Casos Clínicos en Gastroenterología y Hepatología
Más datosImmune checkpoint inhibitors (ICPIs) are a novel class of oncological therapy that improves survival in patients with advanced-stage tumors.1 ICPIs inhibit the T-lymphocyte receptors that downregulate T-cell immunity. ICPIs activate immune population that reacts against tumors but also against non-tumor host cells1 and they can cause immune-related adverse events including hepatotoxicity.2,3 Usually, the interruption of the treatment is sufficient to solve a mild case of hepatotoxicity, but in severe cases it is necessary to start treatment with corticosteroids.3,4 Only a few cases are steroid-resistant and need to be treated with another immunosuppressant as mycophenolate mofetil (MMF).2,4 We present a case of severe steroid-resistant immune-related hepatitis treatment with MMF and review previous cases described.
A 70-year-old woman with a stage-IV melanoma started with itching, jaundice and choluria twelve days after the first doses of nivolumab. Liver analysis found ALT of 141U/l, AST of 123U/l, gamma glutamyl transferase (GGT) of 103U/l, alkaline phosphatase (AP) of 231U/l and Total Bilirubin of 0.4mg/dl. Ultrasound and abdominal tomography were normal. Antinuclear antibodies, anti-liver-kidney microsomal antibody and anti-smooth muscle actine antibodies were negative and hepatitis A, B and C virus serologies were also negative.
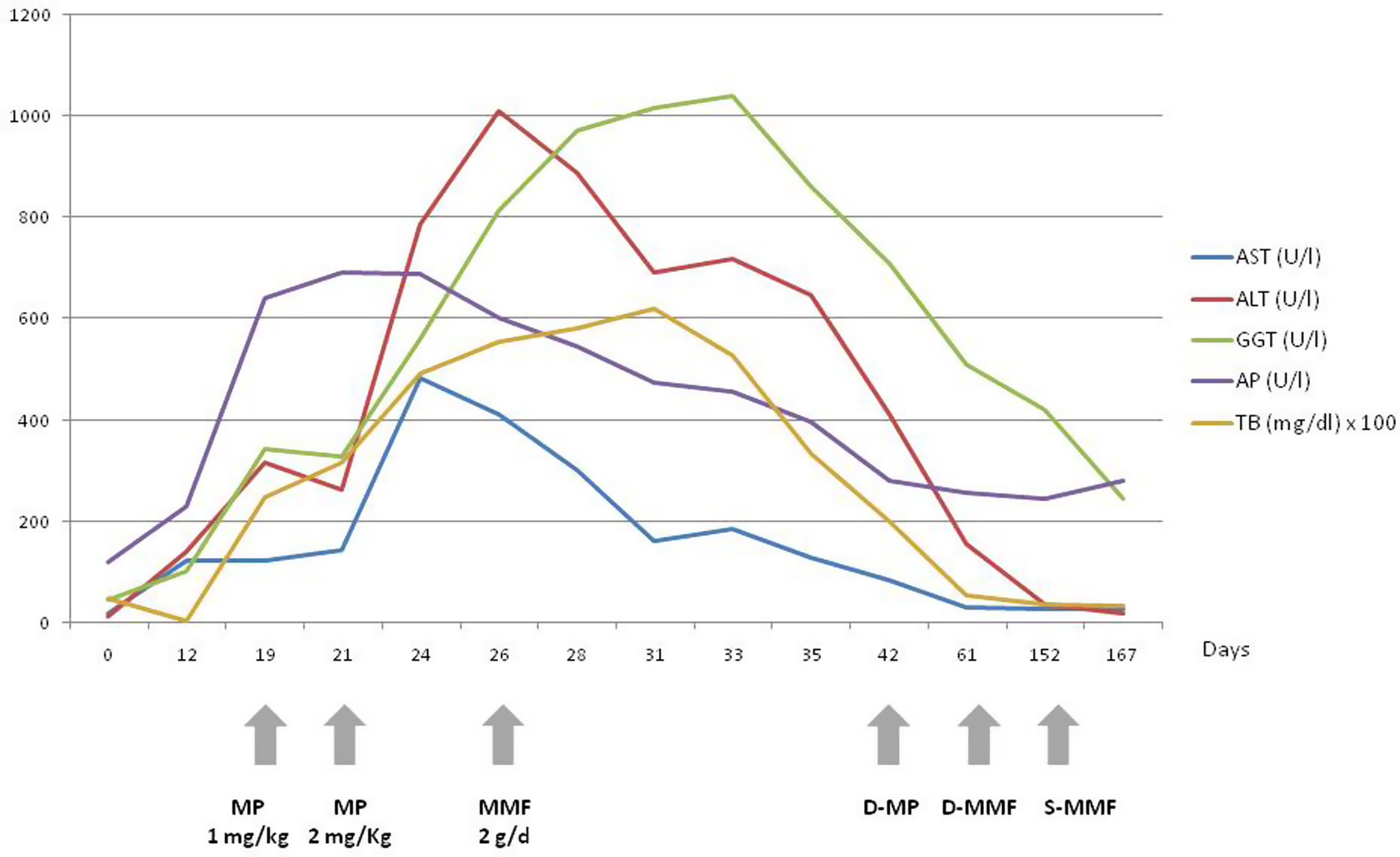
After diagnosis of grade-1 CTCAE (Common Terminology Criteria of Adverse Events) immune-related hepatitis, nivolumab was interrupted. Following a control analysis an increase of ALT, AST, TB with normal clotting was found (grade 3 CTCAE), so methylprednisolone (MP) at dose of 1mg/kg was started. Due to a persistent increase of ALT, AST and TB during the following 48h the dose of MP was increased to 2mg/kg without improvement within the following 5 days, so we decided to start MMF at a dose of 2g/day. After that, a progressive improvement of liver function tests was observed (Supplementary Image 1). During the next two months the patient presented a Coxiella Burnetti respiratory infection and a left hip fracture.
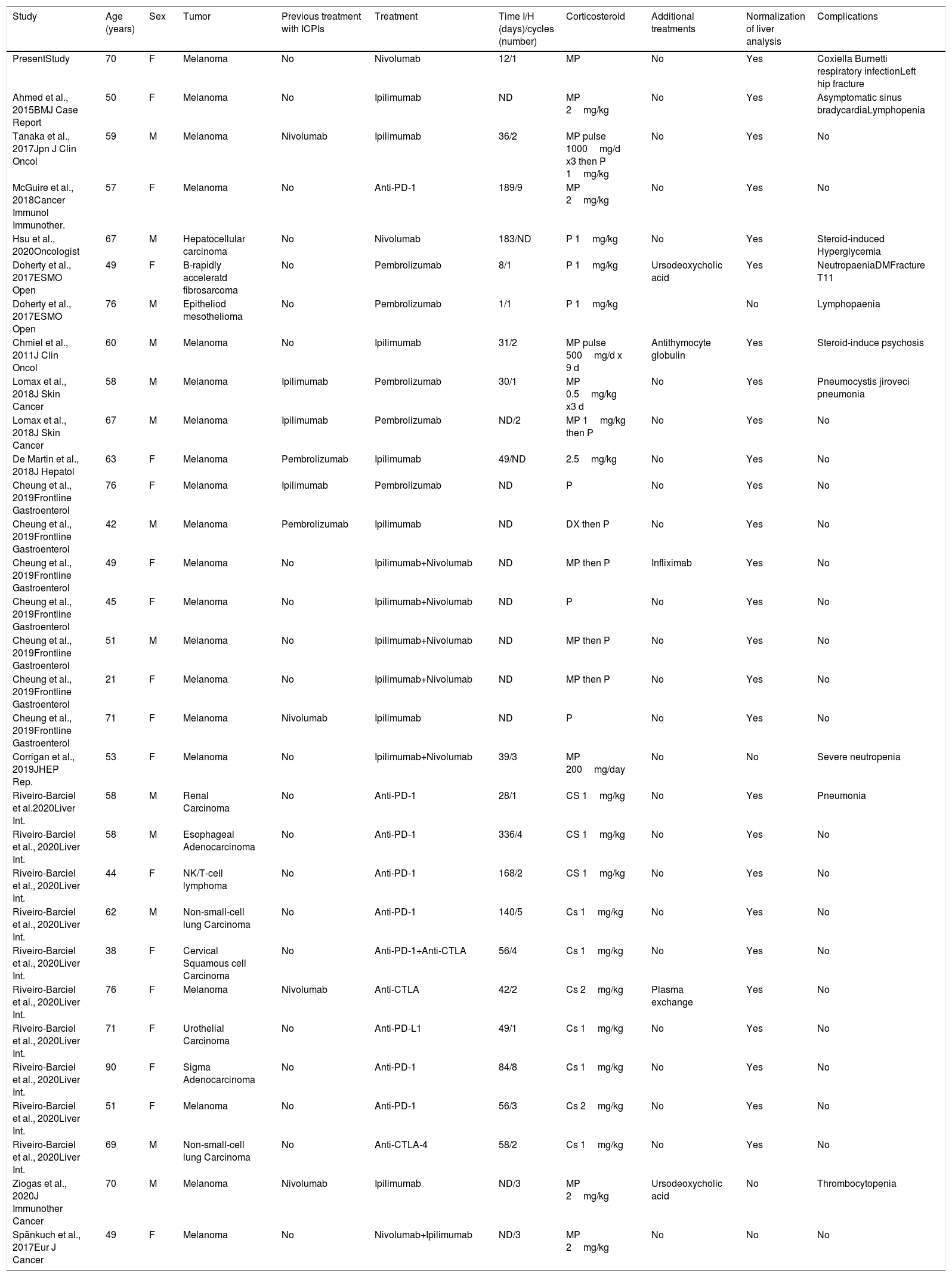
We searched using PubMed the cases of immune checkpoint inhibitors hepatitis or hepatotoxicity. We included every study wherein patients received treatment with MMF: 16 studies were found. We excluded four articles because they showed no description of the characteristics of the patients. A total of 12 studies were included in the analysis. We found a total of 30 previous cases of patients treated with MMF (Table 1). If we include our patient in the analysis, 60.0% were female with a mean age of 58.7 years. The more frequent tumor was melanoma in 20 of cases and 16 patients received combined ICPI therapy. The mean time between ICPIs and hepatotoxicity was 80 days. All patients received corticosteroids. MMF was used in doses of 1 or 2g per day, in five cases associated with other treatments (Plasma exchange, Ursodeoxycholic acid, Antithymocyte globulin or Infliximab). Only four patients did not present an improvement of liver analysis. Ten patients presented complications due to immunosuppression therapy, four of them due to steroid use (hyperglycemia, fractures, steroid-induced psychosis).
Characteristics of patients treated with MMF.
| Study | Age (years) | Sex | Tumor | Previous treatment with ICPIs | Treatment | Time I/H (days)/cycles (number) | Corticosteroid | Additional treatments | Normalization of liver analysis | Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| PresentStudy | 70 | F | Melanoma | No | Nivolumab | 12/1 | MP | No | Yes | Coxiella Burnetti respiratory infectionLeft hip fracture |
| Ahmed et al., 2015BMJ Case Report | 50 | F | Melanoma | No | Ipilimumab | ND | MP 2mg/kg | No | Yes | Asymptomatic sinus bradycardiaLymphopenia |
| Tanaka et al., 2017Jpn J Clin Oncol | 59 | M | Melanoma | Nivolumab | Ipilimumab | 36/2 | MP pulse 1000mg/d x3 then P 1mg/kg | No | Yes | No |
| McGuire et al., 2018Cancer Immunol Immunother. | 57 | F | Melanoma | No | Anti-PD-1 | 189/9 | MP 2mg/kg | No | Yes | No |
| Hsu et al., 2020Oncologist | 67 | M | Hepatocellular carcinoma | No | Nivolumab | 183/ND | P 1mg/kg | No | Yes | Steroid-induced Hyperglycemia |
| Doherty et al., 2017ESMO Open | 49 | F | B-rapidly acceleratd fibrosarcoma | No | Pembrolizumab | 8/1 | P 1mg/kg | Ursodeoxycholic acid | Yes | NeutropaeniaDMFracture T11 |
| Doherty et al., 2017ESMO Open | 76 | M | Epitheliod mesothelioma | No | Pembrolizumab | 1/1 | P 1mg/kg | No | Lymphopaenia | |
| Chmiel et al., 2011J Clin Oncol | 60 | M | Melanoma | No | Ipilimumab | 31/2 | MP pulse 500mg/d x 9 d | Antithymocyte globulin | Yes | Steroid-induce psychosis |
| Lomax et al., 2018J Skin Cancer | 58 | M | Melanoma | Ipilimumab | Pembrolizumab | 30/1 | MP 0.5mg/kg x3 d | No | Yes | Pneumocystis jiroveci pneumonia |
| Lomax et al., 2018J Skin Cancer | 67 | M | Melanoma | Ipilimumab | Pembrolizumab | ND/2 | MP 1mg/kg then P | No | Yes | No |
| De Martin et al., 2018J Hepatol | 63 | F | Melanoma | Pembrolizumab | Ipilimumab | 49/ND | 2.5mg/kg | No | Yes | No |
| Cheung et al., 2019Frontline Gastroenterol | 76 | F | Melanoma | Ipilimumab | Pembrolizumab | ND | P | No | Yes | No |
| Cheung et al., 2019Frontline Gastroenterol | 42 | M | Melanoma | Pembrolizumab | Ipilimumab | ND | DX then P | No | Yes | No |
| Cheung et al., 2019Frontline Gastroenterol | 49 | F | Melanoma | No | Ipilimumab+Nivolumab | ND | MP then P | Infliximab | Yes | No |
| Cheung et al., 2019Frontline Gastroenterol | 45 | F | Melanoma | No | Ipilimumab+Nivolumab | ND | P | No | Yes | No |
| Cheung et al., 2019Frontline Gastroenterol | 51 | M | Melanoma | No | Ipilimumab+Nivolumab | ND | MP then P | No | Yes | No |
| Cheung et al., 2019Frontline Gastroenterol | 21 | F | Melanoma | No | Ipilimumab+Nivolumab | ND | MP then P | No | Yes | No |
| Cheung et al., 2019Frontline Gastroenterol | 71 | F | Melanoma | Nivolumab | Ipilimumab | ND | P | No | Yes | No |
| Corrigan et al., 2019JHEP Rep. | 53 | F | Melanoma | No | Ipilimumab+Nivolumab | 39/3 | MP 200mg/day | No | No | Severe neutropenia |
| Riveiro-Barciel et al.2020Liver Int. | 58 | M | Renal Carcinoma | No | Anti-PD-1 | 28/1 | CS 1mg/kg | No | Yes | Pneumonia |
| Riveiro-Barciel et al., 2020Liver Int. | 58 | M | Esophageal Adenocarcinoma | No | Anti-PD-1 | 336/4 | CS 1mg/kg | No | Yes | No |
| Riveiro-Barciel et al., 2020Liver Int. | 44 | F | NK/T-cell lymphoma | No | Anti-PD-1 | 168/2 | CS 1mg/kg | No | Yes | No |
| Riveiro-Barciel et al., 2020Liver Int. | 62 | M | Non-small-cell lung Carcinoma | No | Anti-PD-1 | 140/5 | Cs 1mg/kg | No | Yes | No |
| Riveiro-Barciel et al., 2020Liver Int. | 38 | F | Cervical Squamous cell Carcinoma | No | Anti-PD-1+Anti-CTLA | 56/4 | Cs 1mg/kg | No | Yes | No |
| Riveiro-Barciel et al., 2020Liver Int. | 76 | F | Melanoma | Nivolumab | Anti-CTLA | 42/2 | Cs 2mg/kg | Plasma exchange | Yes | No |
| Riveiro-Barciel et al., 2020Liver Int. | 71 | F | Urothelial Carcinoma | No | Anti-PD-L1 | 49/1 | Cs 1mg/kg | No | Yes | No |
| Riveiro-Barciel et al., 2020Liver Int. | 90 | F | Sigma Adenocarcinoma | No | Anti-PD-1 | 84/8 | Cs 1mg/kg | No | Yes | No |
| Riveiro-Barciel et al., 2020Liver Int. | 51 | F | Melanoma | No | Anti-PD-1 | 56/3 | Cs 2mg/kg | No | Yes | No |
| Riveiro-Barciel et al., 2020Liver Int. | 69 | M | Non-small-cell lung Carcinoma | No | Anti-CTLA-4 | 58/2 | Cs 1mg/kg | No | Yes | No |
| Ziogas et al., 2020J Immunother Cancer | 70 | M | Melanoma | Nivolumab | Ipilimumab | ND/3 | MP 2mg/kg | Ursodeoxycholic acid | No | Thrombocytopenia |
| Spänkuch et al., 2017Eur J Cancer | 49 | F | Melanoma | No | Nivolumab+Ipilimumab | ND/3 | MP 2mg/kg | No | No | No |
Anti-CTLA-4: anticytotoxic T-lymphocyte antigen 4; Cs: corticosteroids; DM: diabetes mellitus; F: female; M: man; ICPIs: Immune checkpoint inhibitors; MMF: mycophenolate mofetil; MP: Methylprednisolone; ND: not described; P: Prednisone; Time I/H: Time between inmunotheraphy/hepatitis.
ICPIs are commonly used in oncological therapy and between 3% and 10% of cases can cause hepatotoxicity.2 Usually, interruption of the treatment and treatment with corticoisteroids are enough to heal the hepatotoxicity.5 However, some cases are steroid-resistant and another immunosuppressant is needed. We found that MMF was effective in the majority of the cases. ICPIs-induced hepatotoxicity in these cases was developed after 8–12 weeks from the start of the treatment.2,3 A strict follow-up of these patients is recommended for an early diagnosis and treatment of hepatotoxicity. MMF is a reversible inhibitor of inosine monophosphate dehydrogenase and it is used in second-line therapy for autoimmune hepatitis. In the cases that we analyzed, we observed that MMF can be safely used on second line in patients with steroid-resistant immune-related hepatitis with a normalization of the liver analysis in the majority of the cases. Some patients presented complications related to the immunosuppression and steroid treatment. As a consequence, it is important to decrease the dose of immunosuppressant treatment as soon as possible.
In conclusion, MMF is an effective and safe therapeutic alternative for the treatment of steroid-resistant immune-related hepatitis.
Ethics statement and consentThe study followed the criteria of the Helsinki Declaration and the patient has given the written informed consent for publication.
Funding statementThis research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflicts of interestAll authors declare no conflicts of interest.