Biosimilar drugs represent a valuable opportunity for healthcare systems worldwide, offering substantial cost savings while ensuring equivalent efficacy and safety in treating chronic conditions. These savings can be reinvested in ongoing medical innovation.
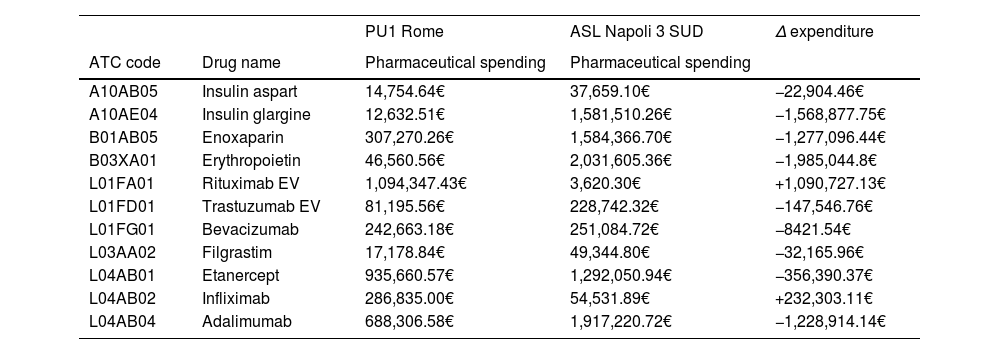
MethodsAn investigation was carried out to evaluate the utilization of key biologic therapies across various clinical indications within two Italian healthcare institutions: Asl Napoli 3 Sud and Policlinico Umberto I in Rome. Information regarding consumption and expenditure was extracted from institutional databases. A comparison with figures from 2021 was conducted to detect any growth in biosimilar adoption throughout 2022.
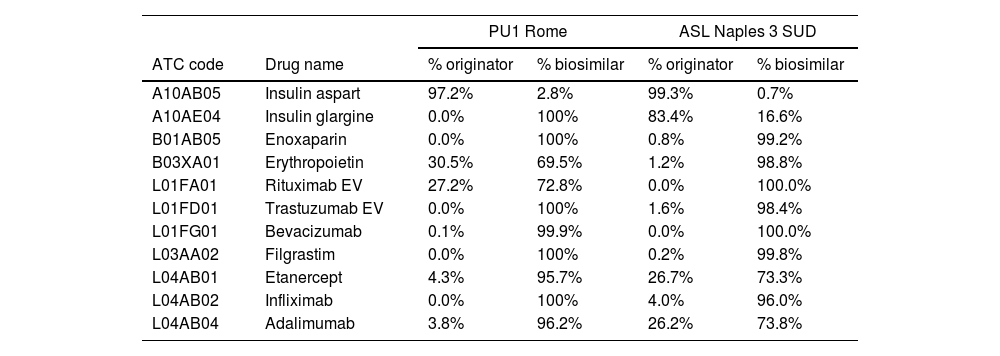
ResultsThe 2022 review demonstrated that most of the examined compounds were administered predominantly as biosimilars, with proportions nearing complete substitution, aside from some notable outliers. In particular, reluctance persisted in prescribing biosimilar adalimumab (73.8%) and etanercept (73.3%) at Asl Napoli 3 Sud, as well as erythropoietin (69.5%) and rituximab (72.8%) at Policlinico Umberto I.
ConclusionThe year-on-year comparison between 2021 and 2022 underscored a growing shift toward biosimilar prescriptions. This favorable direction indicates the likelihood of reaching full implementation soon, with meaningful advantages for the National Healthcare Service and citizens, promoting an efficient and economically viable model of care.
Los medicamentos biosimilares representan una valiosa oportunidad para los sistemas de atención sanitaria de todo el mundo, ya que ofrecen importantes ahorros de costes y garantizan una eficacia y seguridad equivalentes en el tratamiento de enfermedades crónicas. Estos ahorros se pueden reinvertir en los nuevos descubrimientos de innovaciones médicas.
MétodosSe realizó un estudio para evaluar el uso de los principales medicamentos biológicos (originales y biosimilares) en diferentes áreas terapéuticas en dos entidades sanitarias italianas, Asl Napoli 3 Sud y Policlinico Umberto I en Roma. Los datos de costos y consumicion fueron obtenidos de las bases de datos de las empresas. Además, se realizó una comparación con los datos de 2021 para identificar cualquier aumento en la utilización de medicamentos biosimilares durante 2022.
ResultadosEl análisis de 2022 reveló que la mayoría de los principios activos estudiados se han administrado como medicamentos biosimilares, con porcentajes cercanos al 100%, salvo algunas excepciones. En particular, se mantuvo la resistencia en la prescripción de los biosimilares adalimumab (73,8%) y etanercept (73,3%) en Asl Napoli 3 Sud, y de eritropoyetina (69,5%) y rituximab (72,8%) en Policlinico Umberto I.
ConclusiónUna comparación entre 2021 y 2022 destacó la creciente adopción de medicamentos biosimilares. Esta tendencia positiva sugirió el potencial de una utilización plena en el futuro cercano, beneficiando significativamente al Sistema Nacional de Salud y a la población, asegurando un enfoque de atención médica que sea a la vez eficaz y sostenible.