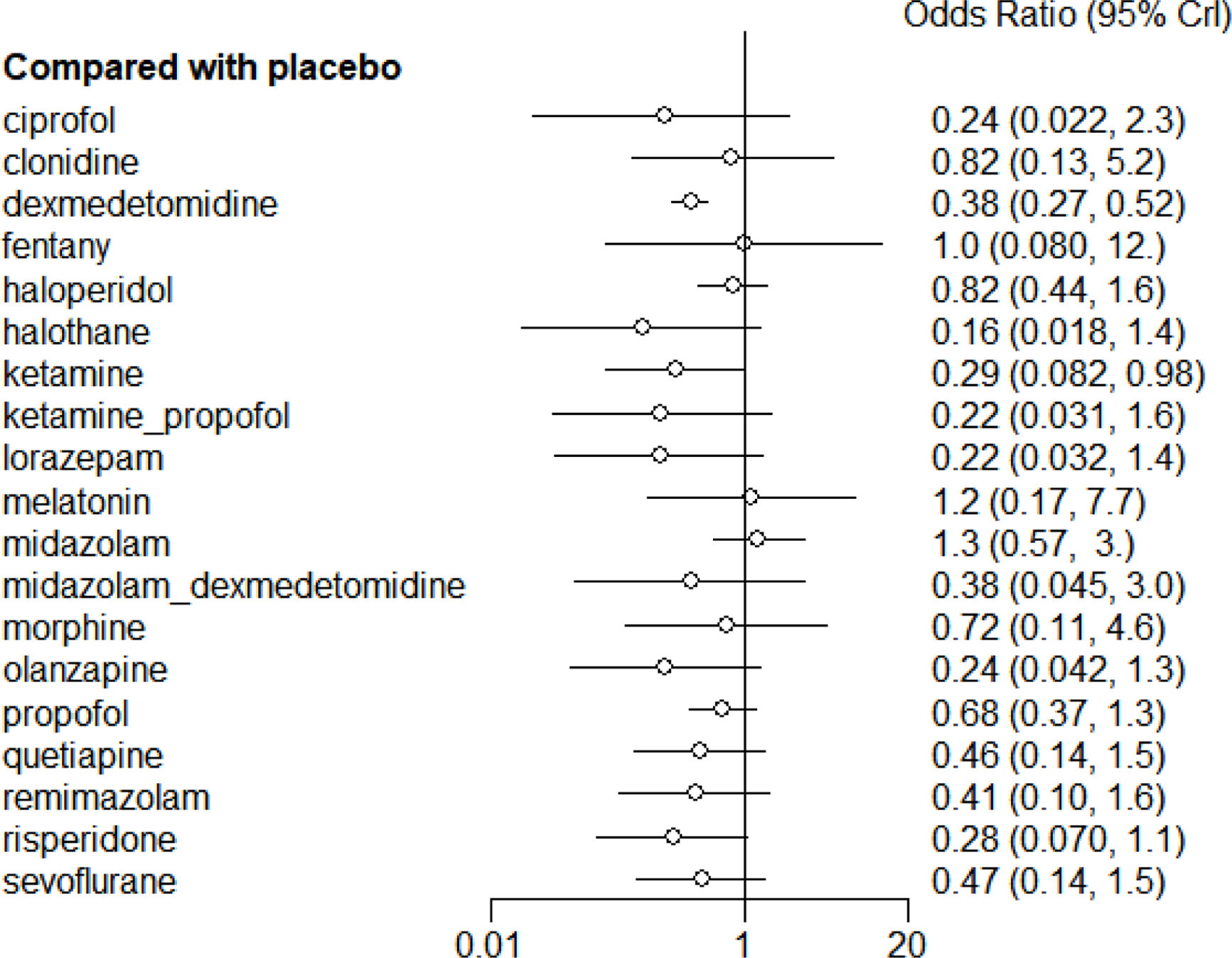
Analyses of outcomesThe main findings of the network meta-analyses are shown in Fig. 2. The thickest line in the figure represents the direct comparison between dexmedetomidine and placebo, indicating the largest sample size for this comparison. Two drug interventions were rated as high certainty of evidence in preventing delirium symptoms compared to the control group: dexmedetomidine (OR=0.38, 95% CI 0.27–0.52) and ketamine (OR=0.29, 95% CI 0.082–0.98) (Fig. 3). According to the trace plots in Fig. S2, when the number of iterations reached over 10,000, the MCMC chain stabilized and showed good overlap. The density plots indicated that when the number of iterations reached 50,000, the bandwidth approached 0 and stabilized. In Fig. S3, both B and W stabilized at a level, and the Gelman–Rubin statistic was approximately equal to 1, indicating good model convergence.
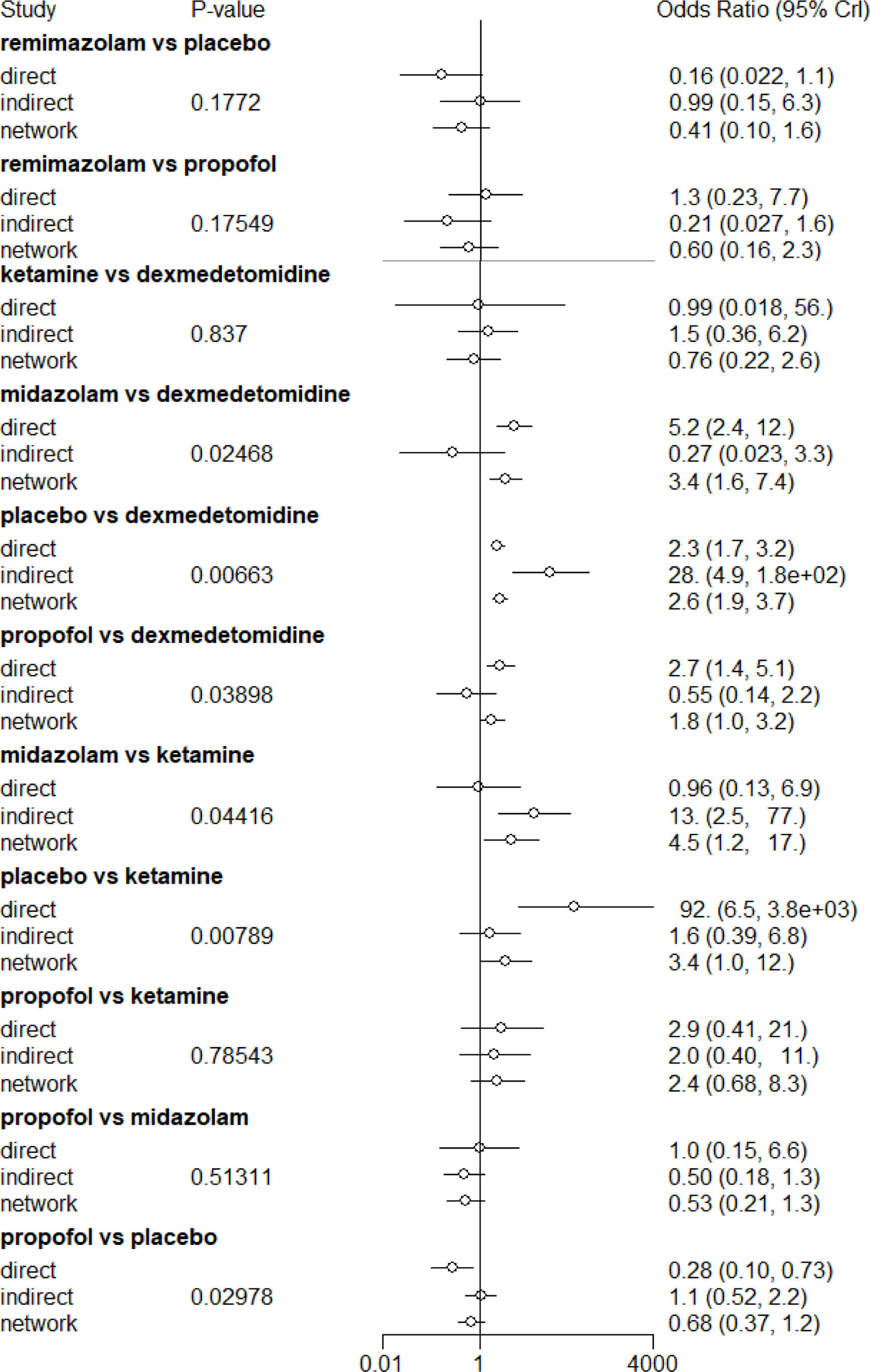
Local consistency check of the model using node-splittingThe node analysis method is used to test the consistency of partial comparison results. From the node analysis diagram (Fig. 4), it can be seen that the indirect comparison results of the interventions ketamine with dexmedetomidine (OR=1.5, 95%CI 0.36–6.20), propofol with ketamine (OR=2.0, 95% CI 0.40–11), propofol with midazolam (OR=0.50, 95%CI 0.18–1.3), remimazolam with placebo (OR=0.99, 95%CI 0.15–6.3), and remimazolam with propofol (OR=0.21, 95%CI 0.027–1.6) show good consistency. However, the indirect comparison results of the interventions dexmedetomidine with midazolam (OR=0.27, 95%CI 0.023–3.3), dexmedetomidine with placebo (OR=28, 95%CI 4.9–1.8e+02), dexmedetomidine with propofol (OR=0.55, 95%CI 0.14–2.2), ketamine with midazolam (OR=13, 95% CI 2.5–77), ketamine with placebo (OR=1.6, 95% CI 0.39–6.8), and propofol with placebo (OR=1.1, 95% CI 0.52–2.2) show heterogeneity.
Heterogeneity testAs shown in Fig. S4, the I2 value for the direct comparison (pair-wise) between dexmedetomidine and placebo was 77.5%, indicating high heterogeneity. The I2 value for the network comparison between dexmedetomidine and placebo was 80.9%, which did not meet the homogeneity assumption, and there were no indirect comparisons (back-calculated) for dexmedetomidine and placebo, resulting in missing indirect comparisons. The I2 value for the network comparison between ketamine and placebo was 83.2%, also failing to meet the homogeneity assumption. Subgroup analysis showed no conspicuous differences based on age or the surgical and ICU subgroups (Figs. S5 and S6). The reasons for heterogeneity may include differences in the interventions studied, variations in outcomes, racial differences, and the small sample sizes of some studies.
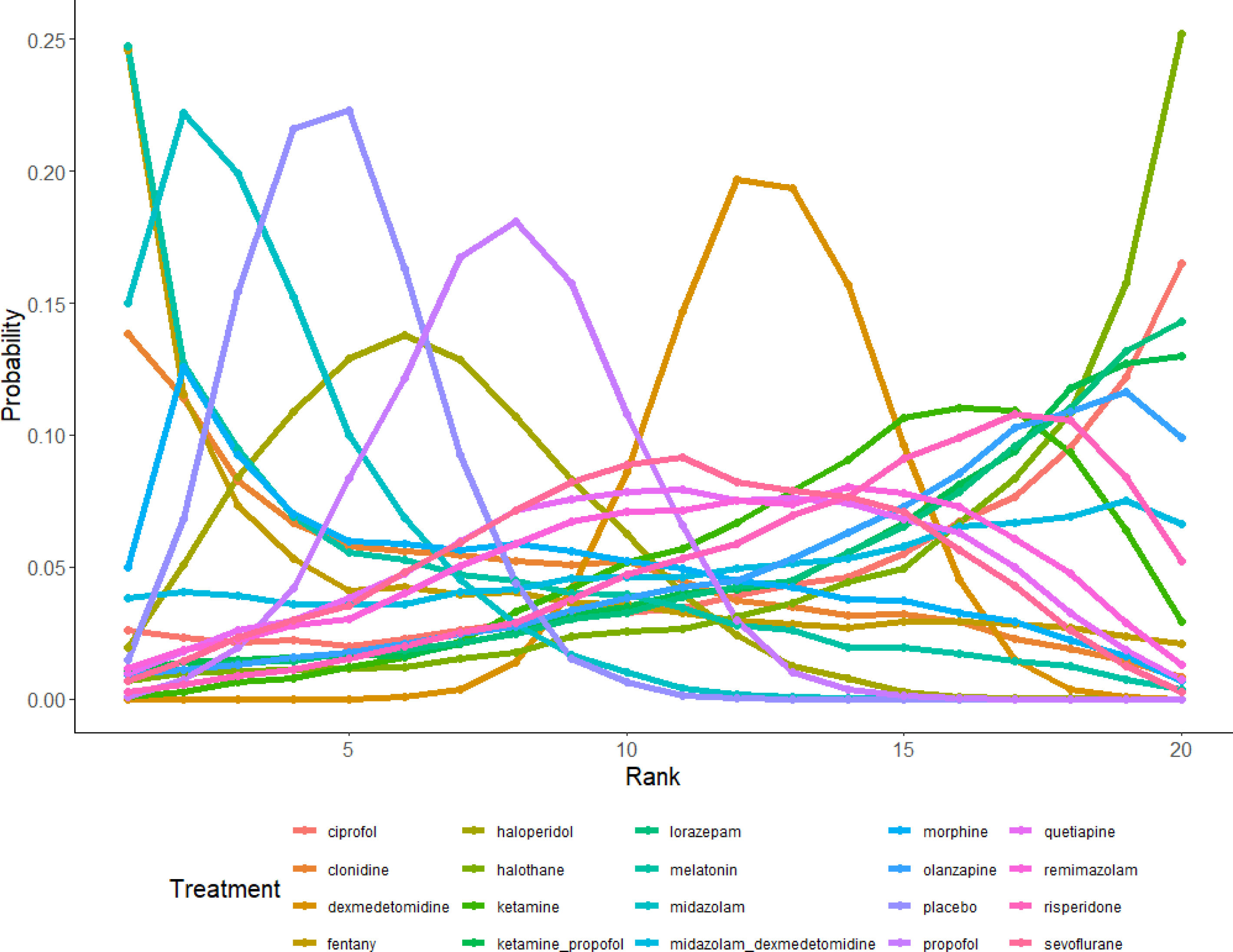
Efficacy rankingBased on interval estimates from both direct and indirect comparisons (Supplementary Table 4), dexmedetomidine was found to be the most effective in preventing delirium. The ranking of interventions is depicted in Fig. S7 and Fig. 5, showing that dexmedetomidine had the highest probability of ranking first, while halothane had the highest probability of ranking last.
DiscussionInterpretation of results and comparison with previous researchIn this systematic review and network meta-analysis, we are the first to conduct an efficacy analysis of 20 drugs for the prevention of delirium. We included 80 studies evaluating the efficacy of 20 drug interventions in preventing delirium. Our findings suggest that two interventions, dexmedetomidine and ketamine, may reduce the likelihood of delirium compared to placebo. Numerous studies have indicated that dexmedetomidine can decrease delirium incidence; however, the heterogeneity was substantial, possibly due to small sample sizes and differences among the studied subjects. This review confirms that, compared to placebo, dexmedetomidine and ketamine reduce the occurrence of delirium. We also note that our findings regarding dexmedetomidine and delirium occurrence align with other systematic reviews.101,76 However, many studies have shown that ketamine does not reduce the incidence of delirium.102–104 Based on the evidence in this review, clinicians may consider using dexmedetomidine for delirium prevention. The evidence network in our review provided further support, but given the risk of bias (e.g., lack of blinding), indirectness, imprecision, and heterogeneity, the quality of evidence is very low, warranting caution in interpreting and applying these results.
Strengths and limitations of this studyThe main strength of this review is the inclusion of a wide range of interventions in the NMA, providing a comprehensive examination of the efficacy of 20 drug interventions for preventing delirium, which enhances the generalizability of the results. Given the large sample sizes and narrow confidence intervals applied in this network meta-analysis, we believe the findings are reliable. However, this review has some limitations. First, there was high heterogeneity in the results, possibly due to variations in gender ratios, racial differences, locations, and concurrent use of other medications among the subjects. Second, some studies had relatively small sample sizes and a limited number of studies, which may affect the applicability and accuracy of the results.
ConclusionThis network meta-analysis compared the efficacy of 20 different drug interventions for preventing delirium. Dexmedetomidine emerged as the only effective medication for delirium prevention. This review provides evidence for clinicians that dexmedetomidine can be used to prevent delirium.
Ethical considerationsThis study does not involve ethics.
FundingThis article has no funding source. The publication fee is to be paid by the corresponding author.
Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Xiangwu Zhou and Chengguo Yin generated the research questions and designed and led the implementation of the review. Xiangwu Zhou, Chengguo Yin, and Chaohuan Chen led the statistical analysis and contributed to the protocol, data extraction, and interpretation of results. All authors approved the final manuscript and bear final responsibility for the decision to submit for publication. Chengguo Yin is the guarantor.