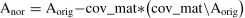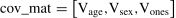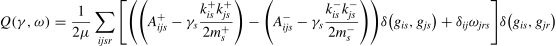Despite both internet gaming disorder (IGD) and gambling disorder (GD) being officially recognized as medical conditions by the World Health Organization, controversies persist. A transdiagnostic study may help inform classification and intervention approaches. IGD and GD may share or have distinct neural and behavioral features. To investigate, resting-state functional magnetic resonance imaging (fMRI) and self-reported behavioral data were collected from 58 individuals with GD, 31 with IGD, and 83 healthy control (HC) participants. After controlling for demographics, both GD and IGD groups scored lower on measures of gambling-related positive play. Neural data revealed reduced brain connectivity in the right rectus/orbital frontal gyrus in GD and IGD groups compared to HC participants. IGD participants displayed increased dynamic brain activity in the left triangular inferior frontal gyrus compared with GD and HC participants. Relatively decreased modular flexibility was also observed in GD but not IGD participants, relative to HC participants. Multiclass classification results showed that the indicators of gambling-related positive play, as well as dynamic brain activity and connectivity patterns, were useful for classifying GD, IGD, and HC participants, outperforming the use of either neural signals or self-report indicators alone. The shared phenotypes of GD and IGD groups provide insight into common features of behavioral addictions, and the combination of self-report and neural measures may provide the most robust approach for classification of diagnostic groups.
Gambling disorder (GD) involves persistent and recurrent problematic patterns of betting that lead to clinical impairment (e.g., sleep disturbances, emotional distress, and financial and relational problems; Ciccarelli et al., 2017; Parham et al., 2012). Internet gaming disorder (IGD) is similarly characterized by persistent and recurrent engagement in video gaming leading to negative outcomes (e.g., poor sleep, emotional distress, and academic problems; Benjet et al., 2023; Bonnaire & Baptista, 2019; Wong et al., 2020). Although both GD and IGD are considered medical conditions by professional health organizations (Chinese Society of Psychiatry, 2001; World Health Organization, 2022), the latter in particular has been recently debated regarding conceptualization, diagnostic symptoms, underlying mechanism(s), and classification (Jin et al., 2019; Kuss et al., 2017; Van Rooij & Kardefelt-Winther, 2017). Research is needed to better understand its prevalence, clinical course, and neurobiological underpinnings (American Psychiatric Association [APA], 2022). Common processes (e.g., dysfunctions in reward processing and inhibitory control; Antons et al., 2020) have been proposed between GD and IGD. Direct comparisons between GD and IGD provide direct insights into shared and unique elements that could help guide intervention development.
Phenotypic features constitute important elements of specific mental disorders (Courtenay et al., 2009; Cuijpers et al., 2010). Compared with healthy control (HC) participants, both individuals with GD and those with IGD have exhibited greater risk preference and higher impulsivity (Argyriou et al., 2017; Moccia et al., 2017; Schluter & Hodgins, 2021; Zhu et al., 2023). In addition, both GD and IGD have been associated with poor behavioral control (e.g., setting limits on time/money spent on playing; Costes & Bonnaire, 2022; Lavoie & Main, 2019). In GD, gambling-related positive play, as the indicator of individuals’ beliefs and behaviors referring to responsible and controlled gambling (Wood et al., 2017), has been found to be negatively correlated with GD (Tong et al., 2020; Zhou et al., 2022; Zhou & Wu, 2024).
Neuroimaging studies have demonstrated that people with GD and individuals with IGD exhibit shared differences from those without in brain activations during task performance and functional connectivity during resting state. For example, in studies examining dual process models using reward and control tasks, people with either GD or IGD have shown different activations from HC participants in the frontal cortex (e.g., orbital frontal cortex, dorsolateral prefrontal cortex /medial frontal cortex), anterior cingulate cortex, and striatum (Crockford et al., 2005; de Ruiter et al., 2012; Ding et al., 2014; Goudriaan et al., 2010; Ko et al., 2009, 2014; Luijten et al., 2017; van Holst et al., 2012; Yao et al., 2017). Resting-state fMRI, that records spontaneous neural activity and can be used to examine functional connectivity within and between brain networks (Hohenfeld et al., 2018; Smitha et al., 2017), suggests analogous neurobiological bases of GD and IGD. For example, altered resting-state functional connectivity between regions of frontoparietal network and that of the limbic network has been observed in people with IGD (See a review; Yan et al., 2021), and functional connectivity differences in frontal areas and part of the limbic network, striatal regions, have been reported in people with versus without GD (Jung et al., 2014; Koehler et al., 2013; Tschernegg et al., 2013).
Despite similarities across GD or IGD in studies investigating each condition separately, few neuroimaging studies have directly examined GD and IGD concurrently. To the authors’ best knowledge, only one resting-state fMRI study, in which 14 participants with internet-based GD, 15 with IGD, and 15 HC participants were compared for their stationary resting-state functional connectivity in default-mode, cognitive control, and reward networks (Bae et al., 2017). Bae et al. (2017) found internet-based GD and IGD groups shared decreased functional connectivity in the default-mode network while exhibiting differences in the other two networks. Specifically, the IGD group had increased functional connectivity within the cognitive control network compared to both the GD and HC groups, and the GD group had increased functional connectivity within reward circuitry compared with both the IGD and HC groups. Further studies, especially those not limiting GD to internet-based GD, are needed as internet-based GD may not generalize to GD more broadly (Russell et al., 2022).
Bae et al. (2017)’s study applied a static analytic approach, one susceptible to loss of information contained in the temporal patterns of spontaneous signals (Hutchison et al., 2013). Large-scale dynamic neural statistics are emerging powerful methods to capture how the human brain adapts to different circumstances to conduct appropriate behaviors across time (Lurie et al., 2020). In particular, both dynamic fractional amplitude of low-frequency fluctuation (fALFF), which depicts the spontaneous brain activity (Zou et al., 2008), and dynamic degree centrality (DC), which quantifies the degree to which one brain node connects to all other nodes, are powerful dynamic neuroimaging statistics (Zuo et al., 2012) and have been used in previous studies of mental disorders (Lu et al., 2020; Sun et al., 2022).
In addition to dynamic spontaneous brain activity and connectivity, brain networks also reconfigure spontaneously to adapt to changing internal and external circumstances (Bassett et al., 2011; He et al., 2018). The multilayer network algorithm is a robust method to probe the reconfiguration of brain networks across time (Mucha et al., 2010). It helps estimate an individual's neural flexibility, which underpins cognitive flexibility (i.e., tendencies to adapt to different circumstances; Ionescu, 2012) at a behavioral level (Bassett et al., 2011; Braun et al., 2015). Previous studies have reported a positive correlation between problem-gambling severity and cognitive inflexibility (Leppink et al., 2016), which was also found in patients with either GD (Álvarez-Moya et al., 2009; Perandrés-Gómez et al., 2021) or IGD (Kim et al., 2017). These results suggest potential altered neural flexibility in people with GD and IGD.
Although there have been efforts using machine learning to explore the use of behavioral and neural markers to statistically classify GD (Genauck et al., 2020, 2021) or IGD (Stavropoulos et al., 2023; Wang et al., 2022), no study to our knowledge has used both phenotypes simultaneously to distinguish GD and IGD from HC groups. The combination of behavioral and neural data, which can provide a more comprehensive picture of distinct and reliable phenotypes of disorders, has been adopted in machine learning to diagnose medical conditions (e.g., Alzheimer's disease; Mirzaei & Adeli, 2022). Thus, a multiclass classification algorithm could be adopted to probe if the neural dynamic properties and controlled behavioral indicators could facilitate the successful classifying of GD and IGD. The findings could potentially be useful in assisting disease classification and clinical diagnosis and to advance interventions.
In this study, we adopted a transdiagnostic approach (Fusar‐Poli et al., 2019), intending to identify similarities across IGD and GD and differences across the disorders. Gambling-related positive play, as one of the potential behavioral phenotypes referring to responsible and controlled gambling, was examined among GD and IGD groups. Dynamic statistics were used to probe potential alternations in neural dynamics in GD and IGD using resting-state fMRI data to identify shared and specific dysfunctional brain circuits. Advanced analytic approaches probed neural flexibility in individuals with GD and IGD using a multilayer network algorithm. The multiclass classification algorithm was used to explore whether these neural and/or behavioral indicators could be helpful for GD and IGD classification.
MethodsParticipants and proceduresAll participants were Chinese adults aged 18 years or above and were recruited via posts on social media, posters and personal referral in the corresponding author's university and local community centers. If individuals were interested in study participation and provided their contact information either in an online form or verbally via personal phone calls or messages, they were scheduled by the trained research assistant for intake involving a clinical interview followed by a self-reported questionnaire survey and an MRI scan. All participants were interviewed by a psychiatrist before MRI scanning. The diagnostic inclusion criteria were based on the fifth edition of the Diagnostic and Statistical Manual (DSM-5;APA, 2013). The exclusion criteria for participants included any self-reported physical disease, psychosis due to neurologic or medical conditions, especially schizophrenia spectrum and other psychotic disorders, manic episodes, bipolar disorder, depressive disorders, anxiety disorders, and substance use disorders, as well as the history of using dopaminergic medications associated with GD (e.g., levodopa, pramipexole, ropinirole and aripiprazole) and family psychosis. According to the psychiatrist's diagnostic assessment of participants’ past-year gambling/gaming experience, 58 participants were diagnosed with GD, 31 with IGD, and 83 with neither (i.e., HC participants), after removing six participants from further analyses due to incomplete data.
Before clinical interviews, all participants provided written consent. All participants completed a questionnaire with items regarding their demographics (age, sex) and responsible and controlled play levels, which were assessed by the Chinese version (Tong et al., 2020) of the Positive Play Scale (Wood et al., 2017). The scale consists of a 7-item beliefs subscale (e.g., “I should be aware of how much money I spend when I gamble”) and a 7-item behaviors subscale (e.g., “I was honest with my family and/or friends about the amount of time I spent on gambling”). Participants answered on a 5-point Likert scale (from 1 = strongly disagree to 5 = strongly agree). The higher total score in both beliefs and behaviors scales represented a higher level of corresponding responsible and controlled gambling.
The study was reviewed and approved by the Research Ethics Committee at the corresponding author's university (reference number: BSERE20-APP014-ICI-01).
MRI acquisitionMRI scans were obtained by a 3T Siemens Magnetom Prisma scanner at the University of Macau. The MRI scanning parameters were the same as in a previous study (Zhou et al., 2023). The parameters for the structural scan were FOV = 256 mm; slice number = 176; TR = 2300 ms; TE = 2.26 ms; slice thickness = 1 mm. A resting-state fMRI scan for each participant lasted 10 min and consisted of 600 vol. Participants were asked to fixate on a white cross with a black background and not to focus on any thoughts. The scanning parameters for resting-state fMRI were FoV = 192 mm; slice number = 65; slice thickness = 2 mm; TR = 1000 ms; TE = 30 ms; phase encoding direction = AP; flip angle = 60.
MRI data preprocessingA MATLAB-based MRI data processing package DPABI (Yan et al., 2016), the preprocessing procedures of which are sourced from statistical parametric mapping (https://www.fil.ion.ucl.ac.uk/spm/), was used to conduct the preprocessing procedures of fMRI data. To avoid the instability of the MRI scanner, the first 10 vol were removed from the original 600 vol. Realignment was performed to address head motion. Several confounding effects were removed for further analyses, including constant, linear, and quadratic trends (Power et al., 2012), 24 head motion parameters (Friston et al., 1996), white matter and cerebral fluid measures, and regressors relating to head motion. Further procedures included normalizing the functional space into a standard MNI space and smoothing the data with a 4 mm Gaussian kernel. In addition, a bandpass filter (0.01 ∼0.1) was adopted to remove noise for the calculation of DC and functional connectivity between pairs of regions of interest (ROIs).
Temporal variation of spontaneous brain activityThe fALFF summarizes the relative power magnitude of a designated frequency range (0.01–0.1 Hz) in spontaneous brain activity (Zou et al., 2008). The full scanning volumes were divided into sliding windows of 30 time points each, with 1 sliding step as recommended as the default parameters by a previous study (Yan et al., 2017), resulting in 561 windows in total for each participant. The fALFF was calculated for each voxel for each participant in each time window. The standard deviation of fALFF across time was calculated to depict the temporal variation of spontaneous brain activity of each voxel.
Temporal variations in brain connectivityThe DC is an index of the overall degree of connecting strength of one brain node to all other brain nodes (Zuo et al., 2012). The full scanning volumes were divided into sliding windows of 50 time points each, with 5 sliding steps. While Hutchison et al.’s review (2013) has identified window length of 30s–60 s bringing the most robust results, they have also highlighted, a too long window length must be avoided to capture the dynamic fluctuations of brain connectivity across time despite the fact that a longer window length can make the connectivity results more stable. Consistent with previous research (Sun et al., 2022; Yang et al., 2022), 50 TRs were therefore, adopted as the window length. In addition, although both 5 TRs and 1 TR were the common sliding step in previous studies (Demirtaş et al., 2016; Espinoza et al., 2019; Pedersen et al., 2018), a longer sliding step (i.e., 5 TRs) was adopted to avoid overloading computational resources for the calculation of dynamic DC, resulting in 109 windows in total for each participant. DC was calculated for each voxel for each participant in each time window. The standard deviation of DC across time was calculated to depict the temporal variation of DC of each voxel.
Further functional analysis would take the clusters showing between-group effects as seeds to calculate the whole-brain-wise functional connectivity in a sliding window of 50 time points, with five sliding steps. The standard deviation of functional connectivity in the resulting 109 time windows was then calculated to index the temporal variance of functional connectivity for each voxel.
Between-group statistical analyses for behavioral measurementsOne-way ANOVA was performed to examine the overall between-group effects of gambling-related positive play beliefs and behaviors in GD, IGD, and HC groups. In addition, the t-test was used to examine the differences in these measurements between each pair of groups. To avoid misinterpretation, linear covariate effects of age and sex were removed from the original scores of gambling-related positive play beliefs and behaviors to obtain normalized scores. The normalized scores of positive play beliefs and positive play behaviors were fed to repeat the abovementioned statistical analyses.
Anor: normalized score; Aorig: original score; V: vector of age, sex, or all-ones vector.
Between-group statistical analyses for voxel-wise dynamic statisticsOne-way ANOVA was performed for both temporal variations of fALFF and DC among three diagnostic groups, controlling for covariates of age and sex. The significant results should survive family-wise error correction (FWE) for multiple comparisons (uncorrected p < 0.001, cluster-level FWE q < 0.05). In addition, an ROI analysis was performed to examine differences between each pair of groups in the brain regions showing whole-brain-wise between-group effects.
After controlling the covariate effects of age and sex, t-test was used to examine the differential effects of dynamic functional connectivity between each pair of groups, such as between the GD and HC groups. The same FWE approach was used to correct for multiple comparisons. Further ROI analysis was performed to understand the differential effects between each pair of groups in each brain region showing between-group effects in the whole-brain-wise exploratory analysis.
In addition, correlation analyses were performed to examine whether the dynamic activity or connectivity would be correlated with positive play scores across all groups. Further linear regression analyses were performed to examine whether the group would moderate the associations between such neural and behavioral features.
Beh: positive play beliefs or positive play behaviors; Vol: neural signals (dynamic fALFF or dynamic DC); GroupID: GD, IGD, and HC were assigned as different categorical values.
Multilayer networkA multilayer network algorithm can be used to capture the flexible modular organization of brain regions across time (Bassett et al., 2011). As shown in the following formula, the spatial resolution parameter γ controls the average number of brain regions to form a module (network), while the temporal resolution parameter ω controls how similar the organization is among different time windows. As suggested by previous studies, both γ and ω were set as 1 in the cost function (Bassett et al., 2011, 2013; Finc et al., 2020). Of note, both positive and negative connections were allowed to be incorporated to the connections with different directions. Only sequential connections between nearby windows (layers) were allowed in the cost function.
Denotes: γ regulates the spatial resolution, while ω is a temporal regulator controlling the between-layers consistency. Kis+(Kis−) indicates the node degree of positive (negative) connections of node i in layer s. m+ (m−) is the sum degree of layer s. δ is a delta function, and the δ function equals 1 when gis and gjs are assigned to the same module. μ is the total degree of all layers (time windows).
For the clustering of brain regions, the full length of the images of each participant was divided into sliding time windows of 50 time points in length with 1 sliding step, consistent with previous studies (Favaretto et al., 2022; Tang et al., 2022; Yang et al., 2022), which resulted in 541 time windows for each participant. The brain regions depicted in the Brainnetome atlas (Fan et al., 2016) were used to extract the average time series for each ROI in each time window, followed by the calculation of the Pearson correlation between each pair of brain regions in each time window. Then a 246 * 246 * 541 matrix for each participant was fed to make an estimate of the modular organization across time windows for each participant.
Modular flexibilityModular flexibility was calculated as the number of times a brain region changed its module belonging in successive windows, divided by the total number of time windows (Bassett et al., 2011). To better summarize the flexibility results at a network level, the average flexibility of 8 brain networks (i.e., visual network, somatomotor network, dorsal attention network, ventral attention network, limbic network, frontoparietal network, and default mode network plus a subcortical network; Thomas Yeo et al., 2011) was calculated for between-group comparisons. Of note, the correspondence between a brain region and Yeo's brain network can be retrieved from the webpage of Brainntome atlas (https://atlas.brainnetome.org/download.html). Then the between-group effects were examined for each network's flexibility with a two-sample t-test after controlling for age and sex. Multiple comparisons were corrected by false discovery rate (FDR).
Multiclass classificationThe scores of beliefs and behaviors of positive play, dynamic fALFF and DC in the brain regions showing group differences, and modular flexibility were used as phenotypes to distinguish among GD, IGD, and HC participants. A naïve Bayesian classifier was used to train the data, followed by a 5-fold cross-validation process to estimate prediction accuracy. The classification process was conducted by an open-sourced MATLAB package (Treder, 2020).
ResultsGroup comparisons for the demographic and behavioral dataThere were significant differences in both age (p < 0.001) and sex (p = 0.01) among groups (Table 1). The average age of GD participants was 37.67 years, IGD participants was 24.48 years, and HC participants was 27.31 years. While GD and HC groups were male-predominant, the IGD group was more closely balanced. The average score of beliefs of positive play was 22.93 in GD, 29.26 in IGD, and 28.57 in HC participants. Regarding behaviors of positive play, the average score was 19.43 in GD, 28.22 in IGD, and 28.77 in HC participants. The ANOVA analyses revealed significant between-group effects regardless of covariate effects of age and sex were controlled (positive play beliefs: F = 8.60, p < 0.001; positive play behaviors: F = 20.73, p < 0.001) or not (positive play beliefs: F = 35.48, p < 0.001; positive play behaviors: F = 60.86, p < 0.001). Specifically, original scores of these beliefs/behaviors in GD group were significantly lower than those in both IGD and HC groups (t > 6.75, p < 0.001). However, there were significant negative correlations between age and positive play beliefs (r = −0.43, p < 0.001) and behaviors (r = −0.37, p < 0.001), and males had lower levels of positive play beliefs (Mmale = 25.70, Mfemale = 28.00, t = −2.50, p = 0.01) and behaviors (Mmale = 23.36, Mfemale = 28.47, t = −4.33, p < 0.001) than females. Thus, as shown in Fig. 1, after controlling covariate effects of age and sex, both GD and IGD groups displayed significantly lower scores of both positive beliefs/behaviors than those in the HC group (t > 3, p < 0.001).
Participants’ demographics and behavioral indicators.
Note: GD = Gambling disorder; IGD = Internet gaming disorder; HC = Healthy control.
The whole-brain analysis revealed between-group effects of dynamic fALFF in the left triangular inferior frontal gyrus (IFG) (Fig. 2A, uncorrected p < 0.001, cluster-level q < 0.05). Further ROI analysis indicated that the variation of dynamic fALFF in IGD participants was higher than those in GD and HC participants (Fig. 2C, p < 0.01). Meanwhile, whole-brain analysis revealed between-group effects of dynamic DC in the right rectus/orbital frontal gyrus (Fig. 2B, uncorrected p < 0.001, cluster-level q < 0.05). Further ROI analysis indicated the DC of both GD and IGD participants was significantly lower than that of HC participants (Fig. 2D, p < 0.01).
Temporal variations in brain activation and connectivity among GD, IGD, and HC participants. A: The left IFG showed significant group effects of dynamic fALFF; B: A bar graph displaying the results of post-hoc ROI analysis of dynamic fALFF; C: The right rectus/orbital frontal gyrus showed significant group effects of dynamic DC; D: A bar graph displaying the results of post-hoc ROI analysis of dynamic DC.
Note: GD = Gambling disorder; IGD = Internet gaming disorder; HC = Healthy control; IFG = Inferior frontal gyrus; ROI = Regions of interest; fALFF = Fractional amplitude of low-frequency fluctuation; DC = Degree centrality. ⁎⁎p < 0.01. The whole-brain-wise significant threshold is FWE cluster-level q < 0.05, uncorrected p < 0.001.
Correlation analyses revealed that dynamic fALFF had a significant correlation with positive play beliefs (Fig. 3A, r = 0.19, p = 0.03) and a positive, although statistically non-significant, correlation with positive play behaviors (r = 0.15, p = 0.09) across all groups. In contrast, dynamic DC did not have any significant correlations with positive play scores across all groups (positive play beliefs: r = −0.05, p = 0.56; positive play behaviors: r = 0.01, p = 0.89). Further linear regression analyses demonstrated that the group (i.e., GD, IGD, and HC) significantly moderated the associations between dynamic activity and positive play scores (Fig. 3B and C) as well as dynamic connectivity and positive play scores (Fig. 3D and E).
Associations between dynamic brain features and self-reported behavioral data. A: Correlations between dynamic brain activity and positive play beliefs across all groups; B/C: The moderated effect of the group in the association between dynamic brain activity and positive play beliefs/behaviors; D/E: The moderated effect of the group in the association between dynamic brain connectivity and positive play beliefs/behaviors.
Note: *p < 0.05, ⁎⁎⁎p < 0.001.
As shown in Fig. 4A and B, when compared to HC, people with GD had higher temporal variability of functional connectivity between the right orbital frontal gyrus and target regions including bilateral IFG, bilateral middle frontal gyrus, right dorsolateral prefrontal cortex, right inferior temporal gyrus and superior temporal pole, right inferior parietal lobule, bilateral supramarginal gyrus, and right precuneus, while people with IGD had higher temporal variability of functional connectivity between the right orbital frontal gyrus and the right lingual gyrus. Further ROI analysis illustrated a more detailed comparison between each pair of groups, indicating significant differences between GD and HC groups, narrow differences between IGD and HC groups, and barely any differences between GD and IGD groups (Fig. 4C).
The between-group effects of temporal variability of functional connectivity of the right OFG. A: The brain regions showing higher temporal variability of functional connectivity with the right OFG in the GD or IGD groups when compared to the HC group; B: The connections with the right OFG showing higher temporal variability of functional connectivity with the right OFG in the GD (blue) or IGD (green) group when compared to the HC group; C: A bar graph for comparisons of temporal variation of functional connectivity with the right OFG in 12 ROIs among three groups.
Note: GD = Gambling disorder; IGD = Internet gaming disorder; HC = Healthy control. l = Left; r = Right; OFG = Orbital frontal gyrus; IFG = Inferior frontal gyrus; MFG = Middle frontal gyrus; DLPFC = Dorsolateral prefrontal cortex; ITG = Inferior temporal gyrus; STP = Superior temporal pole; IPL = Inferior parietal lobule; SupraM = Supramarginal gyrus; Precu = Precuneus; Ling = Lingual gyrus; ROI= region of interest.
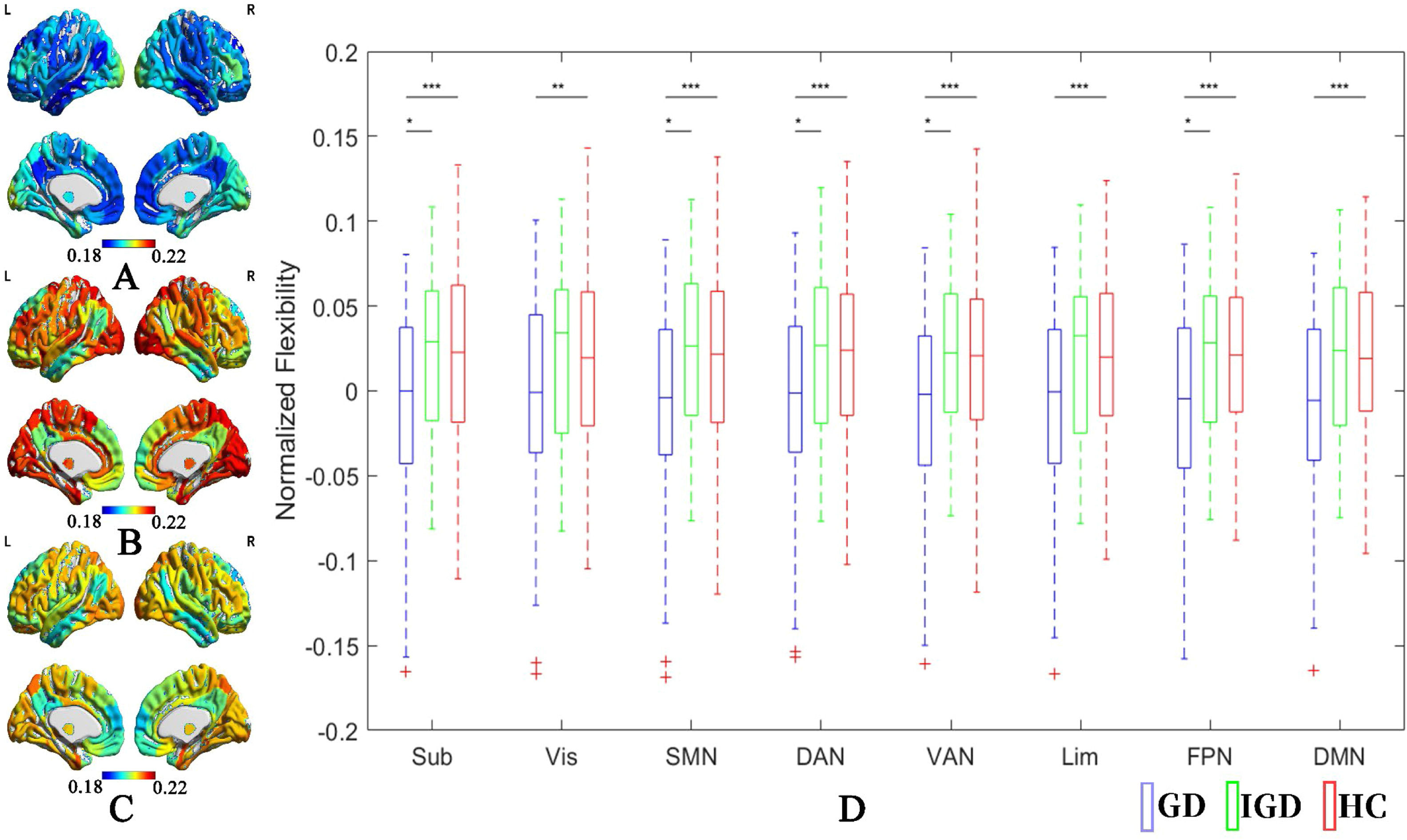
The average modular flexibility across the brain for each group is shown in Fig. 5. Controlling for age and sex, the GD group had significantly lower flexibility in all 8 brain networks when compared to HC participants (FDR q < 0.05), while the IGD group did not reveal any significant differences in modular flexibility compared to HC participants. The IGD group showed higher network flexibility in subcortical, somatomotor, dorsal and ventral attention, and frontoparietal networks when compared to the GD group (p < 0.05, FDR q > 0.05).
Modular flexibility among GD, IGD, and HC groups. A: Average raw modular flexibility in the GD group; B: Average raw modular flexibility in the IGD group; C: Average raw modular flexibility in the HC group; D: The normalized modular flexibility after controlling for age and sex effects in the GD, IGD, and HC groups, as well as the significant levels of between-group comparisons.
Note: GD = Gambling disorder; IGD = Internet gaming disorder; HC = Healthy control. Sub = Subcortical network; Vis = Visual network; SMN = Somatomotor network; DAN = Dorsal attention network; VAN = Ventral attention network, Lim = Limbic network; FPN = Frontoparietal network; DMN = Default-mode network. *p < 0.05, ⁎⁎p < 0.01, ⁎⁎⁎p < 0.001. All p < 0.01 survive false discovery rate correction q < 0.05.
Positive play scores and dynamic fALFF and DC measures could distinguish among GD, IGD, and HC groups (Fig. 6A, 5B). Combining measures of the beliefs and behaviors of positive play, dynamic fALFF, and dynamic DC made the best diagnostic classification of GD and IGD, with an accuracy of 72.30 %, while using behavioral or neural data alone reached lower accuracies (see Fig. 6C). Modular flexibility had little power in predicting group assignments (accuracy: 37.93, Fig. 6C). Combining phenotypes of modular flexibility with phenotypes of positive play and dynamic fALFF, dynamic DC decreased the predictive power to 58.67 % accuracy.
Multiclass classification for the GD, IGD, and HC participants. A: A 3-D scatter plot of the data points of each individual in the dimensions of dynamic fALFF, dynamic DC, and positive play beliefs; B: A 3-D scatter plot of the data points of each individual in the dimensions of dynamic fALFF, dynamic DC, and positive play behaviors; C: The predicting accuracy of diagnosis of GD, IGD, or HC with different sets of phenotypes including phenotypes of only behaviors (Only-beh), of dynamic fALFF and DC (DC-fALFF), of behaviors, dynamic fALFF, and dynamic DC (Beh-DC-fALFF), of modular flexibility (Flexibility), or all mentioned above (all).
Note: GD = Gambling disorder; IGD = Internet gaming disorder; HC = Healthy control; fALFF = Fractional amplitude of low-frequency fluctuation; DC = Degree centrality.
The present transdiagnostic study is the first to compare dynamic brain activity among GD, IGD, and HC groups. Additionally, self-reported measures of gambling-related positive play were compared for the first time among these three groups. To summarize, (a) compared with HC participants, those with GD or IGD showed not only lower levels of beliefs and behaviors of gambling-related positive play after controlling for covariate effects of age and sex, but also relatively decreased dynamic brain connectivity in the right rectus/orbital frontal gyrus; (b) the dynamic brain activity in the left triangular IFG was increased in IGD but not GD participants, while the modular flexibility was decreased in GD but not IGD participants; (c) the classification of GD, IGD, and HC groups reached high accuracy with making use of self-reported (i.e., beliefs and behaviors of positive play) and neural (i.e., dynamic fALFF and DC) data.
Shared characteristics of GD and IGD groupsCompared with HC, both GD and IGD groups reported lower levels of beliefs and behaviors regarding the regulation of behaviors after controlling for the effects of age and sex. Consistent with previous studies showing the negative correlation between GD and gambling-related positive play in community-dwelling individuals who gamble (Tong et al., 2020; Zhou et al., 2022; Zhou & Wu, 2024), this study provided empirical evidence regarding low levels of self-reported responsible and controlled gambling among individuals with GD. At the same time, the study also found that people with IGD had lower levels of self-reported responsible and controlled gambling when controlling for effects of age and sex, even though the unadjusted scores were more similar to the HC group. These findings raise the possibility that responsible and low-risk gambling may show a complex relationship with IGD. However, future studies are warranted to recruit demographically matched participants to investigate further differences in gambling-related positive play between IGD and HC groups, as well as to determine the extent to which individuals with IGD may demonstrate gaming-related decrements in positive play.
The present study found GD and IGD participants shared decreased dynamic brain connectivity in the right rectus/orbital frontal gyrus. The orbital frontal gyrus, including the rectus gyrus, has been implicated in value representation, reward processing, and decision-making (Rolls, 2004), and differences in the reward and value systems (e.g., hypersensitivity to addiction-related rewards, blunted responses to monetary reward anticipation and more risky choices) in GD and IGD have been reported (Hewig et al., 2010; Luijten et al., 2017; Raiha et al., 2020; Wiehler & Peters, 2015). Consistent with several studies revealing dysfunction in the orbital frontal gyrus during decision-making in GD (Brand et al., 2005; Freinhofer et al., 2020), the altered dynamic brain connections in the right orbital frontal gyrus might be one of salient neural factors contributing to disturbed reward and value processes in GD and IGD. Among the limited studies examining brain dynamic connectivity in GD or IGD, one study reported the altered dynamic interactions within frontal-striatal circuits (e.g., inhibitory effective connectivity from the orbital frontal gyrus to the right caudate) in IGD, supporting abnormal reward processing among individuals with behavioral addictions (Zeng et al., 2022). Thus, our results echo the existing literature on involvement of the orbital frontal gyrus in GD and IGD and provide additional knowledge relating to altered dynamic connectivity between the orbital frontal gyrus and other brain regions. Further dynamic functional connectivity analysis revealed that many distributed brain regions reported by a meta-analysis showing altered grey matter in addictive disorders (Zhang et al., 2021) had higher temporal variability of functional connectivity with the right orbital frontal gyrus, further implicating the right orbital frontal gyrus in both GD and IGD groups.
Decreased levels of beliefs and behaviors of responsible and controlled play in GD and IGD groups after controlling for covariate effects of age and sex suggest possible deficits in awareness and ability to regulate and control their own gambling, with impaired impulse control considered as a core feature of addictions (Weinberg, 2013). The GD and IGD groups shared altered dynamic brain connectivity in the right rectus/orbital frontal gyrus, of which these two groups also had higher temporal variability of functional connectivity with other brain regions compared to HC group. Such findings resonate with previous studies that have found that stimulating brain regions with strong connections with the orbital frontal gyrus may disrupt outcome-guided behaviors (Howard et al., 2020). As such, the orbital frontal gyrus may be a key brain region in reward-based decision-making processes in behavioral addiction (Yalcinbas et al., 2021). Thus, the right rectus/orbital frontal gyrus is recommended as one of the core brain regions in future studies for evaluating the effectiveness of neuro-stimulation therapies (e.g., transcranial magnetic stimulation) in behavioral addiction interventions, either directly or through connected brain regions.
Differences between GD and IGD groupsThe dynamic brain activity in the left triangular IFG was higher among IGD than GD and HC participants. This finding was in line with the results of a meta-analysis that showed activity differences in the left IFG among people with IGD, possibly relating to poor executive functioning or impulse control among individuals with IGD (Zheng et al., 2019). Despite some studies having reported the involvement of IFG in GD (Miedl et al., 2015), there has been arguably less consistent evidence concerning IFG functional differences in individuals with GD during executive or general performance (Quaglieri et al., 2020; Raimo et al., 2021). Therefore, the altered dynamic brain activity in IFG may be more consistently linked to difficulties in executive functioning or impulse control in individuals with IGD. In addition, the group moderated the associations between dynamic brain activity as well as connectivity and positive play scores, suggesting that the brain-behavior association is not simply a linear relationship across the disorders. These patterns of brain-behavior associations might differ in individuals with GD or IGD, providing a potential way to distinguish GD and IGD based on such brain-behavior association patterns.
The dynamic functional connectivity analysis revealed more brain regions showing a higher temporal variation of functional connectivity with the right orbital frontal gyrus in the GD group when compared to HC participants, whereas only a narrower portion of brain regions showed a higher temporal variation of functional connectivity in the IGD group compared to the HC group. Higher temporal variation of functional connectivity between the right orbital frontal gyrus and these brain regions might suggest that there are more fluctuating information exchanges affecting value encoding as the orbital frontal gyrus is an important brain region for reward processing and decision-making (Rolls, 2004). The more pervasively higher temporal variation of functional connectivity with the right orbital frontal gyrus in the GD group might suggest people with GD have more pervasive deficits in the reward processes or decision-making, while the people with IGD might have a more tempered deficit in reward processing or decision-making networks, although these possibilities are currently speculative and warrant direct examination.
Previous studies, including a meta-analysis, have found cognitive inflexibility among people with GD (Grant & Chamberlain, 2023; Leppink et al., 2016; Perandrés-Gómez et al., 2021; Van Timmeren et al., 2018). Considering that brain network flexibility may function as the neural underpinning of one's cognitive flexibility at a behavioral level, with the brain network flexibility positively correlating with cognitive flexibility (Bassett et al., 2011; Braun et al., 2015), the current study suggesting neural inflexibility in GD may relate to cognitive inflexibility, and this possibility warrants direct examination. Furthermore, as no altered network flexibility was observed among the IGD versus HC participants, it suggests that alternate mechanisms may be operating in IGD versus GD. Despite cognitive inflexibility (i.e., reflected by the Stroop interference effect) having been reported previously in individuals with IGD (Dong et al., 2015; Kim et al., 2017), the results suggest that some aspects of cognitive flexibility at a neural level may be preserved in IGD. Other studies have noted the enhancement of multitasking and task-switching abilities through online/video games (Anguera et al., 2013; Choi et al., 2021; Ryu et al., 2021), which speculatively may relate to preserved network flexibility in IGD. These results taken together suggest that individuals with IGD may experience deficits in some types of cognitive flexibility (e.g., space rotation flexibility; Podlogar & Podlesek, 2022) but not others. In the context of previous results, our findings also suggest that individuals with GD might show more pervasive deficits of cognitive flexibility than those with IGD, although this speculative possibility warrants future direct examination. For example, further studies are warranted to examine whether individuals with GD exhibit more network inflexibility than individuals with IGD during task performance.
Benefits of combining neural and self-report indicators for GD and IGD classificationTo the best of our knowledge, the present study is the first to adopt multiclass classification, based on both neural and self-reported data, to explore the accuracy of simultaneously classifying GD, IGD, and HC participants. Several prior studies have used brain or non-neuroimaging data to perform classification analysis to distinguish either GD and HC (Genauck et al., 2020, 2021) or IGD and HC (Stavropoulos et al., 2023; Wang et al., 2022) groups. However, their predicting power stems from the differences between people with addictive disorders and their healthy counterparts, and do not consider alterations across different kinds of behavioral addictions (i.e., GD and IGD).
Our results showed that the combination of self-reported data (i.e., gambling-related beliefs and behaviors regarding responsible and controlled play) and dynamic fALFF and DC could reach 72.30 % accuracy in distinguishing GD, IGD, and HC groups, while incorporating the phenotypes of modular flexibility for the multiclass diagnoses decreased the accuracy to 58.67 %, possibly due to similar levels of network flexibility between IGD and HC participants. Our findings suggest that these self-report and neural indicators may constitute relevant distinguishing characteristics in GD and IGD. Thus, the combination of both self-report and neural phenotypes may provide a foundation for developing and facilitating approaches for accurate and reliable GD and IGD classification in the future.
Study limitations exist. First, a relatively small number of participants, especially female participants, were recruited, thus limiting the generalization of our results. Although the sex and age distribution indicated the current sample was a relatively representative one for GD and IGD (e.g., both the GD and IGD groups contained more men than women; Marraudino et al., 2022; Subramaniam et al., 2015), future studies are suggested to recruit larger samples with similar male-to-female ratios and wider ranges of age and ethnicity to investigate the reproducibility and generalizability of these results. Second, some potential confounders were not collected (e.g., emotional distress and the frequency of drinking/smoking), and these may impact dynamic brain activity and connectivity. Despite the exclusion of individuals with other psychiatric conditions (e.g., depressive, anxiety and substance use disorders), future studies may consider these factors and evaluate or control for their potential effects. Third, the present study did not incorporate gaming-specific positive play, with a scale having been developed and published after data collection in the present project (He et al., 2024a, 2024b). The use of this and other scales (e.g., those evaluating general impulse control or reward and punishment sensitivities) are recommended in future studies to examine further how best to use brain and behavioral data to distinguish GD, IGD, and HC participants. Fourth, our classification procedures might pose a data leakage problem (Kapoor & Narayanan, 2023), which might exaggerate the prediction performance of our study. Considering the sample sizes across groups, such procedures were adopted to provide preliminary evidence for supporting that the combined use of both neural and behavioral features may assist in the diagnosis of GD and IGD. However, future studies may take more comprehensive procedures by selecting features in a separate training set.
In conclusion, this transdiagnostic study is the first to compare the dynamic neural activity and self-reported gambling-related positive play measures among GD, IGD, and HC participants. Results revealed that GD and IGD groups shared altered dynamic brain connectivity and, after controlling for sex and age, had reduced levels of gambling-related responsible and controlled play, suggesting possible transdiagnostic features across different types of addictive disorders. Also, these two groups had disorder-specific patterns of differences in dynamic brain activity and network flexibility. In addition, using neural and self-reported measures achieved high accuracy in distinguishing GD, IGD, and HC participants, which may offer a potentially useful approach to assist in classifying GD and IGD.
Ethics approvalThe procedures of this study were carried out in accordance with the Declaration of Helsinki and its later amendments or comparable ethical standards. The ethics approval was obtained from the affiliated university of the corresponding author.
Consent to participateInformed consent was obtained from all participants.
Data availabilityThe datasets generated during and/or analysed in the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statementHui Zhou: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Yuwen He: Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Lulu Liu: Writing – review & editing. Jingwen Yin: Investigation, Writing – review & editing. Anita Yingxin Xiong: Investigation, Project administration. Ka Heng Leong: Investigation, Project administration. Anise M.S. Wu: Conceptualization, Writing – review & editing, Supervision, Funding acquisition. Marc N. Potenza: Writing – review & editing.
The project was supported by the research grants of the University of Macau [grant numbers MYRG2022-00130-FSS, MYRG-CRG2022-00003-FSS-ICI, CRG2020-00001-ICI, MYRG-GRG2023-00074-FSS]. The funding source had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. This work was performed in part at SICC, which is supported by SKL-IOTSC, University of Macau.