Introduction
The use of intravenous drugs is one of the main risk behaviors for the transmission of hepatitis C virus (HCV) and human immunodeficiency virus (HIV) 1,2. As a result, there is a high prevalence of these infections among intravenous drug users (IDUs), with estimates of 30-60% for HCV and 1-30% for HIV 3,4.
The prognosis of HIV disease has improved considerably since 1996 when highly active antiretroviral therapy (HAART) became available. This treatment has lengthened the life expectancy of patients with access to this therapy 5,6. In high-income countries, the number of new HIV infections is estimated to have slowed down 7,8. However, transmission of this disease depends enormously on individual behavior, i.e. personal habits that are subject to changes and variations in line with social circumstances, beliefs, lifestyles and the availability of information 9,10; hence, the patterns of seroprevalence may change considerably 11. This has led the Joint United Nations Program on HIV/AIDS (UNAIDS) to state that, following a major cut in prevention efforts, it is now essential to boost advisory and preventive services if a new peak in HIV transmission is to be avoided in these countries 12.
Hepatitis C infection is five times more widespread than HIV 1, and has remained unchanged even in communities where the rate of HIV infection has fallen 13. In industrialized countries HCV infection has become one of the major health problems among current and former IDUs. Although the sexually-transmitted infection rates of HCV are lower than those of HIV 14, HCV transmission through injection is much more effective 1,15, thus, IDUs are one of the main groups affected by this disease. In the acute phase, hepatitis C has few or no symptoms, but serious diseases, such as hepatic cirrhosis or carcinoma, can develop over a series of decades 1,16 and around 85% of cases can become chronic 17. The asymptomatic course makes it difficult to stage the disease, particularly when there has been no monitoring, and this prevents the implementation of healthcare measures.
With the increase in life expectancy of HIV-infected persons, HCV appears as the most frequently associated pathogen. The prevalence of HCV infection among individuals living with HIV is around 40%, with some studies reporting up to 94% 16. It has been suggested that HIV and HCV co-infection leads to higher mortality rates and a greater likelihood of sexual or mother-to-child transmission of HCV, as well as an accelerated course of HCV infection 3,18-21.
In view of the above factors, former and current IDUs, whether regular or only occasional users, constitute a vulnerable group that require an ongoing review of prevention strategies, healthcare provision, treatment and damage limitation vis à vis HIV and HCV. In general, IDUs are not a homogeneous group and depend on the social circumstances of their environment, the drug market, type of drug use, etc. This often makes it difficult to locate and study IDUs and determine the incidence of HIV and HCV 22 in this population. This study has compiled information from the streets through interviews with the most socially marginal groups to determine drug use patterns, risk behavior, and the incidence of infectious diseases. The aim of this effort is to analyze the self-reported prevalence of HCV and HIV in a sample of socially excluded injecting drug users, as well as factors associated with the presence of these diseases.
Methods
Design and study population
This is a cross-sectional study on the risk behavior and life styles of socially excluded drug users. The data considered in this paper is encompassed within a European research project (Excludes project) on risk behavior and life styles among socially excluded persons, drug users, heterosexuals, and homosexuals at risk for HIV/AIDS in Europe 23. The following cities took part in this project: Seville and Granada, Spain; Cologne, Germany; Vienna, Austria; Brussels, Belgium; Athens, Greece; Dublin, Ireland; London, England; Lisbon, Portugal and Perugia, Italy. The initial sample included 1879 participants, mainly heroin and/or cocaine users (92.3%), with a mean age of 30.19 years. Only those participants that had consumed heroin, cocaine or any derivative of these substances intravenously over the past year were considered for the present study.
Participants and data collection
The sample contained 1131 participants (71.5% men) with a mean age of 30 years who had injected heroin and/or cocaine over the past year. Data were compiled between July and October 1998 (England, Italy and Portugal) and between July and October 2000 (Austria, Belgium, Greece, Ireland, Germany and Spain). Structured, face-to-face interviews were held, using a World Health Organization questionnaire 24 on risk behavior for HIV transmission and drug use that was adapted to local conditions and the aims of the study. The interviews were conducted by outreach workers and privileged access interviewers (PAIs, i.e. former drug users) 25-28, in the locations where participants with the required profile were most frequently found (social service centers, town hall, street, squares and other common meeting points. This approach, combined with the "snowball" technique, allowed contact with a population that did not use regularly (or at all) the available social and health services, from which drug users are often recruited.
Statistical analysis
Two variables, the self-reported serological status for HCV and for HIV, were analyzed. The three possible replies for these variables were positive, negative or unknown. Information on HCV was compiled by asking participants whether they formerly or currently had the disease. For HIV, subjects were asked whether they had ever had a blood test for HIV/AIDS and, if so, what the result had been. First, a descriptive analysis was made to ascertain the final HCV and HIV case count overall and for each country involved in the project. Comparisons were made with the following groups of variables to provide insight into which factors are linked to HCV and HIV infection: sociodemographic characteristics, history of treatment for drug addiction, and risk behavior for acquiring HCV, HIV and related diseases. ANOVA was used to enable comparisons between the continuous variables (age, age at onset of drug use, and how long heroin and/or cocaine had been used), and the chi-square test was used for the nominal variables. Lastly, a stepwise binary logistic regression model was developed between the independent variables and HCV or HIV self-reported positive versus negative status. Variables with a P value of < .05 were entered in the model, and retained with a P value of < .1. Gender, age and country were forced into the model regardless of their significance. The Windows SPSS statistical package was used to perform the analysis 29.
Results
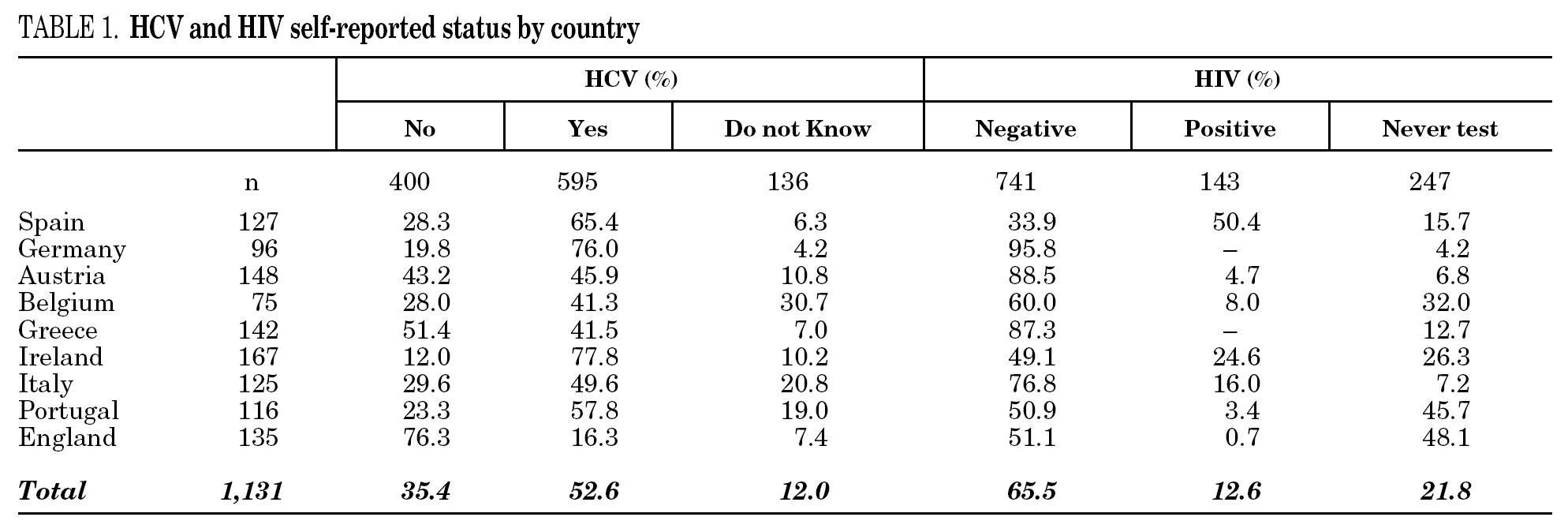
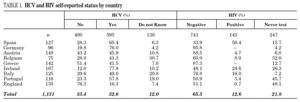
Among the 1131 injecting drug users, 595 (52.6%) reported HCV-positive status and 143 (12.6%) HIV-positive status (table 1). One hundred thirty-six participants (12%) stated they were unaware of their HCV status, and 247 (21.8%) had never been tested for HIV. Prevalence of reported HCV-positive status was over 50% in Ireland, Germany, Portugal, and Spain. Belgium and Italy had the highest percentages of patients unaware of HCV status. No German or Greek participants reported HIV-positive test results and a large proportion of participants from Portugal, England, Belgium and Ireland claimed they had never been tested. The highest HIV-positive rates were reported in Spain (50.4%), Ireland (24.6%) and Italy (16%). The total reported HCV-positive and HIV-positive cases among those who knew their status was 59.8 and 16.2, respectively.
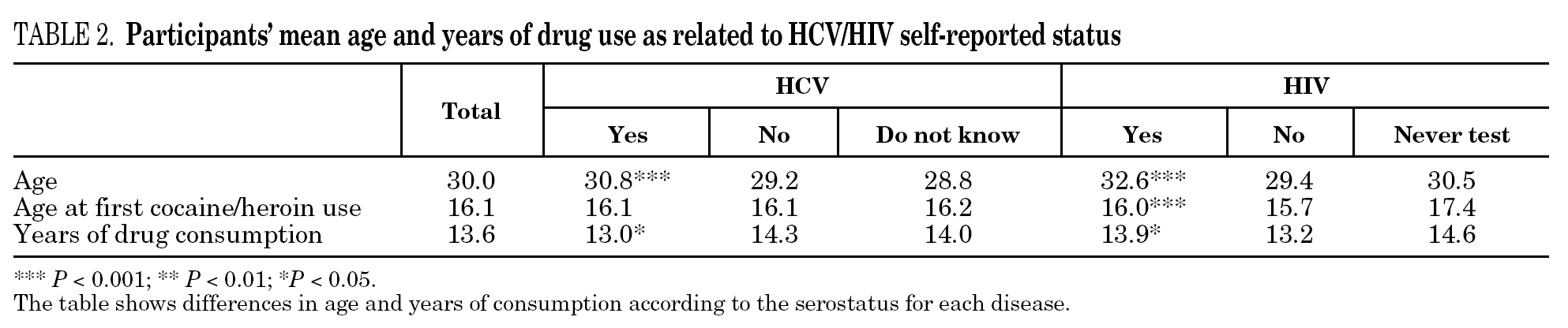
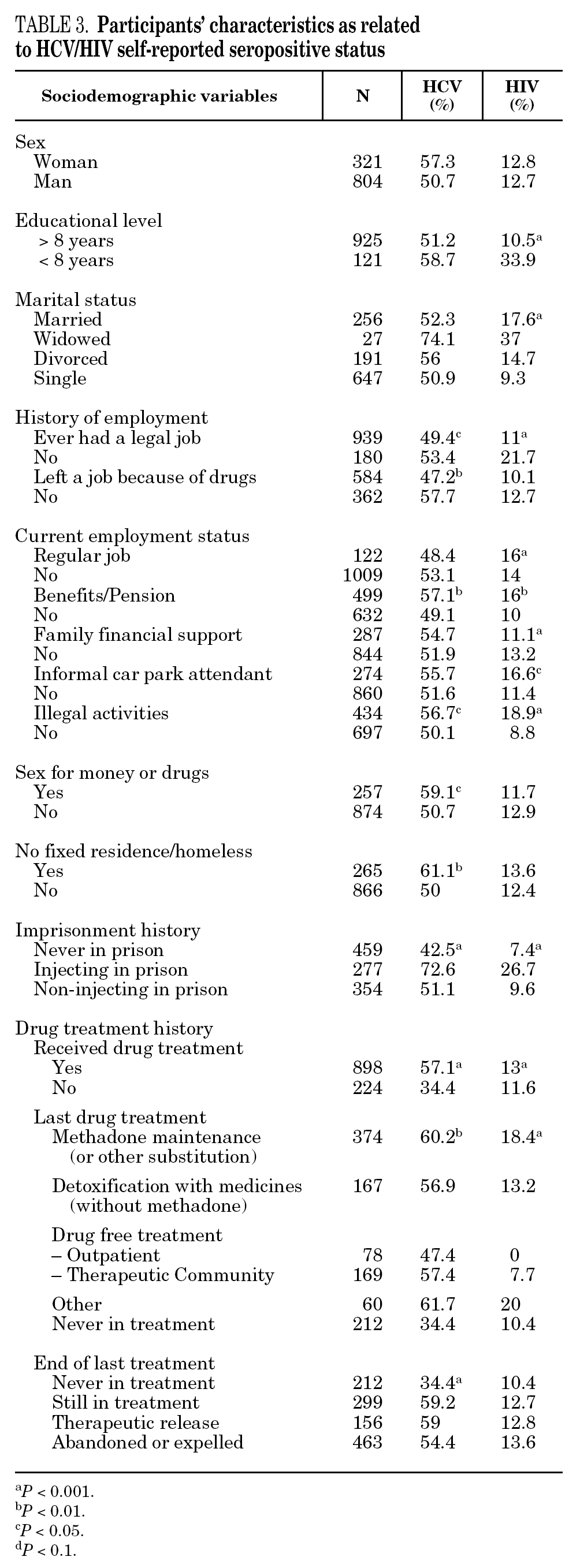
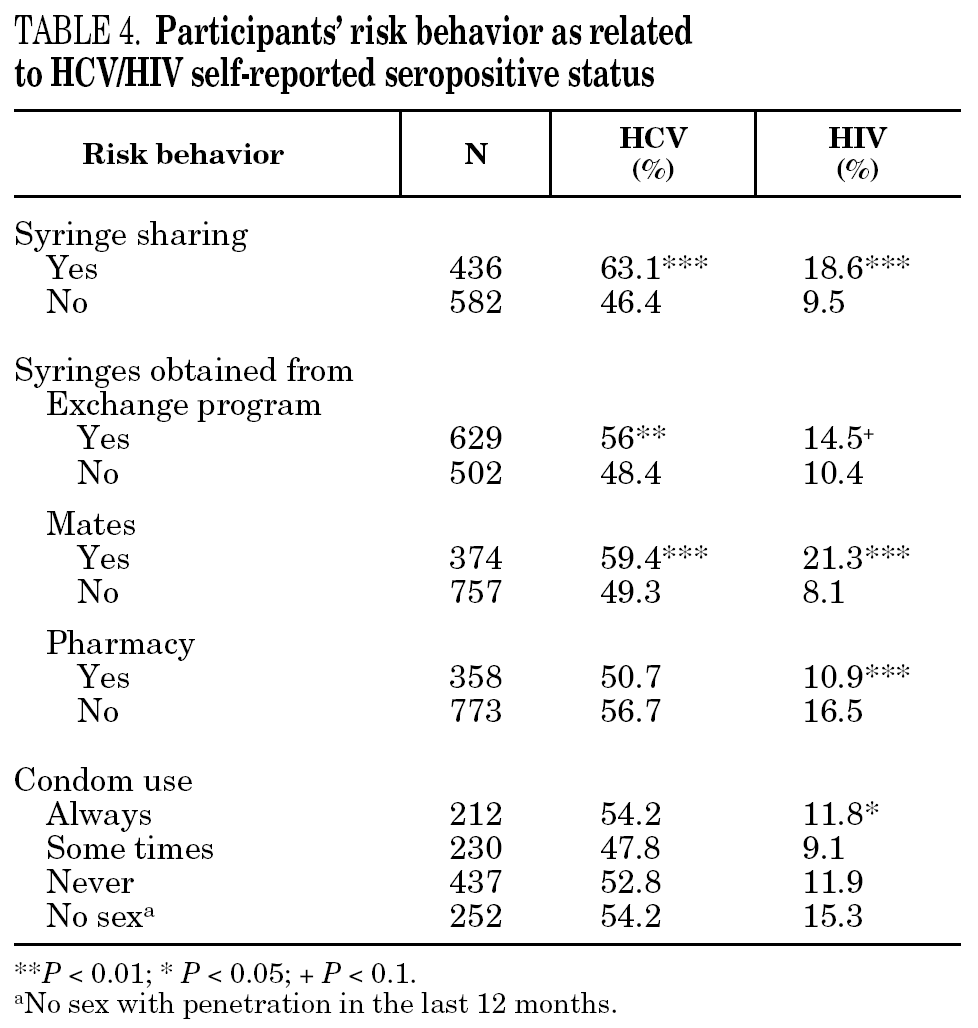
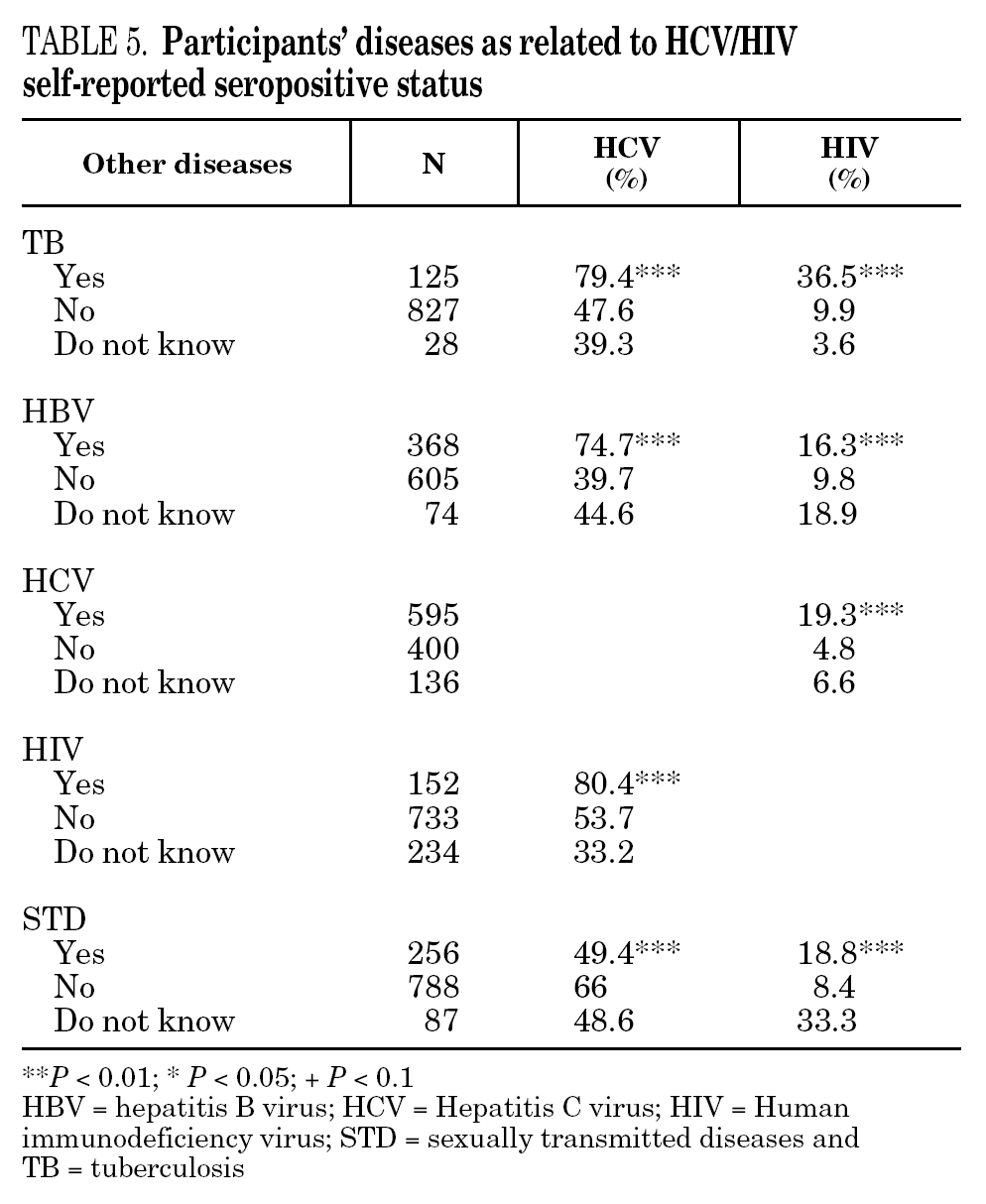
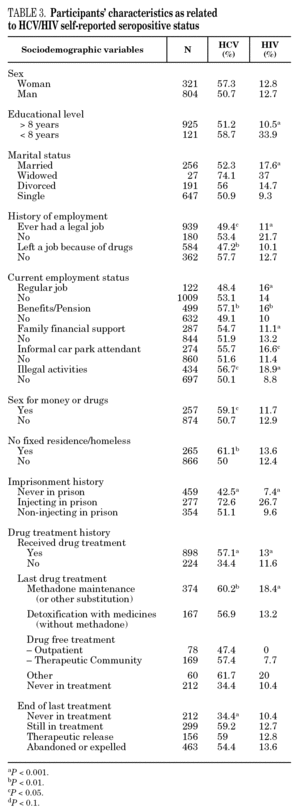
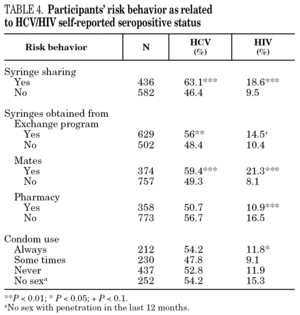
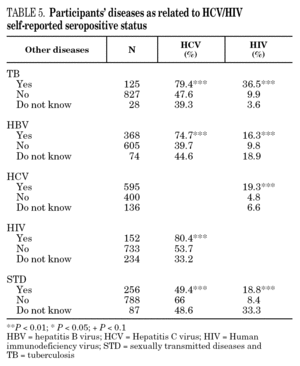
The reported prevalence of HCV and HIV infections increased with age and years of drug use, and showed a statistically significant association (table 2). Chi-square comparisons in tables 3, 4 and 5 summarize the variables that were significantly associated with reported HCV and HIV status, and the prevalence of these infections at each level. High prevalences of HCV and HIV were found in participants who had completed less than 8 years of education, were widowed, had injected drugs while in prison, had received drug addiction treatment, had shared needles or obtained syringes from mates, had never or only occasionally used condoms, and those reporting tuberculosis (TB), sexually transmitted disease (STD), or hepatitis B (HBV) infection. A large proportion of HIV-positive participants (80.3%) were co-infected with HCV (n = 122, 10.9% of the total sample).
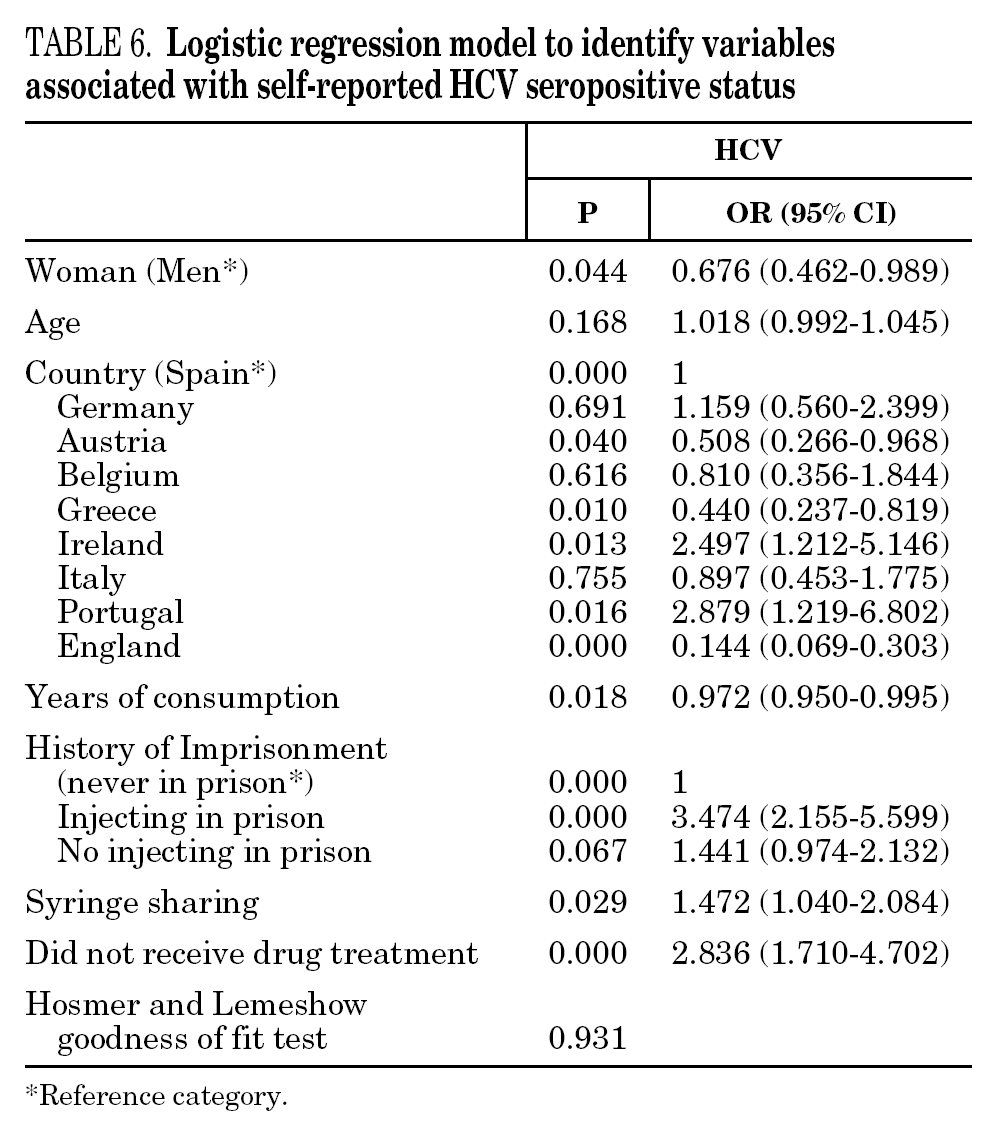
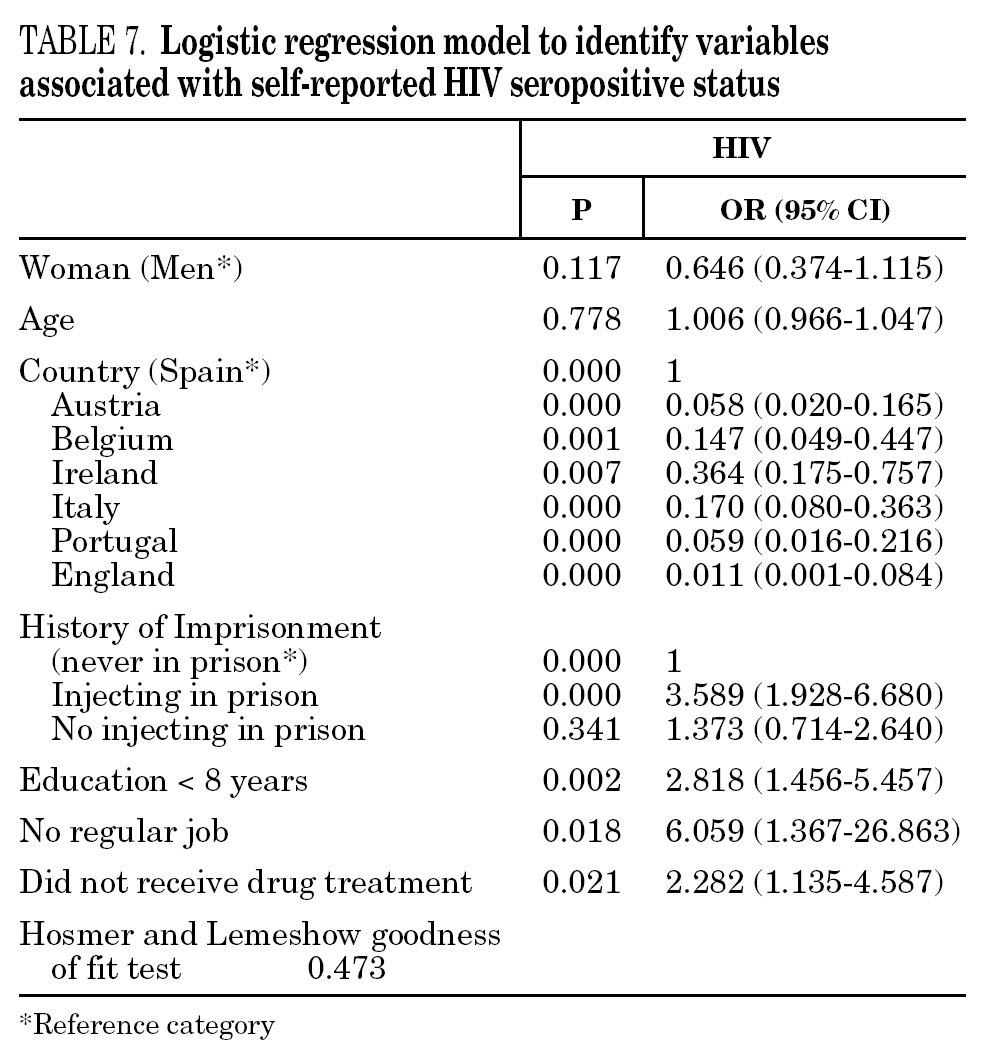
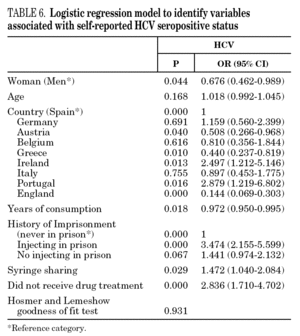
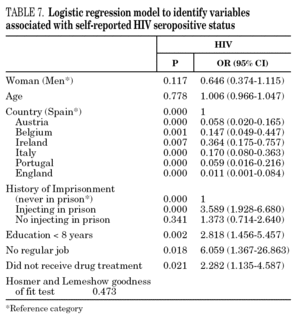
In the final logistic regression model for reported HCV-positive status (table 6), women were less likely than men to report positive status, and age was not associated with this factor. Longer drug use, injecting while in prison, shared needles, and never having received drug treatment were positively associated with HCV infection. Results of the final logistic regression model for reported HIV-positive status are presented in table 7. Participants reporting positive HIV status were usually older, had injected drugs while in prison, had completed less than 8 years of schooling, had no regular employment and had not received drug treatment. Goodness of fit was not significant for either model.
Discussion
In this sample of socially excluded drug users who had injected drugs over the past year, we found a high prevalence of HCV infection, a relatively lower rate of HIV infection, and a high incidence of HCV infection among HIV-positive participants. These results are consistent with data provided by previous studies, which report HCV infection as one of the main health problems among IDUs, stabilization of the HIV infection rate, and an increase in co-infection by these viruses 8,30-35.
The highest incidences of HCV and HIV were reported by participants in a poorer social situation (lower educational level, widowed status, unemployed, informal and illegal activities, imprisoned) and those with a higher prevalence of other infectious diseases. Among the participants, 71% reported TB, HCV, HBV, HIV, or STD infection, and 43% had two or more of these diseases concomitantly (data not presented). These results place intravenous drug use within a framework of social exclusion that goes hand in hand with a dramatic deterioration in health. The use of illicit drugs is one of the main causes of social exclusion 36, although many young people might already be considered socially excluded before they start to use drugs 37. Drug use and its associated problems form a part of a broader process that entails marginalization, vulnerability, and a lack of integration. All these issues are related to the health of IDUs and of those they interact with. Most interviewees in this study underwent some type of treatment for their drug use (79.9%), which suggests that treatment is available through the healthcare system and that IDUs have access to therapy and harm reduction advice 38. However, in general terms, the results are modest and treatment compliance rates are low, possibly because drug addicts must cope not only with their addiction, but also with the process of social exclusion they are immersed in, i.e., unemployment, chronic disease, loss of emotional bonds marginalization, and so on 39. To curb behavior that renders people more vulnerable to infection, action must be taken on a complex set of circumstances that require a multidisciplinary approach 9. Hence, any course of action for these groups should, as far as possible, be built into integrated and coordinated plans that take a broad approach to the main issues involved.
The main form of HCV transmission among IDUs is by needle sharing, which is also among the main sources of HIV infection. Despite the recognized danger of this practice, sharing injecting equipment may continue among different generations of IDUs, and the reasons may remain unclear 40. Although fewer IDUs in this sample had shared needles over the past year (59.3%), those that did share needles showed a high prevalence of HCV and HIV. As in other studies 8,41-45, IDUs who had been in prison and continued injecting drug use had the highest prevalence of HCV and HIV. Sharing injection material, wherever it is done, is the true marker of HCV infection risk. Nevertheless, injecting drugs in prison worsens the situation, since very few prisons have syringe exchange programs 46,47, and this favors assiduous sharing of injection material. In addition, serving a prison sentence is often part of the marginalization process (toward or within), which many opiate addicts experience. From this point on, the possibility of attaining social integration becomes more remote 4. The high percentage of illicit drug users and the high incidence of infectious disease among prison populations increases the vulnerability of this population 48. This suggests that healthcare provision within prisons furnishes an extremely useful opportunity to ensure assessment, healthcare and treatment for an excluded group that will continue to be largely marginalized when released from prison 49-51.
Regular, adequate use of condoms is an effective method for preventing sexual transmission of HIV 52. Over half the participants who had engaged in sexual relations during the past year stated that they never used a condom. However, there were fewer HIV- and HCV-positive participants among them. Several studies have shown irregular condom use in a large proportion of drug users 53. There are, however, certain differences according to the kind of sexual partner; i.e., condoms are used less often among stable couples than among occasional partners 54,55, and there is wider use of condoms among sex workers 56,57. These results emphasize two major issues: first, the risk of sexual transmission of these and other diseases in the non-IDU population, and second, a slightly greater use of the condom among IDUs who self-report as HIV- or HCV-positive when compared with negative IDUs. Education promoting consistent use of the condom continues to be an essential tool for the prevention of disease transmission. Improvements may be feasibly achieved by our interventions if the circumstances that enhance their use in vulnerable populations, such as current and former IDUs, were better understood.
The multivariate models proposed in this paper suggest that social exclusion indicators (lower educational level, loss of family bonds, unemployment) have a greater weight for acquiring HIV infection, while the drug use indicators (longer addiction, needle sharing) have a greater bearing on HCV. HIV infection takes an average of 8 to 11 years to develop into the acquired immunodeficiency syndrome (AIDS) 58, although, in the absence of treatment, some HIV-positive participants develop the syndrome earlier. As for HCV, patients can remain symptom-free for a long interval; for instance, serious liver disease may appear up to 20 or 30 years after the initial infection 1. We can say that quality of life is jeopardized more quickly in HIV infection and that there is a greater social stigma attached to this disease than to HCV, although both raise major barriers to social integration. Moreover, as HCV is very effectively transmitted through blood, long-lasting use of illicit drugs and an injecting habit may have a greater bearing on this disease than on HIV 59.
This paper has several limitations, mainly due to the cross-sectional design, which does not enable inferences to be drawn in terms of causality. The study was carried out between 1998 and 2000; this may be a reason why the results might not reflect the present situation. In this work, HIV and HCV serology is self-reported. This prevents our knowing the 'real' status of the participants, and we should consider a potential bias related to memory failure or prejudices that can make declaration of these infections difficult. In addition, the way in which the question on hepatitis C was posed gives rise to a greater likelihood of false-negatives, i.e. people who have not been tested for HCV report they are HCV-free.
The considerable differences in HIV seroprevalence among cities in different countries could be due to a methodological bias. Nevertheless, some epidemiological studies indicate that there is considerable regional variation between and within countries in relation to HIV prevalence and incidence in IDUs 60. The percentage of HIV-seropositive individuals for each country in this study are reasonably within the ranges reported by other studies on the prevalence of HIV among IDUs 61. The differences observed could reflect a complex interaction between several factors, such as the patterns of drug consumption, how and when methadone treatment, preventive measures, and harm reduction programs were implemented, and the general HIV prevalence rates in each region 60.
Is important to highlight the large number of participants in this study that did not know their HIV or HCV serologic status. The reasons for this are difficult determine, but it can be argued that socially excluded people tend to benefit less from social and health services. This situation can imply a bias in the results, by which there would be an underestimation of the seroprevalence of the sample. It is crucial to know the serostatus of a target population for epidemiological vigilance and to plan preventive strategies adapted to the profiles of specific subpopulations 7,62. In addition, individuals who do not know whether they have a disease will not be able to benefit from the available treatments.
Despite these and other limitations, we believe that this study provides insight into the characteristics of IDUs reporting their HCV and HIV status in several European cities, and into the factors linked to these two diseases.
Correspondence:
Dra. E. Oviedo-Joekes.
Escuela Andaluza de Salud Pública.
Cuesta del Observatorio, 4.
Campus Universitario de Cartuja. 18080 Granada. España.
Correo electrónico: eugenia.oviedojoekes.easp@juntadeandalucia.es
Manuscrito recibido el 16-11-2005; aceptado el 21-7-2006.