Edited by: Em. Professor Gualberto Buela-Casal
(University of Granada, Granada, Spain)
Dr. Katie Almondes
(Federal University of Rio Grande do Norte, NATAL, Brazil)
Dr. Alejandro Guillén Riquelme
(Valencian International University, Valencia, Spain)
Last update: December 2025
More infoPoor sleep is pervasive in modern society. Poor sleep is associated with major physical and mental health consequences, as well as with impaired cognitive function. Less is known about the relationship between sleep and emotional and interpersonal behavior. In this work, we investigate whether poor sleep impairs empathy, an important building block of human interaction and prosocial behavior. We aimed to capture the effects of poor sleep on the various aspects of empathy: trait and state, affect and cognition.
Study 1 (n = 155) assessed daily habitual sleep over several days, and global sleep quality in the past month. Participants who reported worse sleep quality exhibited lower empathic caring and perspective-taking traits. Study 2 (n = 347) induced a one-night disruption of sleep continuity to test a causal relationship between sleep and empathy. Participants in the sleep disrupted condition had to briefly wake up five times over the night, whereas the sleep-rested controls slept normally. In the next morning, participants’ empathy and prosocial intentions were assessed. Participants in the sleep disruption condition exhibited lower empathic sensitivity and less prosocial decision-making than sleep-rested controls.
The main contribution of this work is in providing a robust demonstration of the multi-faceted detrimental effects of poor sleep on trait and state empathy. Our findings demonstrate that poor sleep causally impairs empathic response to the suffering of others. These findings highlight the need for greater public attention to adequate sleep, which may impact empathy on a societal level.
Statement of Significance
This work comprehensively investigated the causal interaction between poor sleep and empathy and identified the following novel findings:
Lower self-reported sleep efficiency, assessed over several nights, is strongly correlated with lower empathic sensitivity traits across individuals.
Experimental sleep disruption during a single night causally impairs state empathic sensitivity to other's pain and consequentially reduces prosocial decision-making the following morning.
Taken together, the present findings reveal that empathic response to the suffering of other humans is causally influenced by poor sleep.
Sufficient and adequate sleep is essential for maintaining physical health (Cappuccio et al., 2010; Leger & Bayon, 2010), mental health (Ben Simon et al., 2020), and cognitive function (Jewett et al., 1999; Killgore, 2010). Recent research highlights that good health relies on both the quantity and quality of sleep, including aspects like continuity and nighttime awakenings. Yet sleep deficiency is highly prevalent throughout many industrialized societies. Approximately 15–20 % of the global adult population suffers from sleep disruption, insomnia, and insufficient sleep duration (Kocevska et al., 2020). Poor sleep, characterized by declines in both quality and quantity, is highly prevalent, particularly among parents of young children (Varma et al., 2020), the elderly (André et al., 2019), shift workers (Akerstedt & Wright, 2009), patients with obstructive sleep apnea (Kimoff, 1996) or traumatic brain injury (Lucke-Wold et al., 2015) and during stressful life events (Mandelkorn et al., 2021). Importantly, poor sleep in these patient populations has been independently associated with mortality-consequential conditions like hypertension (Krause et al., 2023; Ren et al., 2022), cognitive decline and degenerative dementia (André et al., 2019; Kaneshwaran et al., 2019; Wu et al., 2023), depression, anxiety, and suicidality (Ehlers et al., 2023; Finan et al., 2019; Martin et al., 1996; Yiyue et al., 2023).
Research to date focused mainly on the health and cognitive consequences of poor sleep for the individual. However, as humans are a prosocial species, it is important to understand the potential impact of poor sleep on one's interaction with others, and in particular, their prosocial behavior, a domain carrying robust social consequences. Only a few studies have directly tested the potential impact of insufficient sleep on social behavior (Holbein et al., 2019). Our goal here is to examine whether poor sleep affects empathy, a fundamental building block of social interaction. Empathy is defined as the ability to understand and share the thoughts and feelings of others (Batson et al., 1991; Gordon-Hecker et al., 2024; Singer et al., 2004). It enables individuals to function beyond self-centeredness and serves as an essential precursor of prosocial behavior (Preston & de Waal, 2001; de Waal, 2008). Empathy can be divided into trait and state constructs (Cuff et al., 2016). Trait empathy refers to a stable and enduring personality characteristic that reflects an individual's general tendency to understand and respond to the emotions and experiences of others. It can be influenced by both genetic and environmental factors (Abramson et al., 2020; Derntl et al., 2010). On the other hand, state empathy refers to empathic variations within an individual over time. State empathy can be influenced by changes in one's immediate environment or social setting or even one's current mood (Hodges & Wixwat, 2022; Westman et al., 2013). Mechanistically, both trait and state empathy operate via at least two main routes. The first is known as “empathic concern”. It is an affective route that reflects one's ability to feel concern for unfortunate others (Uzefovsky et al., 2015; Zaki & Ochsner, 2012). A second route of “perspective-taking” is more cognitive and refers to the ability of the perceiver to adopt other people's points of view and to evaluate that person's state (Decety, 2015; Decety et al., 2016; Shamay-Tsoory et al., 2009)
Several reasons motivate our overarching hypothesis that poor sleep impairs multidimensional aspects of empathy. First, reduced sleep quality and duration impair basic cognitive operations that contribute to empathy-related decision-making, such as attention, motivation, memory, and executive function (Alhola & Polo-Kantola, 2007; Choshen-Hillel et al., 2021). Second, poor sleep impacts affective and emotional processes (Gordon & Chen, 2014), including mood regulation (Mendoza, 2024), emotional information processing, and numerous aspects of sociality (Ben-Simon & Walker, 2018; Grèzes et al., 2021; Tempesta et al., 2018). Even modest variations in sleep quality and duration from night to night predict worse next-day affective outcomes, such as anxiety, withdrawal from interacting with others, and lack of desire to help others in need (Ben-Simon et al., 2020;Ben Simon et al., 2022). Third, sleep loss has been shown to impair brain activity across functional circuits that are independently linked to empathy (Guo et al., 2023; Krause et al., 2023; Muzur et al., 2002; Zhang et al., 2021). Among these circuits is the ‘social cognition network’ (Krendl & Betzel, 2022), which is activated when empathizing with others and includes the medial and posterior cingulate cortex as well as the temporoparietal junction. Furthermore, data points to the involvement of the nociceptive regions from the ‘pain matrix,’ such as the anterior insula, sensorimotor cortex, and anterior cingulate cortex, which is activated when witnessing another's pain (Fallon et al., 2020).
Emerging evidence from small cohorts (n's of 15–35) offers tentative support for a link between sleep and empathy. For instance, total sleep deprivation for over two days reduces self-reported emotion recognition (Killgore et al., 2008). Moreover, one night of total sleep loss diminishes emotional empathy in response to pictures of facial expressions, compared to controls (Guadagni et al., 2014). Self-reported poor sleep over the last month is associated with lower empathic sensitivity to negative images (Guadagni et al., 2017). In contrast, a larger study (n=86) using 3-hour of sleep restriction reported no impact on empathic responses to images of people in pain (Tamm et al., 2017). Similarly, five hours of sleep restriction for five consecutive nights did not, on average, reduce the motivation to empathize (Amicucci et al., 2021).
Most previous studies documented impairments in empathy resulting from extreme sleep deprivation interventions, such as not getting any sleep for 24 hours. The present research tests the effect of relatively mild disruption to sleep duration and continuity, which are far more common in real life. For example, a parent who wakes up several times during the night to care for a newborn will experience reduced sleep continuity (i.e., sleep fragmentation) as well as insufficient sleep duration. Interestingly, experimental and ecological variations in sleep continuity have been recently linked to other forms of social behavior, such as voting tendency, and moral behavior (Barnes et al., 2015; Gunia et al., 2021; Holbein et al., 2019).
Our overarching hypothesis is that poor sleep impairs empathy for the suffering of other individuals. Study 1 tests the cross-sectional association between self-reported habitual sleep and trait empathic abilities, including empathic concern and perspective-taking. We hypothesize that poorer self-reported sleep is associated with lower empathy traits. Study 2 complements Study 1 by employing an experimental approach to investigate the causal effects of sleep disruption on state empathy. We hypothesize that the disruption of sleep reduces empathy.
MethodsStudy 1Participants. Participants were recruited online through Amazon Mechanical Turk (MTurk). They were each paid between $4.25 and $5.75, depending on the number of daily surveys they have completed. Enrollment was restricted to those with IP addresses in the United States, and a prior online MTurk approval rating of 95 % or higher. Exclusion criteria included the confirmed diagnosis of a sleep disorder and/or a current diagnosis of an Axis 1 psychiatric disorder. A total of 171 eligible participants were enrolled in the study. Participants who reported extreme habitual sleep duration (less than 3 hours or more than 12 hours) or very low sleep efficiency(<65 %) across the 4 days were also excluded. The final sample included N = 155, 41 % female, Mage = 36.99, SDage = 9.75, yielding a total of 472 valid sleep logs across the study period.
Procedure. Participants enrolled in a multi-day sleep study which included a multi-dimensional measurement of trait empathy (Interpersonal Reactivity Index, IRI (Davis, 1983), see more details below under Measures), measurement of overall sleep quality in the past month (PSQI questionnaire (Buysse et al., 1989)) and demographic questions including age and sex (see Fig. 1 for study design). Participants were then asked to complete daily sleep diaries (Simon et al., 2022), quantifying their sleep across four consecutive nights. To ensure good recall, participants were requested to complete the sleep diary as close as possible to their wake-up time. All assessment days were weekdays, to avoid weekend changes in sleep patterns.
Experimental Design, Study 1 and Study 2
In Study 1 (top), 155 participants completed sleep logs across a 4-day assessment of habitual sleep together with a collection of demographics and trait empathy scores. In Study 2, participants’ empathic responses were evaluated either after a night of rested sleep (N=168) or after a night of sleep disruption (N=179). Empathic responses were assessed using reactions to images and to two scenarios depicting individuals in pain.
- •
PSQI- Sleep quality during the last month was assessed using the Pittsburgh Quality Sleep Index (PSQI; (Buysse et al., 1989)); higher scores represent poorer subjective sleep quality; any score greater than 5 is considered indicative of poor sleep.
- •
IRI - The Interpersonal Reactivity Index is a multidimensional measure of trait empathy including a set of related scales, each probing a separate facet of empathy (Davis, 1983). (1) Perspective Taking, measures one's ability to adopt another's point of view, (2) Fantasy, measures one's tendency to imagine the senses and feelings of fictitious characters, (3) Empathic Concern, measures one's feelings of concern for unfortunate others, and (4) Personal Distress, measures one's feelings of unease and anxiety upon seeing other people's misfortune. In general, perspective-taking and empathic concern are considered the positive facets of empathy, as they relate to approach behaviors and pro-sociality (Decety et al., 2016). The personal distress facet is considered negative and is related to self-focus and avoidance behavior, and may even be a barrier to empathic interactions (Kim & Han, 2018).
- •
Sleep Logs – Daily sleep logs asked participants each morning about their sleep the previous night. Each log was available online only during a specific time window in the morning (until 1:00 pm local time), and participants were requested to complete the survey as close as possible to their wake-up time. Each log included the following 5 questions: 1) What time did you go to bed last night? 2) How long did it take you to fall asleep last night? 3) What time did you wake up this morning? 4) How many times did you wake up during the night? 5) If you woke up during the night, how long did it take you to get back to sleep? From these logs, sleep duration and sleep efficiency were calculated for each night and later averaged to obtain habitual sleep parameters per participant.
- •
Personal details – sex, age, and local time zone.
Self-reported sleep efficiency was calculated using participants’ daily sleep diaries, based on the percent of time asleep out of total time in bed (i.e., total time in bed minus sleep latency and time spent awake after sleep onset). Sleep duration was calculated as the total time elapsed from sleep onset to wake time minus sleep latency and time spent awake after sleep onset. Global sleep quality was measured using the total score of the PSQI, with a score higher than 5 denoting poor sleep quality. The average sleep efficiency across participants and days was M = 90 %, SD = 8.5 %. The average sleep duration was M = 7.51 hours, SD = 1.40. The average sleep quality over the last month, measured by the PSQI, was M = 6.95, SD = 3.80. These sleep metrics were in line with data from other studies on the general population (Hirshkowitz et al., 2015; Mollayeva et al., 2016).
Data Analysis. Analyses focused on self-reported sleep efficiency and sleep duration, given previous work linking these sleep parameters to social and emotional functioning (Ben Simon et al., 2020). Pearson's correlations were calculated between self-reported sleep efficiency and sleep duration and the four sub-scales of the IRI: Empathic Concern, Perspective-Taking, Personal Distress, and Fantasy. To ensure specificity, additional regression models were further adjusted for age and sex in each model. For global sleep quality, a t-test was used to compare empathy traits among poor and good sleepers.
The data and materials are publicly available at https://osf.io/3rfht/?view_only=6528f871d7f34525a87276f0424f4c84.
All statistical analyses were performed in IBM SPSS version 29.0.2.0 and the Pingouin library implemented in Python (Vallat, 2018).
The study was approved by the local human studies committee of the University of California Berkeley, with all participants providing written informed consent.
Study 2Participants. Six hundred and thirty adults living in the UK participated in a multi-part study on the online platform Prolific. Participants who failed to complete all parts of the study were excluded from the analysis (N=283). The final sample consisted of N = 347, 70 % female, Mage = 35.49, SDage = 12.44.
Procedure. Participants were invited to take part in a multi-part study (see Fig. 1 for study design). They were told that they may be asked to wake up during the night. All participants completed a background questionnaire between 6:00 to 8:00 pm. This questionnaire included a PSQI questionnaire (Buysse et al., 1989), and demographic questions (see below). The participants were then randomly assigned to one of two conditions- sleep disruption or sleep-rested. Participants in the sleep disruption condition were asked to wake up and complete a 1-minute questionnaire every two hours between 10:00 pm - 6:00 am. Each wake-up questionnaire consisted of a 10-item I- PANAS-SF (Thompson, 2007), and a question on how tired the participant felt at the moment on a 1–5 scale (see below). As pre-registered, only participants who completed at least 4 of the 5 wake-up questionnaires were included in the analysis. No additional exclusion criteria were applied. Participants in the sleep-rested condition were not asked to wake up or complete any questionnaires during the night.
The following morning, between 8:00 - 10:00 am, participants in both conditions were asked to complete a final 6-minute empathy questionnaire. The empathy questionnaire included two empathy-related decision-making scenarios, followed by an empathy-for-pain task and questions regarding sleep quality that night (see below).
The wake-up and empathy questionnaires were sent to the participants via their private inboxes on the Prolific platform at the allotted times. In both conditions, participants were encouraged to set an alarm clock to help them wake up and remember to complete their questionnaire(s) on time. The experimental team ensured that participants responded to the questionnaires at the allotted time range by verifying the timestamps of participants’ responses through Qualtrics’ software.
Participants in the sleep-rested condition were paid 2.5 GBP, whereas participants in the sleep disruption condition were paid 10 GBP (see (Barnes et al., 2015; Gunia et al., 2021) for a similar design and compensation scheme). Participants who did not complete all parts of the study received partial compensation. Since we expected a higher attrition rate in the sleep disruption condition, we recruited more participants in this condition (n=373), than in the sleep-rested condition (n=257). Indeed, the attrition rate in the sleep disruption condition was greater than in the sleep-rested condition (52 % vs. 35 % respectively). The final samples were similar in size (n=179 in the sleep disruption condition, n=168 in the sleep-rested).
MeasuresBackground questionnaire- •
PSQI- see Study 1.
- •
MCTQ- Chronotype was assessed using the Munich Chronotype Questionnaire (MCTQ (Roenneberg et al., 2003)). The MCTQ uses the midpoint of sleep onset and offset to estimate participants’ chronotypes. Participants were classified as “morning chronotype” or “evening chronotype” based on a median split.
- •
Personal characteristics- sex, age, weight, height, family status, hours slept last night.
- •
I-PANAS-SF- participants were asked to rate on a scale of 1 to 5 the extent to which they felt 10 different emotional states (Thompson, 2007).
- •
Sleepiness assessment - participants were asked to rate on a scale of 1 to 5 “How tired are you right now?”
- •
Pain scenarios. Participants were presented with 2 written hypothetical scenarios in a fixed order, the first describing an employee in pain and the second describing a friend in pain (see appendix for the full scenarios). In each scenario, the participants were asked to:
- (1)
Estimate the pain that the protagonist felt on a scale of 0–100.
- (2)
Rate the level of empathy they felt for the protagonist on a scale of 1 (No empathy at all) to 7 (Very high empathy).
- (3)
Indicate what they would have recommended to the protagonist, ranging from 1 (“Wait and see if the pain passes”) to 4 (“Seek immediate medical treatment”).
- (1)
- •
Empathy for pain pictures- This task was designed to measure pain-related affective empathy (Choshen-Hillel et al., 2022; Jackson et al., 2005a). Twelve digital color pictures were presented in random order, each showing a right hand or foot. Ten of the pictures served as target items, showing a hand or foot in a painful situation. Two additional pictures served as attention checks, showing a hand or foot in a non-painful situation. These two pictures also allowed us to test whether the effect is unique to reactions to painful stimuli. Participants were asked to rate the intensity of their emotional reaction when seeing each of the 12 pictures, using a 9-point self-assessment manikin (SAM) scale (see Fig. 2). For each participant, we calculated an empathy-for-pain score by averaging their ratings for the 10 target pictures. The two non-pain pictures were averaged separately.
Fig. 2.Example of a trial in the empathy for pain task, Study 2
A representative picture from the Empathy for Pain task used in Study 2 (from (Jackson et al., 2005b)) along with the Self-Assessment Manikin (SAM) scale used in this study.
- •
Subjective sleep quality- Participants were asked to report how many hours they slept that night and rate their sleep quality on a 1 (“Very bad”) to 6 (“Very good”) scale.
Sleep characteristics and demographic variables by condition are presented in Table 1. As can be seen from the table, participants in the sleep disruption condition reported lower sleep quality and shorter sleep duration, indicating a successful manipulation.
Demographics and sleep characteristics in Study 2 by condition.
| Sleep disruption condition | Sleep-rested condition | Difference | |
|---|---|---|---|
| Age# (M, SD) | 34.67 (11.65) | 36.36 (13.20) | p = .104 |
| Gender# (% female) | 70 | 68 | p = .747 |
| PSQI# (M, SD) | 7.26 (3.62) | 6.42 (3.03) | p = .020 |
| Morning chronotype# (%) | 35 | 40 | p = .261 |
| Evening chronotype# (%) | 40 | 34 | p = .219 |
| Sleep quality* (M, SD) | 2.78 (1.16) | 4.35 (1.19) | p < .001 |
| Sleep duration* (M, SD) | 5.49 (1.68) | 6.74 (1.26) | p < .001 |
Data Analysis. We compared the measures of the pain scenarios and the empathy for pain task between the two experimental conditions by t-test or Mann-Whitney test as appropriate. In order to rule out the possibility that the difference between conditions resulted from differences in PSQI, for each of the empathy measures, we conducted multivariable regression analyses with condition and PSQI as predictors.
The sample size, hypotheses, exclusion criteria, and analysis plan for Study 2 were all pre-registered at https://aspredicted.org/blind.php?x=43ii2k.
The data and materials are publicly available at https://osf.io/3rfht/?view_only=6528f871d7f34525a87276f0424f4c84.
All statistical analyses were performed in IBM SPSS version 29.0.2.0 and and the Pingouin library implemented in Python.
The study was approved by the Hebrew University Business School IRB committee.
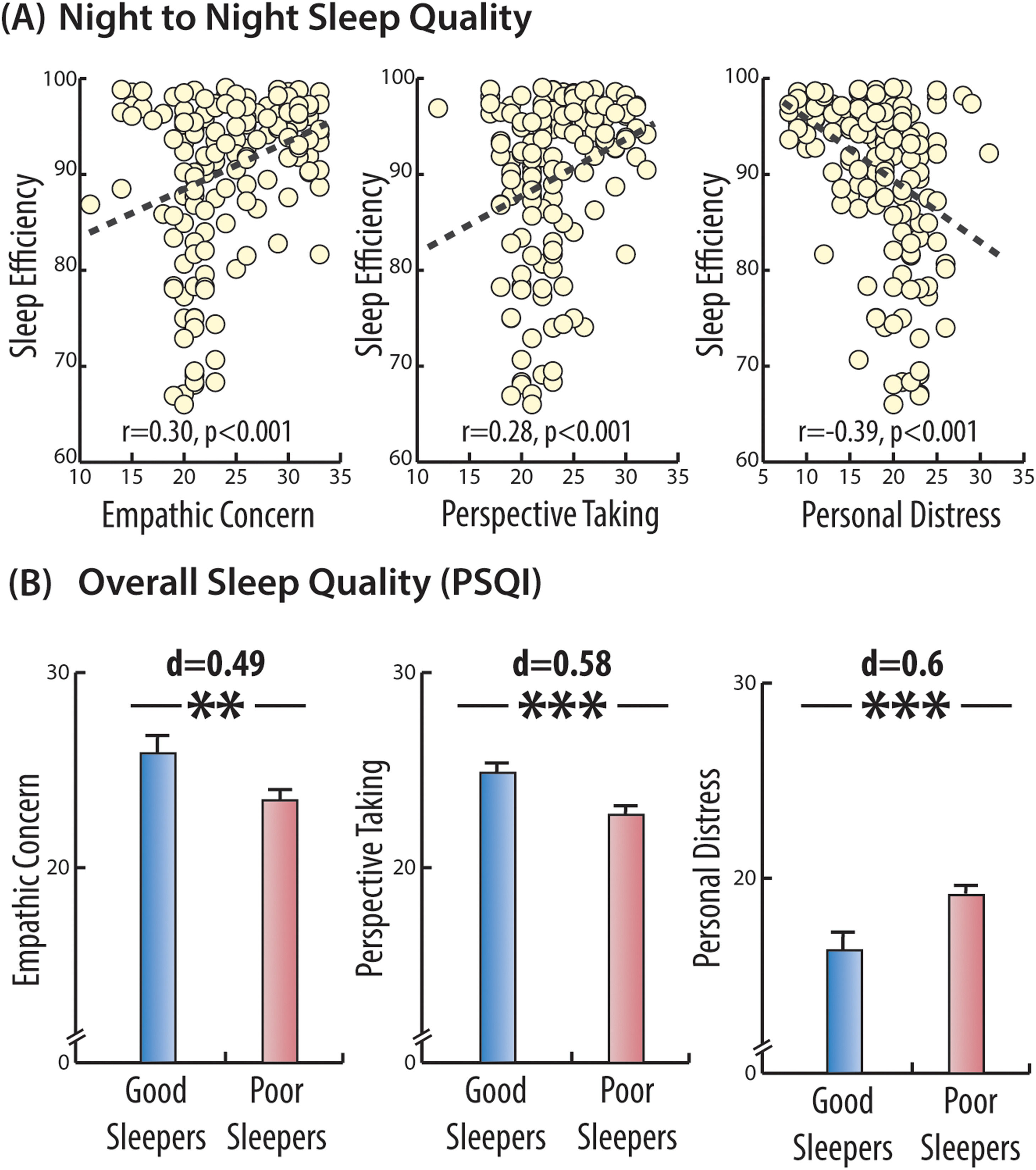
ResultsStudy 1Worse self-reported sleep efficiency was associated with lower levels of Empathic Concern (r = 0.30, p < .001) and Perspective-Taking skills (r = 0.28, p < .001) but with higher levels of Personal Distress (r = -0.39, p < .001, see Fig. 3 a). Self-reported sleep efficiency was not associated with the Fantasy scale (r = 0.09, p = .258). Shorter sleep duration was positively associated with lower Empathic Concern (r = 0.197, p = .014), but not with the remaining sub-scales (|r’s| < 0.03, p’s > 0.687).
Cross-sectional associations between self-reported sleep efficiency and trait empathy, Study 1.
Several key domains of the Inter-Personal Reactivity Index (IRI) were significantly associated with better sleep efficiency, as assessed in Study 1 using both daily sleep logs (top panel) as well as global sleep quality (PSQI, bottom panel). These associations include a positive correlation with Empathic Concern and Perspective Taking as well as a negative correlation with the domain of Personal Distress (top panel). Asterisks denote significant differences between groups (*p < .05; ** p < .01 and *** p < .005).
To examine the specificity of sleep parameters on empathy, we entered both self-reported sleep efficiency and sleep duration into a regression model together with age and sex, with empathic concern set as the key outcome variable. In this model, self-reported sleep efficiency was significantly associated with increased empathic concern, (β = 0.157, t(155) = 3.37, p < .001), however, sleep duration was not a significant predictor of empathic concern (β = 0.007, t(155) = 1.68, p = .100). The model suggests that the association between self-reported sleep efficiency and empathic concern is independent of any impact age or sex might inflict on empathic abilities.
Global sleep quality and trait empathyNext, we examined whether sleep quality over the past month, as assessed by the PSQI, was also related to empathy traits. We tested whether “good sleepers” (PSQI <5, N=51) had higher empathy scores than “poor sleepers” (PSQI≥5, N=104). Good and poor sleepers did not differ in terms of age (MPoor = 36.8, SD = 9.3 vs. MGood = 37.4, SD = 10.7, t(88.25) = -0.355, p = .723, Cohen's d = 0.06) nor gender (χ2=0.01, p = .922). Similar to the association found for the night-to-night sleep efficiency, here too, worse sleep quality over the past month was associated with lower empathy scores, both in terms of Empathic Concern (MPoor = 23.45, SD = 4.81 vs. MGood = 25.86, SD = 5.21, t(92.65) = -2.78, p = .007, Cohen's d = 0.49) and Perspective-Taking skills (MPoor = 22.71, SD = 3.58 vs. MGood = 24.86, SD = 3.9, t(92.27)= -3.31, p = .001, Cohen's d = 0.58). "Poor sleepers" also had higher Personal Distress scores, (MPoor = 19.13, SD = 4.4 vs. MGood = 16.29, SD = 5.34, t(83.73) = 3.27, p = .002, Cohen's d = 0.60, see Fig. 3 b). There was no difference on the Fantasy Scale (MPoor = 21.05, SD = 4.7 vs. MGood = 21.70, SD = 4.37, p = .392).
Taken together, these findings support a cross-sectional association between worse self-reported sleep quality, assessed at both short and long-term scales, and lower empathic care and perspective-taking skills. The association between worse sleep quality and higher personal distress is in line with the previously described association between poor sleep and self-focus or neuroticism traits (Duggan et al., 2014). In the next study, we experimentally manipulated sleep to test its causal effect on state empathy the following day.
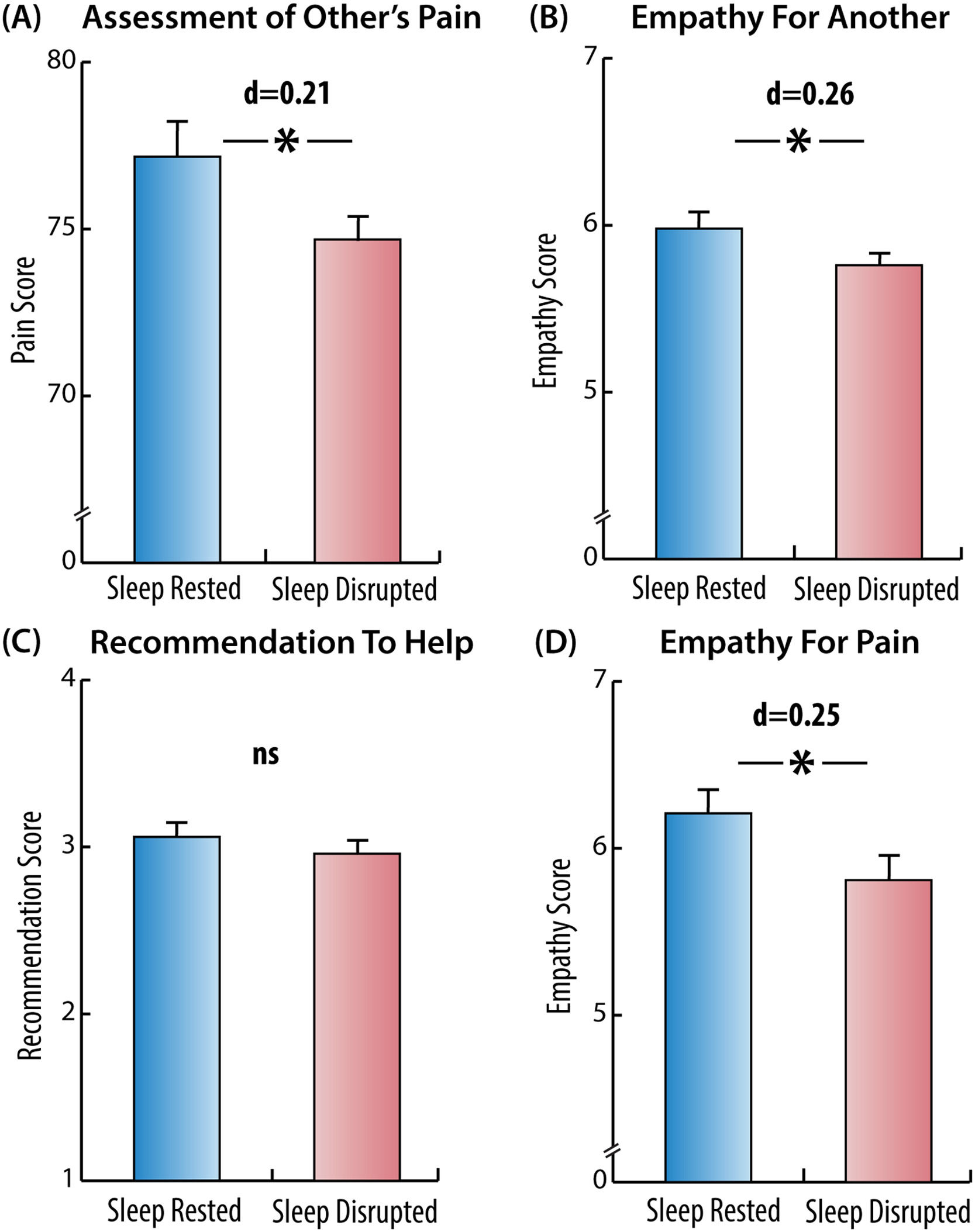
Study 2Pain scenariosAcross the two scenarios, participants assessed the protagonists’ pain as less intense in the sleep disruption than in the sleep-rested condition (M = 74.69, SD = 11.29 vs. M = 77.17, SD = 12.19, t(345) = 1.96, p = .050, Cohen's d = 0.21) (Fig. 4 A). Participants in the sleep disruption condition also reported lower empathy for the protagonist (M = 5.76, SD = 0.86 vs. M = 5.98, SD = 0.89, t(345) = 2.40, p = .017, Cohen's d = 0.26) (Fig. 4 B). These results suggest that poor sleep impairs a cognitive, perspective-taking, aspect of state empathy for pain.
Effect of sleep disruption on empathy measures, Study 2.
Sleep disruption significantly impaired several empathy measures, as assessed in Study 2. Empathy measures include: (A) assessed levels of the protagonists’ pain in the scenarios, (B) reported levels of empathy for the protagonists in the scenarios, (C) recommendations given to the protagonists in the scenarios, (D) emotional responses to pictures of people in pain. Asterisks denote significant differences between groups (p <0.05).
Across the two scenarios, however, participants did not make different recommendations to the protagonist in the two conditions (M = 2.96, SD = 0.65 vs. M = 3.06, SD = 0.67, Mann-Whitney U = 1.35, p = .177) (Fig. 4 C), which would indicate that poor sleep does not impair cognitive decision making uniformly. A post-hoc analysis of each scenario separately demonstrated that in the first scenario (employee), the recommendations did not differ between the two conditions (M = 2.89, SD = 0.86 vs. M = 2.93, SD = 0.84, Mann-Whitney U = 0.34, p = .734). In the second scenario (friend), participants in the sleep disruption condition made weaker recommendations than sleep-rested (M = 3.03, SD = 0.72, M = 3.18, SD = 0.71, Mann-Whitney U = 2.05, p = .040). Thus, albeit post hoc, this analysis suggests that the impact of poor sleep on empathic sensitivity is greatest for empathy-evoking situations, i.e. situations in which the individual has a relationship with another person, and therefore an interactive social bond and an implicit social contract (to empathize with and receive empathy from).
Empathy for pain taskParticipants in the sleep disruption condition exhibited reduced emotional responses to people in pain, compared to the sleep-rested condition (M = 5.81, SD = 1.62 vs. M = 6.21, SD = 1.56, t(345) = 2.36, p = .019, Cohen's d = 0.25). The responses to the two non-pain depicting pictures did not differ between the conditions (M = 1.81, SD = 1.23 vs. M = 1.71, SD = 1.26, t(345) = -0.80, p = .423, Cohen's d = 0.07). This experimental result suggests a causal impact of disrupted sleep on the affective empathic response of an individual to the suffering of another person (Fig. 4 D).
Global sleep quality and state empathySince the PSQI, measured before the night of the experiment, differed between the conditions, it is possible that chronic poor sleep quality have influenced, to some degree, the different state empathic responses between the experimental conditions. Therefore, linear regression analyses were performed with the condition, the PSQI score, and their interaction as predictors of each of the empathy measures. The analyses demonstrated that even after controlling for PSQI, experimental sleep disruption still significantly predicted the change in empathic measures. Specifically, compared to the sleep-rested participants, sleep-disrupted participants assessed the protagonists’ pain in the scenarios as lower (β = -0.123, t(342) = -2.29, p = .023). Furthermore, their empathy for the protagonists was diminished (β = -0.130, t(342) = -2.41, p = .017), and they had reduced emotional reactions to the pain pictures (β = -0.121, t(342) = -2.24, p = .026).
PSQI did not predict any of the empathy measures: assessment of the protagonists’ pain in the scenarios, empathy for the protagonists, the recommendations, or the emotional reactions to the pain photos (all β’s ≤ 0.092, all p's ≥ 0.085). Finally, PSQI did not interact with the condition for any of the empathy measures (p’s > 0.716), indicating that those with chronic poor sleep quality were not affected by acute sleep disruption differently than those without.
DiscussionThis paper presents two key findings linking poor sleep with reduced empathy. Our first study assessed self-reported sleep efficiency daily over several days, and sleep quality globally for the last month. Our findings indicate that participants who report worse sleep efficiency exhibit significantly lower empathic caring and perspective-taking traits. An experiment that employed a sleep disruption manipulation supported a causal effect of poor sleep on empathic responses. Participants who had to briefly wake up five times a night, expressed less empathy in response to images and scenarios depicting people in pain, compared to participants whose sleep was uninterrupted. Sleep disruption also led participants to make less empathic decisions in response to certain scenarios.
The main contribution of this work is in providing a robust demonstration of the multi-faceted effect of poor sleep on empathy. Empathy is a complex construct characterized by stable traits and ad hoc fluctuations, with affective and cognitive components. Our work is unique in capturing the effects of poor sleep on the various aspects of empathy. Study 1 demonstrated the association between short- and long-term poor sleep on trait empathy, whereas Study 2 demonstrated the effect of poor sleep on state empathy. Each of the two studies examined affective measures (empathic concern trait, responses to images depicting painful situations) along with cognitive ones (perspective-taking trait, decision-making responses). Additionally, a sleep-induced impairment in empathy in Study 2 was evident both at the perception level (i.e., understanding another person's pain) and at the action level (i.e., offering help). Taken together, our findings add to an emerging literature (Ben-Simon & Walker, 2018; Simon et al., 2022) suggesting that poor sleep not only affects basic cognitive and emotional processing but also higher-order interpersonal interactions and social behaviors.
Our findings demonstrate that poor sleep causally impairs empathy. In Study 1, empathy traits were associated with self-reported sleep efficiency. In Study 2, disruptions to sleep continuity- affecting both sleep duration and sleep quality, but without imposing sleep restriction-demonstrated a clear effect on state empathy. Our findings contribute to the growing evidence that, in some cases, disrupted sleep continuity impairs the mental and physical recovery typically supported by adequate sleep (Bonnet & Arand, 2003;Forbes et al., 2008; Hawkley et al., 2010;Kurina et al., 2011; Hoopes et al., 2021; Fjell et al., 2023).
The relationship between global sleep quality (measured via PSQI) and empathy deserves further attention. In Study 1, PSQI was associated with several measures of empathic traits, suggesting a link between habitual sleep quality and basic empathic tendencies. However, in Study 2, baseline PSQI did not predict empathic responses the following morning after an acute sleep disruption. These seemingly inconsistent findings may potentially align: the impact of a single night of disrupted sleep on empathic responses may be so profound that it masks any baseline differences. Thus, both good and poor sleepers experience diminished empathy following acute sleep disruption.
The literature on sleep stages and emotional regulation may offer insights into the mechanisms underlying the effect of poor sleep on empathy. Specifically, rapid-eye-movement sleep (REM) has been associated with the processing and integration of emotional experiences overnight (Gujar et al., 2011; Tempesta et al., 2019). Furthermore, REM sleep has been shown to recalibrate the brain's sensitivity to specific emotions. For example, a nap containing REM sleep can reverse negative emotional reactivity to anger and fear while enhancing positive emotional responses (Gujar et al., 2011). Selective suppression of REM sleep increases next-day negative affect and enhances amygdala responses to social exclusion, underscoring the importance of REM sleep in emotional regulation (Glosemeyer et al., 2020). Beyond the importance of REM sleep to emotional calibration, recent findings also established a role for non-REM (NREM) Slow Wave Activity (SWA) in offering complementary effects on mood regulation and prosocial behavior. Both the amount spent in deep NREM sleep, as well as the electrical brainwave quality of that deep sleep indexed in SWA, have been shown to predict lower anxiety, better mood and increased prosocial behavior the next day (Ben-Simon et al., 2020; Finan et al., 2019; Studler et al., 2024). Given that frequent interruptions to sleep reduce both non-REM slow wave and REM sleep amounts (Finan et al., 2015; Zavodny et al., 2006) it is possible that interrupted sleep prevents the calibration of emotional and social processing necessary for intact empathic responses the next day. These hypotheses require further testing through PSG-recorded sleep disruption, which can be performed in a sleep laboratory but not through the large-scale online setting used in the current study.
The present studies examined variations in habitual sleep either during a normal day-to-day routine (Study 1) or following repeated awakenings during a single night (Study 2). Both mimic common and mild sleep disruptions that are pervasive in modern society, including everyday life events such as caring for young children or suffering from a viral illness or chronic pain. These stand out from the standard sleep deprivation manipulations (Guadagni et al., 2014; Killgore et al., 2008), which restrict sleep in an extreme way over many hours (sometimes days). Notably, despite the relatively mild manipulation of sleep used in this study, we find significant changes in empathy measures, with dire implications for numerous social contexts requiring empathy.
The implications of this work are particularly important in the context of medical providers (e.g., nurses, paramedics, and physicians), who suffer from continuous sleep disruption (Choshen-Hillel et al., 2021) and whose empathy is critical to successful medical outcomes and patient well-being (Decety, 2020). In a related study, we have shown that night shift work, which includes sleep disruption and sleep deprivation, as well as fatigue and stress, makes physicians less empathic to patients’ pain (Choshen-Hillel et al., 2022). Whereas different psychological mechanisms could explain the night shift effect on empathy, the present study causally demonstrates the crucial role of a rested night of sleep. The present findings may suggest that improving health professionals’ sleep can have beneficial effects on patient care.
Strengths and limitationsThe current work has notable strengths, including the causal manipulation of sleep continuity and diverse measures of empathy in large samples of the general population, from two different countries, with significant generalizability and applicability. Nonetheless, the study is not without limitations. The observational nature of Study 1 precludes determining the direction of the association between diminished empathic care, reduced perspective taking, and increased distress, to poorer self-reported sleep. We have suggested here that poor sleep impairs one's ability to focus on the other's perspective, care for them, and even act prosocially. However, it is possible that variations in certain empathic traits lead to poorer sleep. Nonetheless, Study 2 supports our directional hypothesis via an experimental intervention. It is worth noting that empathy measurements in study 2 could be driven by both trait and state abilities. Given that trait empathic abilities and susceptibility to sleep disruption vary among individuals, these factors could impact the empathic assessment in the morning. The large participant pool suggests that these tendencies would be equally distributed between the two groups, although we cannot entirely rule out an attrition bias. The observed differences in several outcomes, despite the uncontrolled setting and interpersonal variance, underscore the robustness of our findings. Future studies should employ a baseline assessment of empathy traits to accurately evaluate the association between a good night of sleep (or disrupted sleep) and state empathy alone. Finally, the self-reported nature of the measures of sleep assessments used in this work warrants attention, as people's self-reflection on their sleep is not always accurate (sleep state misperception) (Volkovich et al., 2016; Walton et al., 2024), especially among patients with chronic insomnia (Valko et al., 2021). It is possible that individuals with limited introspection about their sleep also struggle with extrospection, which is essential for empathy. This raises the possibility that biases in sleep reports - rather than actual sleep - are linked to empathy. The relationship between sleep misperception and empathic abilities is an intriguing idea for future research employing objective measures of sleep. However, the experimental design of Study 2, which directly manipulated sleep and did not rely on self-reports, strongly points to objective sleep disruption as the cause of impaired empathic responses the following morning.
ConclusionsOur modern society suffers from a sleep-loss epidemic (Centers for Disease Control and Prevention (CDC), 2011). The present findings reveal that our empathic response to the suffering of other humans is causally influenced by poor sleep. That is, our society may also be suffering from an empathy deficit endemic. Indeed, survey evidence has indicated a general trend toward decreasing empathic behavior in numerous industrialized societies (Konrath et al., 2011). Since empathic behaviors represent a fundamental binding factor of us, a prosocial species, there may be a need for greater public health education regarding the importance of sufficient and uninterrupted sleep.
Disclosure statementFinancial disclosureNone.
Non-financial disclosureNone.
Declaration of generative AI and AI-assisted technologies in the writing processDuring the preparation of this work, the authors used Chat GPT4 in order to improve the writing style. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the published article.
This work was supported by United States Israel Binational Science Foundation (BSF) grant 2022114 (AGH, SCH, MW, AP); Israel Science Foundation (ISF) grants 2824/22 (AGH) and 354/21 (AP and SCH), and the Recanati Fund at the Hebrew University Business School at the Hebrew University (SCH).