Introduction
In 1982, the Morbidity and Mortality Weekly Report from the Centers for Disease Control (CDC) provided information on possible transmission of acquired immunodeficiency syndrome (AIDS) by contaminated blood and blood products 1. Since then, public and private health institutions, blood banks and manufacturers of diagnostic tests have collaborated to develop a blood safety vigilance system to reduce transfusion-related human immunodeficiency virus (HIV) transmission.
At the beginning of this effort, most HIV-contaminated blood units were discarded by surrogate marker screening 2. In 1985, the first specific HIV type 1 (HIV-1) antibody test was licensed and blood donors then underwent double screening: a) an extensive pre-donation questionnaire to determine donor eligibility and b) post-donation HIV-1 antibody testing. The most commonly used method for detecting HIV antibodies (HIV Ab) has been enzyme-linked immunosorbent assay (EIA). The performance of HIV screening assays has improved substantially over the years. First-generation HIV tests, based on HIV-1 whole-virus lysate, were able to detect only anti HIV-1 IgG. Subsequently, replacement of viral lysate antigens with recombinant proteins and synthetic peptides that detect anti HIV-1 and HIV-2 IgG (second-generation tests) increased the sensitivity of the assay, particularly in the early phase of seroconversion. Later, third generation HIV Ac testing, and HIV-1 p24 assays, which detect the p24 core antigen produced by the HIV-1 gag gene, were developed and introduced in blood safety vigilance. When the HIV-1 antigen (p24 protein) test is implemented together with HIV-1/2/O antibody screening, the window period is lowered to 16-17 days 3. New combined antigen/antibody testing, the so-called fourth generation tests, are more sensitive than third generation antibody tests; nevertheless, their analytical sensitivity for p24 Ag (p24 Ag) is lower than that of single antigen tests 4. Nucleic acid amplification technology can improve the effectiveness of HIV detection and shorten the potentially infectious window to 10-12 days 3. However, this testing technology has an elevated cost and requires separate laboratory areas and highly trained personnel.
Detection of HIV Ab has been mandatory in Argentina since 1991, and the Asociación Argentina de Hemoterapia e Inmunohematología (AAHI; Argentine Society for Hemotherapy and Immunohematology) recommended routine screening for p24 Ag in 1997, when p24 Ag tests were commercially available in our country. Use of the p24 Ag assay was implemented on 4 September 1997 to ensure a safer blood supply for our hospital patients who needed transfusions.
The aim of this retrospective study is to describe the results obtained during double HIV screening (antibodies to HIV 1/2/O and p24 Ag) among our blood donors, and clinical correlations with these results, and to report the single case of HIV seroconversion detected over the time since screening has been practiced.
Methods
Blood donors
All blood donors at our blood bank are unpaid; most of them are occasional directed donors who voluntarily donate blood for the transfusion needs of a relative or friend (allogeneic donors). When patients are able to donate blood for themselves, autologous blood donations are also drawn. Prior to blood donation, all prospective donors receive an oral explanation about the donation process and utility of the transfusion, and are confidentially evaluated about clinical and infectious conditions according to the AAHI recommendations. Blood pressure, pulse, temperature, weight and hematocrit are checked. Donors must complete a general health history questionnaire. A more detailed version of this questionnaire was implemented in January 2002. Donors are then asked to sign a written informed consent form allowing serologic screening and have the opportunity to ask questions. All blood donors can voluntarily choose to leave at any point of the process without donating, and have the chance to exclude themselves as blood donors during the time following the donation (self-deferral form, implemented in 2001). Donors with repeatedly reactive or positive serological test results are routinely recalled by mail citation. If they return, an extensive and more detailed questionnaire is given to detect risk behavior or a disease history that was denied or omitted during the pre-donation interview. Additional serological tests are performed when necessary. These individuals are referred to the Infectious Disease Service to be given information on follow-up diagnostic assessment and the available medical, preventive and psychosocial services.
Between 4 September 1997 and 30 September 2003, a total of 30,132 consecutive blood donations were completed and screened for transfusion-transmitted infections, including presence of HIV Ab and p24 Ag. Individual data that we report in this paper were obtained from the pre-donation questionnaire and the clinical files of the Infectious Disease Service.
Samples
All fresh serum specimens were immediately examined on the same day as collection by screening tests. Initially reactive samples were kept at 4 °C and retested in duplicate within the three following days. Repeatedly reactive specimens were frozen at 20 °C and thawed once prior to confirmatory testing.
HIV antibodies and HIV-1 antigen: dual testing algorithm
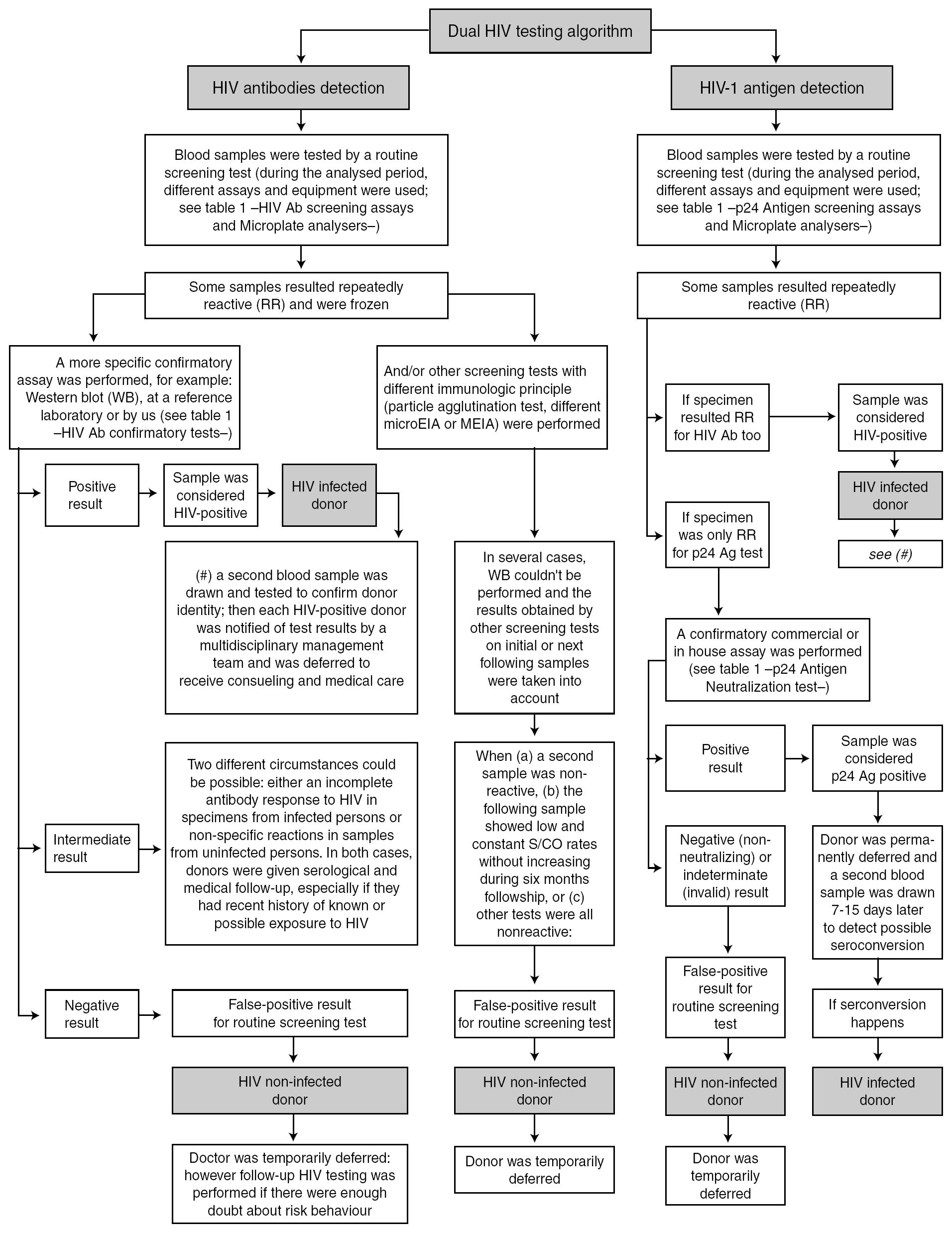
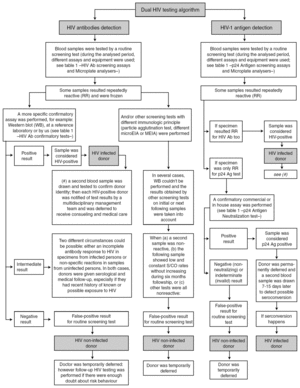
The routine blood donor testing algorithms for HIV Ab and HIV-1 Ag are shown in figure 1. Some additional considerations are mentioned:
Figure 1. Routine blood donor testing algorithm for HIV Ab and HIV-1 Ag.
Samples from blood donors were always analyzed by two HIV screening methods (one for HIV Ab and the other for HIV-1 Ag). According to the 1997 revised UNAIDS-WHO recommendations 5, HIV antibody testing strategy I was applied, and tests having the highest possible sensitivity were used to minimize the rate of false-negative results and to ensure transfusion safety. Because the fourth-generation HIV Ab tests we used have a low analytical sensitivity for p24 Ag, this assay was used only to detect HIV Ab.
In accordance with CDC guidelines 6 and the manufacturer's instructions, any sample with an initially reactive result was retested in duplicate. If the result for either duplicate test was reactive, the specimen was reported as repeatedly reactive and the blood unit was discarded.
When confirmatory assays could not be performed, one or two additional screening tests were used, in keeping with proposed WHO HIV antibody testing strategies II or III 5 for diagnostic purposes, before notifying any repeatedly reactive HIV result to a blood donor.
Western blot (WB) interpretation was done according WHO criteria 7. When a sample showed an inadequate band pattern to meet the recommended criteria for WB positive status, it was considered indeterminate 8.
Neutralization of viral antigen by specific antibody is an accepted confirmatory method for p24 Ag repeatedly reactive samples. This neutralization assay was performed promptly 9,10 because of the potential deterioration of HIV antigen during sample storage.
Other serological screening tests
Besides anti HIV and p24 Ag testing, eight other screening tests were performed on each blood donor sample to detect anti HBc and HBsAg for hepatitis B, anti HCV, anti HTLV I-II, anti Brucella abortus, serologic markers for syphilis by treponemal or nontreponemal methods, and anti Trypanosoma cruzi by two different methods (mandatory) to detect Chagas' disease.
Statistical analysis
All statistical calculations were performed on a personal computer using Microsoft Excel software or the MEDCALC demo for Windows. Results are expressed as mean ± SD and/or 95% CI, where applicable. When the number of samples was large enough, the test of hypothesis for two populations (TH) was used. Data were analyzed with the 2 ×2 chi-square test with Yates' correction, or by Fisher's exact test, where applicable. Significance was set at a P-value of less than.05. Specificity was calculated as the ratio between true-negative results and HIV non-infected donors (true-negative + false-positive donors) 5.
Results
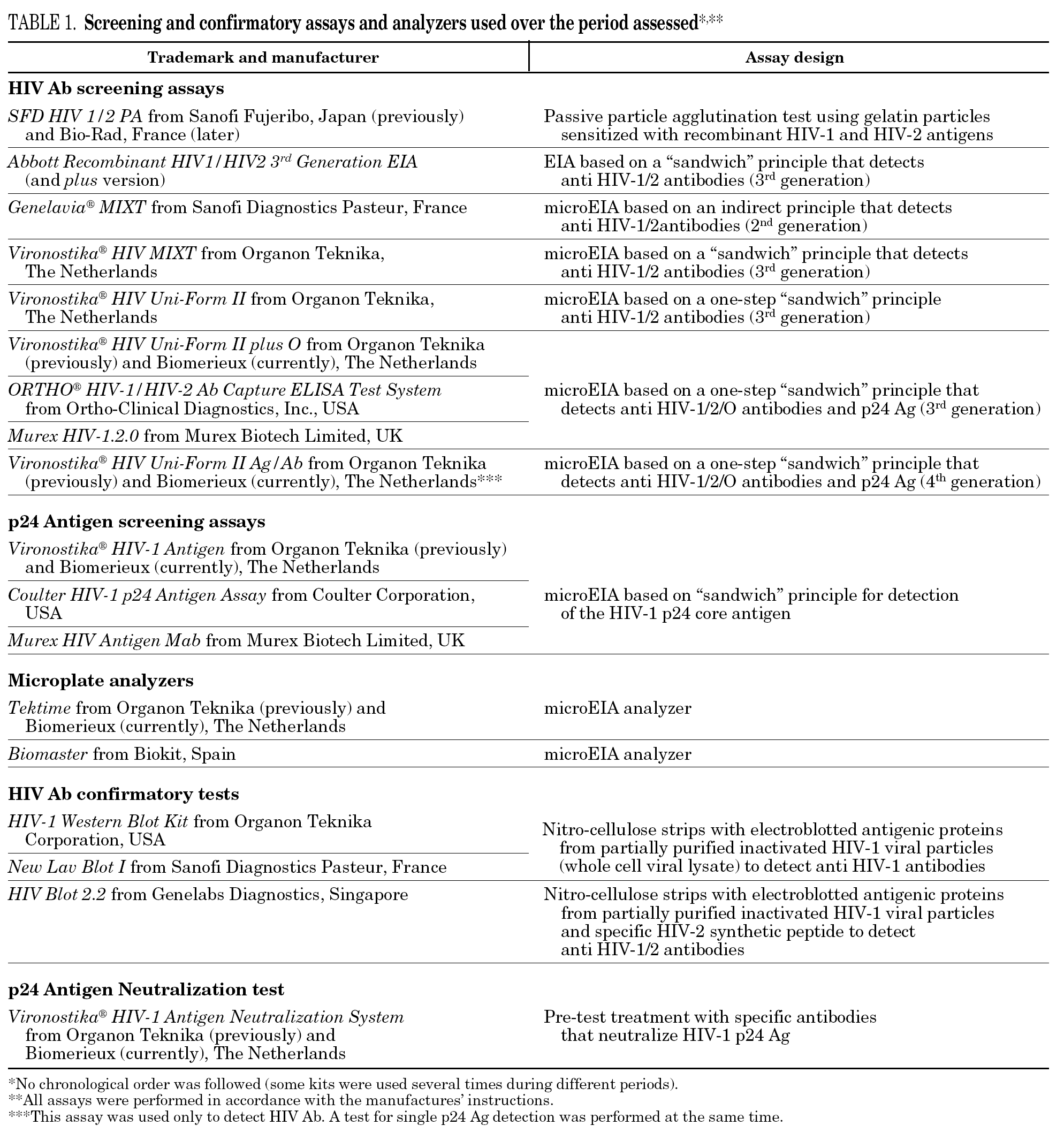
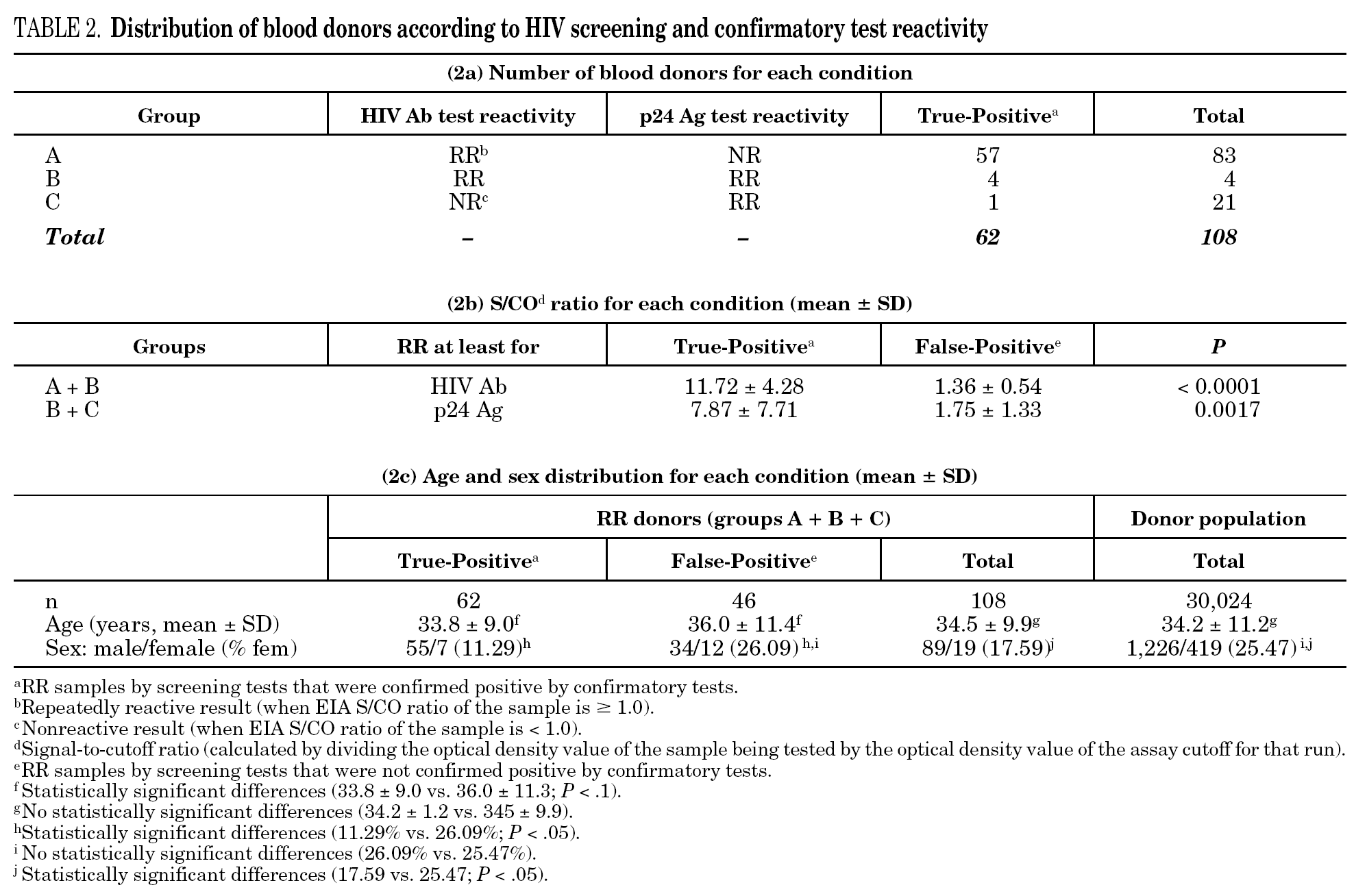
Among the 30,132 blood donor samples screened, 108 (0.3623%; 95% CI, 0.3611-0.3636) were repeatedly reactive by HIV Ab and/or p24 Ag screening tests, mainly EIA (table 1). Donors were classified into three groups according to the screening and confirmatory test results, which are summarized in table 2a. Among the 108 HIV Ab and/or p24 Ag repeatedly reactive donors only 62 (57.41%) were confirmed as true-positive, which yields 0.2084% (62 of 30,132; 95% CI, 0.2072-0.2096) of true HIV-infected donors.
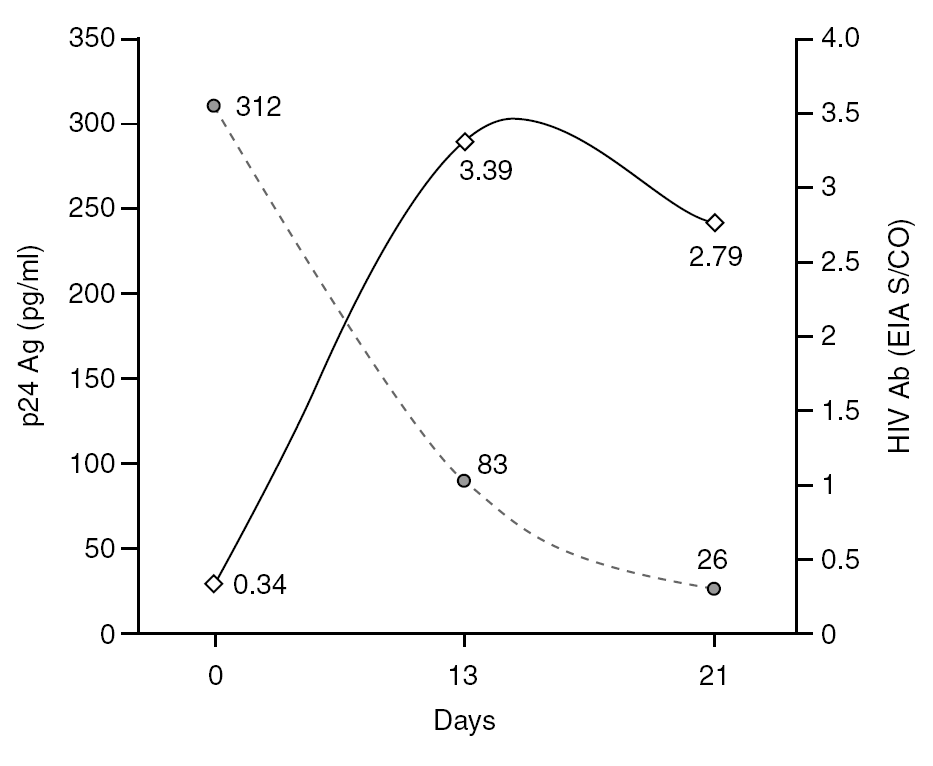
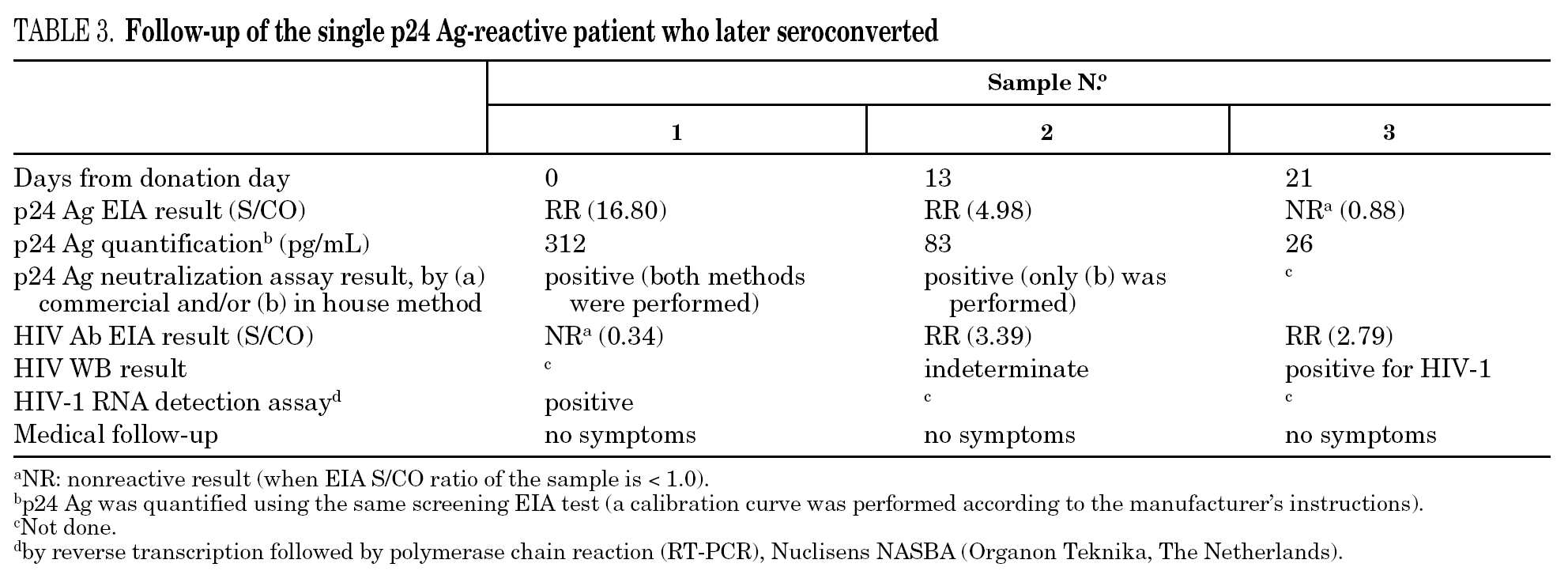
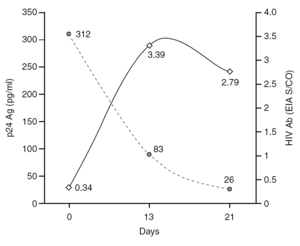
The percentage of true-positive results was significantly higher (P < 0.0001) in HIV Ab repeatedly reactive donors (groups A + B: 61 of 87 [70.11%]) as compared to p24 Ag repeatedly reactive donors (groups B + C: 5 of 25 [20.00%]). Among our 21 donors who tested repeatedly reactive by p24 Ag EIA and nonreactive by HIV Ab testing (group C), only one donor was found to be positive by neutralization assay (4.76%). This donor was recalled and a serological and virological HIV follow-up was performed on the initial and two additional successive specimens in coordination with the Infectious Disease Service. It was not possible to establish the date of the sexual event that triggered the infection. The donor showed evidence of seroconversion in a second sample 13 days after the donation (fig. 2 and table 3) 11.
Figure 2. Evolution of p24 Ag concentration and HIV Ab EIA S/CO from donation day (day 0).
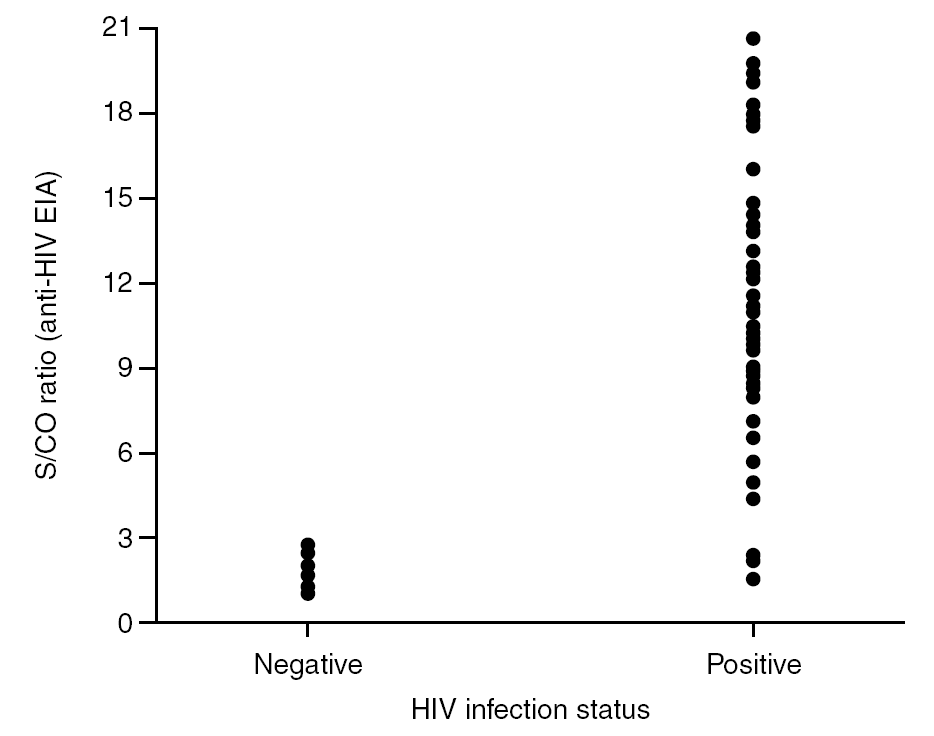
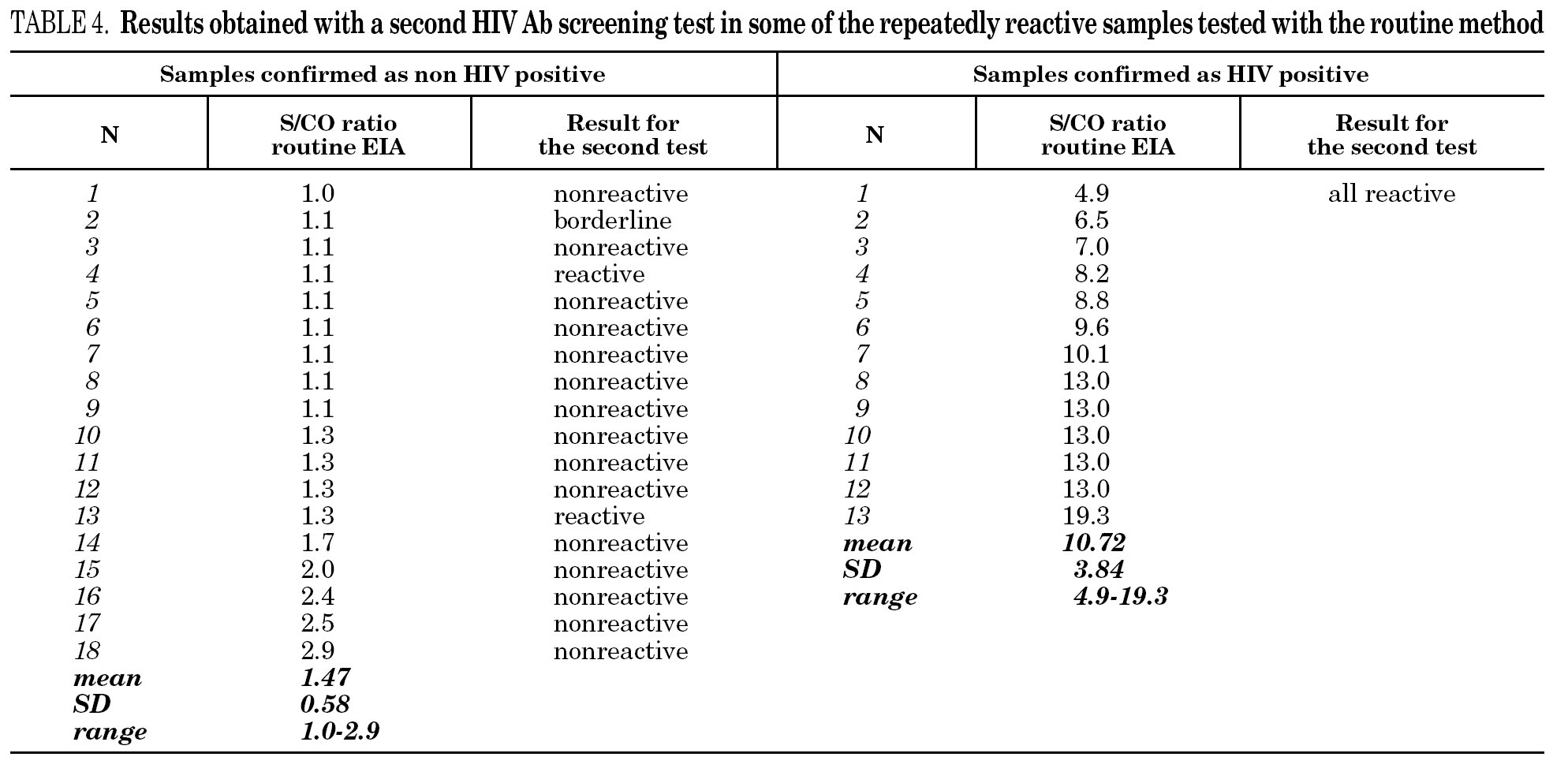
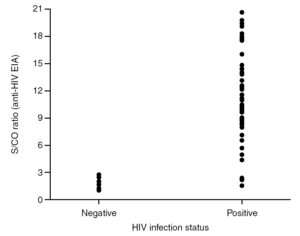
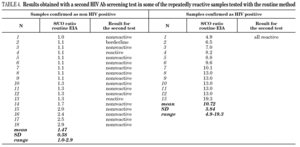
It is known that the proportion of screening-test-reactive results that are positive by confirmatory tests increases as the screening-test-reactive average S/CO ratio increases. In our donor population, there were significant differences in the S/CO ratio (P < 0.0001) between EIA true-positive HIV Ab repeatedly reactive donors and false-positive donors (11.72 ± 4.28 vs. 1.36 ± 0.54) (groups A and B, table 2b). The same was true among p24 Ag repeatedly reactive donors by EIA (7.87 ± 7.71 vs. 1.75 ± 1.33; P = 0.0017) (groups B and C, table 2b). The proportion of HIV Ab repeatedly reactive donors by EIA that tested positive by WB was 11.1% for samples with an average S/CO ratio < 3.0 and 100.0% for those with an average S/CO ratio $ 3.00 (fig. 3). In addition, 31 of 85 (35.6%) HIV Ab repeatedly reactive samples were tested by a second screening test; another EIA, a microparticle enzyme immunoassay, or an agglutination test (table 4). All 13 samples with an S/CO ratio ≥3.0 (10.72 ± 3.84) on the first test were also reactive on the second test and positive by WB. However, the 18 samples that had an S/CO ratio < 3.0 (1.47 ± 0.58) with the routine screening test were nonreactive (15), reactive (1) or borderline (2) with the second test. All these donors were confirmed as HIV non-infected.
Figure 3. Distribution of S/CO ratios from HIV Ac EIA screening versus HIV infection status.
At the time of blood donation, all donors had denied high risk behaviors for HIV infection and, therefore, completed the donation process. Nevertheless, some of them were found to be repeatedly reactive for HIV Ab and/or p24 Ag, including one donor who self-deferred (he was found to be false-positive for HIV Ab). All the repeatedly reactive donors (n = 108) were requested by mail to return.
The true-positive donors (n = 62) were divided into three groups: 1) 17 donors (27.4%) who did not come to the appointment, six of whom had given a false address; 2) 21 donors (33.9%) for whom additional information could not be obtained because they decided to receive medical care outside of our hospital; and 3) 24 donors (38.7%) who returned and were referred to the Infectious Disease Service. After extensive lifestyle questioning, it was found that 89.5% of the HIV-infected donors who returned possessed risk factors for retroviral infection, including unprotected heterosexual (47.4%), homosexual (15.9%) and bisexual (15.9%) activity and intravenous drug use (10.5%). A high proportion of infected donors (70.0%) did not know their partner's HIV serostatus and only one infected donor admitted sexual activity with a risk partner. The seroconverting donor, the only true-positive donor from group C (p24 Ag repeatedly reactive by EIA only), was a 29-year-old man who later admitted heterosexual promiscuity.
No statistically significant differences in average age were observed between HIV Ab and p24 Ag non-reactive donors and HIV Ab and/or p24 Ag repeatedly reactive donors (34.2 ± 11.2 vs. 34.5 ± 9.9 years; table 2c). However, between true-positive HIV Ab and/or p24 Ag donors and false-positive donors (33.8 ± 9.0 vs. 36.0 ± 11.3, a significant (although low) difference (P < .1) was found by TH.
A significantly smaller percentage of female donors was seen in the HIV Ab and/or p24 Ag repeatedly reactive group in comparison with non-reactive donors (17.59% vs. 25.47%) (P < .05). Similarly, the proportion of women was significantly lower (P < .05) in the true-positive groups as compared to the false-positive group (11.29% vs. 26.09%). The proportion of women in the false-positive group was similar to that of the negative group (26.09% vs. 25.47%, non-significant).
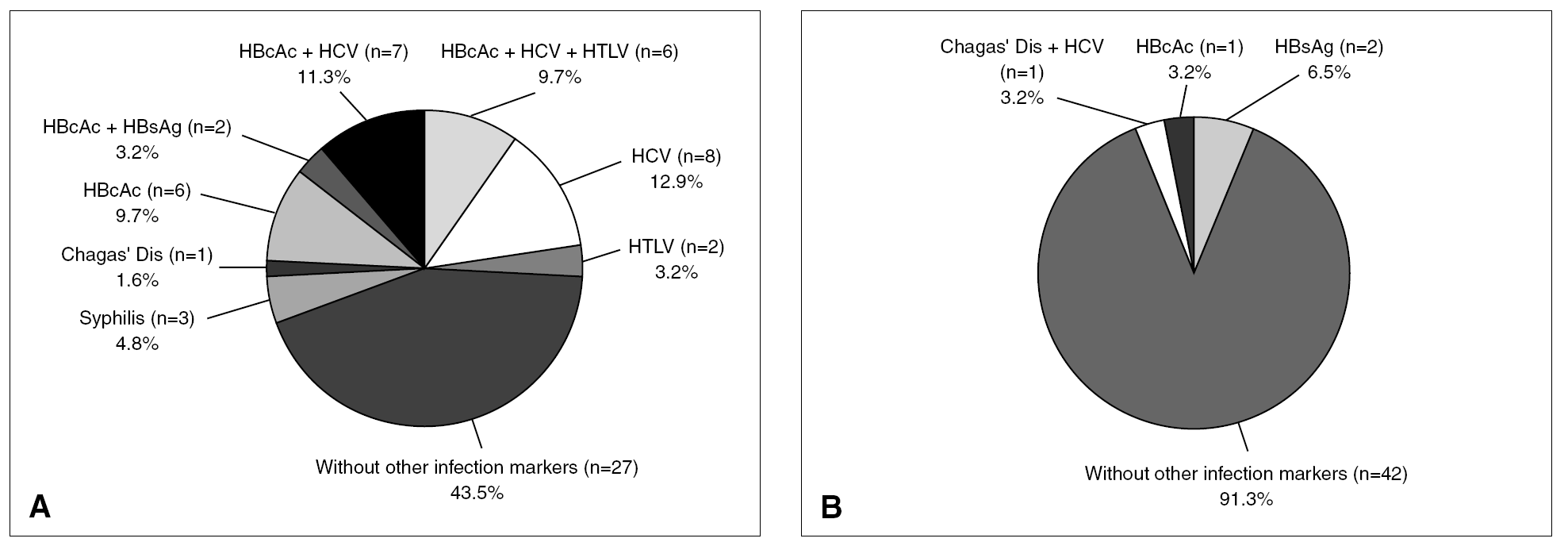
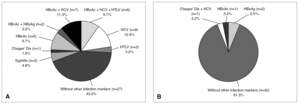
Among the total repeatedly reactive donor population, only 7.7% were reactive for two or more serological markers of infection (coinfections). However, among the 62 true-positive HIV donors, 35 (56.5%) tested repeatedly reactive for other screening reactions (3 for syphilis; 1 for Chagas' disease; 6 for anti HBc; 2 for anti HBc and HBsAg; 7 for anti HBc and anti HCV; 6 for anti HBc, anti HCV and anti HTLV I-II; 8 for anti HCV and 2 for anti HTLV I-II) and 43.5% were reactive only for HIV tests (fig. 4, part A). This proportion of coinfections was significantly higher (TH, P < .01) for HIV true-positive donors than for the overall reactive donors. Of 46 false-positive HIV donors, 8.7% were found to be reactive for two or more serological markers of infection (proportion similar to that of the overall infected donor population) whereas 91.3% showed reactivity (false) only for HIV serological markers (fig. 4, part B). Thirty-one of 35 (88.6%) HIV true-positive donors who presented coinfections were infected with other blood-transmissible viruses (HBV and/or HCV and/or HTLV).
Figure 4. Additional reactivity from true (A) and false-positive (B) HIV Ab and/or p24 Ag donors.
Discussion
Our total rate of HIV repeatedly reactive donors (0.3623%), including HIV Ab and/or p24 Ag reactive donors, was similar to values reported by other centers in our city 12 and Brazilian cities 13,14, but was higher than values reported by other blood banks in Argentina and the rest of Latin America 15-19. The rate of confirmed HIV-infected donors (0.2084%) was intermediate among other public and private Latin American blood donation centers 13,17,20,21, but much higher than rates from other countries with a repeat donor population, such as the USA and France 22.
Our data (80% of false-positive results among p24 Ag repeatedly reactive donors) are consistent with the concept that that most repeatedly reactive p24 Ag screening results in the blood donor population are expected to be false-positive or invalid 9,18. In contrast, most of the repeatedly reactive results in the HIV Ab assays were confirmed as positive (70.11%). These findings yield a specificity of 99.93% for p24 Ag and 99.91% for HIV Ab (the kits used report 100% sensitivity; hence all nonreactive samples were considered true negative). The specificity values obtained coincide with the values provided in the kit inserts and are compliant with the WHO minimum standards 5. Although these specificity values for screening methods are acceptable, we think it is desirable to improve this parameter in order to minimize unnecessary donor deferrals.
To confirm repeatedly reactive samples for HIV Ab screening tests, supplemental or confirmatory tests, such as WB, should be performed. One-third of our HIV Ab repeatedly reactive donors were false positive; thus, it is very important to confirm HIV infection by additional tests before reporting reactive results to the donor to avoid the psychological distress associated with a false-positive HIV result. Other blood banks have expressed similar considerations 13. Given the fact that confirmatory testing (such as WB) may sometimes be unavailable because of its high cost, and to minimize the number of specimens that require additional testing, we believe that in cases with an S/CO ratio ≥3.00 (or using another specific S/CO ratio established with a larger number of samples), WB can be replaced by a second screening test, in keeping with WHO recommendations 5. If a sample is reactive on both these tests, it is considered HIV Ab-positive without wasting time and money performing WB testing. However, samples with low S/CO ratio (< 3.0) must be tested by WB to define the true HIV status, as is recommended in the CDC algorithm for HCV 23.
Only one case of HIV seroconversion was detected among the 30,132 tested donors; that is, 3.3 cases per 100,000 donations. It has been estimated that one p24 Ag reactive/HIV Ab nonreactive donor will be present in every 88,000-175,000 donations in the Latin American replacement blood donor population 18; therefore, it is probable that we will have to wait several years to detect a second seroconversion case in our donor population. p24 Ag is usually detectable a mean of six days earlier than HIV Ab 24. Based on the data in figure 2 it can be inferred that the donor might have seroconverted between the 4th and 6th day after donation, when p24 Ag was detected. Other blood banks in Argentina have reported cases of p24 Ag true-positive donors who later seroconverted 25,26. These results support the policy of continuing with p24 Ag testing among blood donors, despite the fact that this antigen is only detectable during a short time.
Among the group of true-positive HIV donors, a large number did not return after the mail citation or lied about their address. The low percentage of positive HIV donors that returned is similar to that observed by others 14. It may be that the donors who did not return knew about or suspected their positive serological status and, nevertheless, came to the blood bank because of pressure by their family or work partners. Another possibility is that these individuals used the donation opportunity to obtain free HIV testing, which in our setting is only prescribed after a medical interview (these individuals may not have wanted a physician consultation) 27. Most of our infected donors were young men practicing high-risk behavior (unprotected sex activity and intravenous drug use), as has been reported by other blood banks and infectious disease centers 17,20,28,29. It is known that donors generally do not reveal their risk factors during the interview 30.
There was no clear demographic profile to distinguish between repeatedly reactive or infected donors and nonreactive ones. Furthermore, there were no significant differences in the mean age between nonreactive and repeatedly reactive donors for both HIV Ab and p24 Ag testing; however, among repeatedly reactive donors there was a small age difference (P < .1) between true- (younger) and false-positive donors.
A high association was found between HIV infection and other diseases, particularly blood-transmitted infections (coinfection with other blood-transmissible viruses was common). Similar coinfection patterns have been reported by other blood banks 17,19-21 and health centers 28,29. In contrast, coinfection was rare among our HIV-negative donors, also in keeping with data from other blood banks 19. It is clear that a HIV Ab and p24 Ag screening by improved methods and kits, more rigorous donor selection, and an increase in the proportion of voluntary and repeat donors versus replacement donors is necessary to improve transfusion safety. However, there still remains a small but significant transfusion risk for HIV and other viral agents. To reduce this residual risk, progressive introduction of nucleic acid amplification technology is necessary in blood screening 31. Considering that RNA testing at a sensitivity of 50 copies/mL would detect HIV infection approximately 7 days before p24 Ag testing 32, implementation of nucleic acid amplification for HIV screening in our blood bank should be done as soon as possible to minimize the serological diagnostic window of recent HIV infection and reduce the residual transfusion-transmissible infection risk. Several publications have reported the benefits of this new technology in blood donor screening, in addition to serological testing 31,33-37. However, before introducing these techniques, associated issues must be resolved, such as the higher HIV screening cost per blood unit, and the need for separate laboratory areas and highly trained personnel for this purpose. A clear public health policy in favor of implementing nucleic acid amplification technology in blood banks would be an important advance to achieve this goal.
Acknowledgements
The authors thank Dr. Luisa Carnevali (chief of the Hemotherapy and Immunohematology Unit, Juan A. Fernández Hospital, Government of Buenos Aires City) for her kind cooperation and Dr. Pedro Cahn, Dr. Héctor Pérez and Lic. Florencia Ameal (Infectious Disease Unit, Juan A. Fernández Hospital, Buenos Aires) for clinical data collection and critical review of our manuscript, as well as Dr. Beatriz Livellara (Molecular Biology Laboratory, Italian Hospital, Buenos Aires) who performed the molecular study of our single HIV pre-seroconversion donor.
Contributions
The authors contributed equally during the preparation of this paper and the order of appearance of the names does not reflect differences in amount of work put into the writing of manuscript.
Disclosures
Conflict of interest: none.
Redundant publications: no substantial overlapping with previous papers.
Correspondence:
Dra. S.A. Gendler.
Migueletes, 1127 2.º B. 1126 Buenos Aires. Argentina.
E-mail: gendlersa@yahoo.com.ar
Manuscrito recibido el 6-11-2005; aceptado el 8-6-2006.