The genus Aspergillus contains more than 300 species, which are divided into closely related groups called sections. Molecular studies have revealed numerous cryptic species within different sections of this genus, which have different profiles of antifungal susceptibility and lack diagnostic morphological features. However, there are few studies on the prevalence and in vitro antifungal susceptibility of the cryptic species of this genus. The aim of this study was to investigate the distribution of Aspergillus spp. among clinical samples, and to study their in vitro susceptibility to different antifungal drugs.
MethodOver a period of 2-years (2014–2015), a total of 379 strains of the genus Aspergillus were isolated. Most of the isolates were classified as respiratory colonizations; no cases of invasive aspergillosis were found. The strains were identified by MALDI-TOF mass spectrometry, and susceptibility testing was performed by the EUCAST reference procedure.
ResultsTwenty species belonging to 8 sections were identified, being A. fumigatus the most prevalent (44.1%). The prevalence of cryptic species was 15.3%, with a clear predominance of A. tubingensis. Among the tested antifungal drugs, amphotericin B was the less active in vitro, followed by triazole drugs and echinocandins. The cryptic species had minimun inhibitory concentrations (MICs) higher than the corresponding type species.
ConclusionsAccurate identification of the genus Aspergillus at the species level and in vitro antifungal susceptibility testing are necessary because, as it has been shown, some species of this genus may show resistance profiles against available antifungal drugs.
El género Aspergillus contiene más de 300 especies, que se dividen en grupos estrechamente relacionados llamados secciones. Los estudios moleculares han revelado la existencia de numerosas especies crípticas dentro de las diferentes secciones de este género, las cuales tienen diferentes perfiles de sensibilidad antifúngica y carecen de características morfológicas diferenciales de diagnóstico. Sin embargo, hay pocos estudios sobre la prevalencia y la sensibilidad antifúngica in vitro de las especies crípticas de este género. El objetivo de este estudio fue investigar la distribución de Aspergillus spp. en muestras clínicas, y estudiar su sensibilidad in vitro a diferentes fármacos antimicóticos.
MétodosDurante un período de 2 años (2014-2015), se aislaron un total de 379 cepas del género Aspergillus. La mayoría de los aislamientos se clasificaron como colonizaciones respiratorias; no encontrándose casos de aspergilosis invasiva. Las cepas se identificaron mediante espectrometría de masas MALDI-TOF, y las pruebas de sensibilidad antifúngica se realizaron mediante el procedimiento de referencia EUCAST.
ResultadosSe identificaron 20 especies pertenecientes a 8 secciones, siendo A. fumigatus la más prevalente (44,1%). La prevalencia de especies crípticas fue del 15,3%, con un claro predominio de A. tubingensis. Entre los fármacos antimicóticos probados, la anfotericina B fue la menos activa in vitro, seguida de los fármacos triazoles y las equinocandinas. Las concentraciones mínimas inhibitorias (CMIs) de los antifúngicos fueron más elevadas frente a las especies crípticas que frente a las especies tipo correspondientes.
ConclusionesLa identificación precisa de Aspergillus a nivel de especie y las pruebas de sensibilidad a antifúngicos in vitro son necesarias porque, como se ha demostrado, algunas especies de este género presentan diferentes perfiles de resistencia frente a los fármacos antimicóticos disponibles.
Since the genus Aspergillus was first described in 1729 by the Italian botanist Micheli, more than 300 species have been described.1 These species are characterized by their spores. They are widely distributed in the air and the environment, being responsible for a wide spectrum of diseases, from allergies to invasive infections, particularly in immunosuppressed patients.2 Traditionally, in clinical mycology laboratories, the identification of Aspergillus spp. has been routinely based on determination of macroscopic and microscopic morphological characteristics.3 These identification methods do not allow discrimination between morphologically closely related species (cryptic species) within the genus, so current recommendations for species level identification within the Aspergillus sections include the use of molecular methods based on comparative sequencing.4,5
Accurate identification at the species level is important because some of these cryptic species, such us A. calidoustus (Aspergillus section Usti) and A. lentulus (Aspergillus section Fumigati) show decreased susceptibility to multiple antifungal drugs.6,7 Therefore, a correct identification should guide the choice of the most appropriate antifungal therapy, until the antifungal susceptibility testing results are available.
Finally, it is important to highlight how medical advances have managed to increase the survival of patients under immunosuppressive conditions, increasing the risk of developing an invasive fungal infection.8,9 In fact, Aspergillus spp. is the main cause of invasive mycosis caused by molds in immunosuppressed patients.
Despite all of the above, there are few prevalence studies involving these criptic pathogens. Therefore, the aim of this study was to analyze the distribution of Aspergillus spp. isolated from clinical samples and to describe their in vitro antifungal susceptibility, in a Spanish tertiary care hospital.
MethodsAspergillus spp. isolates and clinical dataWe included all 379 strains of Aspergillus spp. isolated from clinical samples over a 2-year period, from January 2014 to December 2015, in the Clinical Microbiology Laboratory of the University Hospital Virgen del Rocío (Seville, Spain).
The laboratory information system and the medical records of the patients were used to obtain the demographic data, information on underlying disease, dates and type of samples, and clinical assessment of each isolation. Regarding the latter, and in similar way to that described by Alastruey-Izquierdo et al.,13 we classified Aspergillus spp. isolates into two groups: the clinically relevant ones, which received targeted antifungal treatment; and the colonizers, which did not receive it.
Strains collectionThe identification of the Aspergillus spp. strains by molecular methods (gene sequencing and mass spectrometry) and the antifungal susceptibility testing were carried out in different periods and retrospectively, from a collection of strains based on suspensions of conidia in sterile distilled water. These were obtained from cultures grown on Sabouraud-chloramphenicol agar plates (SCA; Oxoid, Basingstoke, UK) after 5–7 days of incubation at 30°C. The vials were stored at room temperature. Finally, the strains were recovered by inoculating an aliquot of distilled water suspension of conidia on SCA, making 5 marks per plate and incubating for 48h at 30°C.
Strains identificationAll 379 strains were identified prospectively, during the study period, by morphological observation using the identification key described by de Hoog et al.10
One hundred seventy nine strains were selected to be identified by sequencing of beta-tubulin and calmodulin genes (because they offer unknown phenotypic characteristics). Finally, an in-house reference database was constructed in the matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonik GmbH, Germany) from 42 sequenced clinical isolates and 11 reference strains.11 This new database included 23 different species (7 non-cryptic and 16 cryptic species), of which 12 species, all cryptic, are exclusively present in our library and are not found in the manufacturer's library.
The remaining 200 strains were identified by MALDI-TOF MS, exclusively using our in-house extended and previously validated database.11 For this, the isolates were grown on SCA for 48h of incubation at 30°C, and were processed by a standardized extraction procedure with ethanol and formic acid. All isolates were spotted and identified in triplicate by MALDI-TOF MS. In order to correctly consider the identification of the strains, at least two of the three identifications were required to coincide in the name of the species and obtain scores ≥2.0. The strains identified with score <2.0 were reidentified by partial sequencing of the beta-tubulin gene.
Antifungal susceptibility testingMicrobroth dilution testing was performed as outlined by the European Committee for Antimicrobial Susceptibility Testing (EUCAST) version 9.3, 2015.12 The antifungal agents tested were amphotericin B (Sigma-Aldrich Química), voriconazole (Pfizer S.A., Madrid, Spain), posaconazole (Merck & Co., Inc., Rahway, NJ), caspofungin (Merck & Co., Inc., Rahway, NJ) and anidulafungin (Pfizer S.A., Madrid, Spain). Aspergillus fumigatus ATCC 204305 and Aspergillus flavus ATCC 204304 were used as quality-control strains.
A range of concentrations from 0.03 to 16mg/L of amphotericin B, voriconazole, caspofungin and anidulafungin and, from 0.015 to 8mg/L for posaconazole were studied. Plates were incubated at 35±2°C for 48h. Visual readings were performed at 48h with the help of a mirror. The endpoint for amphotericin B, voriconazole and posaconazole was the antifungal concentration that produced a complete inhibition of visual growth at 48h (minimum inhibitory concentration, MIC). For the echinocandins (caspofungin and anidulafungin), the endpoint was the antifungal concentration that produced a visible change in the morphology of the hyphae compared with the growth control well (minimum effective concentration, MEC). Tests were performed in triplicate. We performed the susceptibility testing on 82 strains, which included all cryptic species of our collection and a maximum of 5 strains of each non-cryptic species, since the aim of the study focused on cryptic species.
As with the document “Clinical breakpoints-fungi (v 8.1)” of EUCAST, only breakpoints are established for certain Aspergillus spp., and none of them are for cryptic species. We decided not to apply them, and just described the results obtained for every species.
ResultsOut of 184,558 clinical samples processed for fungal culture over a 2-year period in our laboratory, 351 samples (0.2%) from 280 different patients were positive in culture for Aspergillus spp. A total of 379 strains of Aspergillus spp. were isolated from a variety of samples including respiratory tract (n=273), otic exudates (n=62), skin/nails scales (n=6), bone biopsy (n=1), conjunctival exudate (n=1), skin burn (n=1), peritoneal drainage (n=1), pharyngeal exudates (n=3), pleural fluid (n=1), vitreous humor (n=1) and wound exudate (n=1). Among the respiratory tract samples, sputum (90.11%) was the most frequent, followed by 4.76% bronchoalveolar lavage, 4.76% transbronchial aspirates fluids and 0.37% bronchial brushings. There were 26 samples in which multiple isolates of Aspergillus spp. were cultured, including 24 samples with double isolates and two with triple isolates.
The male/female ratio of the 280 patients with Aspergillus spp. cultured was 1/19. The mean age of the patients was 53.86±24.18 years (range: 1–94 years). Pulmonary disease was present in 38.21% patients as underlying disease, followed by cystic fibrosis in 17.50%, while immunosuppressed patients accounted for 5%.
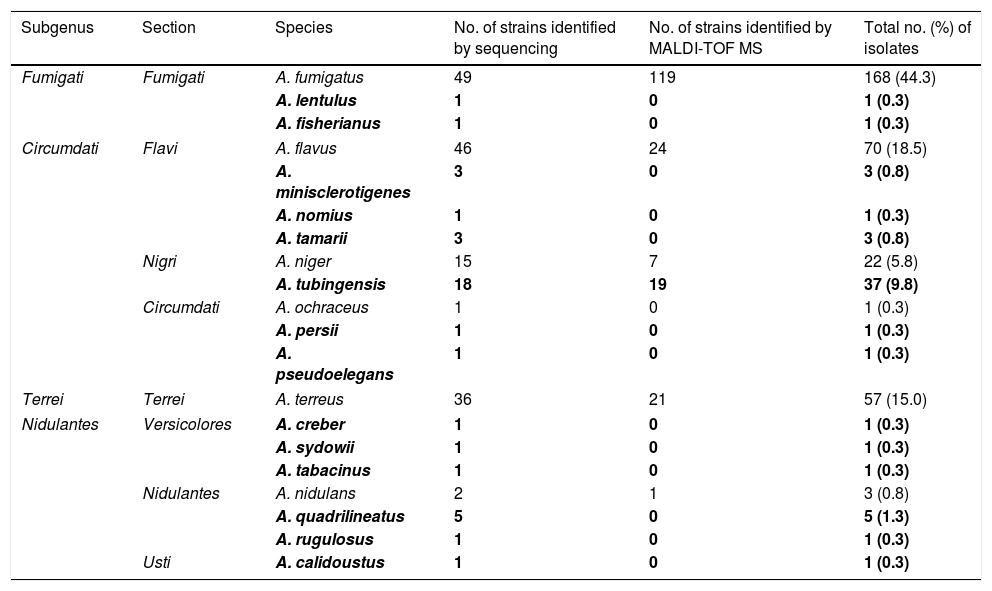
The identification of the 200 strains of Aspergillus spp. by MALDI-TOF MS, with the new database, allowed obtaining a correct identification at the species level (score ≥2.0) in 191 (95.5%) of them. The nine remaining strains obtained a score between 1.7 and <2.0, being reidentified by partial sequencing of the beta-tubulin gene. Finally, twenty different species from 8 sections were identified (Table 1). One-hundred seventy strains were classified as Aspergillus section Fumigati: 168 A. fumigatus, 1 A. lentulus and 1 A. fisherianus. Aspergillus section Flavi included 70 A. flavus, 3 A. minislclerotigenes, 1 A. nomius and 3 A. tamarii. Aspergillus section Nigri included 22 A. niger and 37 A. tubingensis. Aspergillus section Terrei included 57 A. terreus. Aspergillus section Nidulantes included 3 A. nidulans, 5 A. quadrilineatus and 1 A. rugulosus. Aspergillus section Versicolores included 1 A. creber, 1 A. sydowii and 1 A. tabacinus. Other sections represented were Circumdati (1 A. ocraceus, 1 A. pseudoelegans and 1 A. persii) and Usti (1 A. calidoustus). In our study of 379 strains, 58 (15.3%) corresponded to cryptic species, being A. tubingensis the most frequent.
Distribution of Aspergillus species (n=379) and number of isolates.
| Subgenus | Section | Species | No. of strains identified by sequencing | No. of strains identified by MALDI-TOF MS | Total no. (%) of isolates |
|---|---|---|---|---|---|
| Fumigati | Fumigati | A. fumigatus | 49 | 119 | 168 (44.3) |
| A. lentulus | 1 | 0 | 1 (0.3) | ||
| A. fisherianus | 1 | 0 | 1 (0.3) | ||
| Circumdati | Flavi | A. flavus | 46 | 24 | 70 (18.5) |
| A. minisclerotigenes | 3 | 0 | 3 (0.8) | ||
| A. nomius | 1 | 0 | 1 (0.3) | ||
| A. tamarii | 3 | 0 | 3 (0.8) | ||
| Nigri | A. niger | 15 | 7 | 22 (5.8) | |
| A. tubingensis | 18 | 19 | 37 (9.8) | ||
| Circumdati | A. ochraceus | 1 | 0 | 1 (0.3) | |
| A. persii | 1 | 0 | 1 (0.3) | ||
| A. pseudoelegans | 1 | 0 | 1 (0.3) | ||
| Terrei | Terrei | A. terreus | 36 | 21 | 57 (15.0) |
| Nidulantes | Versicolores | A. creber | 1 | 0 | 1 (0.3) |
| A. sydowii | 1 | 0 | 1 (0.3) | ||
| A. tabacinus | 1 | 0 | 1 (0.3) | ||
| Nidulantes | A. nidulans | 2 | 1 | 3 (0.8) | |
| A. quadrilineatus | 5 | 0 | 5 (1.3) | ||
| A. rugulosus | 1 | 0 | 1 (0.3) | ||
| Usti | A. calidoustus | 1 | 0 | 1 (0.3) | |
Note: Species highlighted in bold correspond to cryptic species.
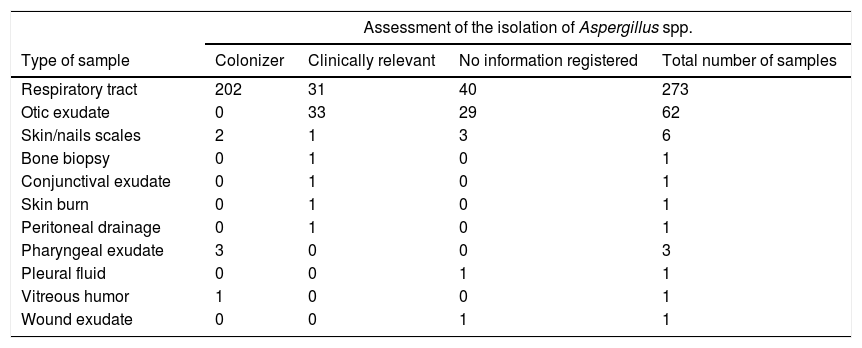
A total of 277 (78.9%) of the 351 samples had clinical information on the assessment of the isolation of Aspergillus spp. in the medical records (Table 2). Two hundred and eight (75.1%) of the isolates were considered as colonizers, and in the remaining 69 (24.9%) samples, the isolates were considered clinically relevant. These strains were isolated from the following samples: respiratory tract samples (n=31) otic exudates (n=33), bone biopsy (n=1), skin burn (n=1), conjunctival exudate (n=1), peritoneal drainage (n=1) and skin scales (n=1). Cryptic species were isolated in 24.63% of the clinically relevant cases: A. tubingensis was the most frequent with 14 isolates (all from otic exudates), followed by A. nomius, A. minisclerotigenes and A. persii, with only one isolate per species (in bone biopsy, otic exudate and respiratory tract sample, respectively).
Clinical relevance of Aspergillus spp. in different types of samples according to the information recorded in the medical history.
| Assessment of the isolation of Aspergillus spp. | ||||
|---|---|---|---|---|
| Type of sample | Colonizer | Clinically relevant | No information registered | Total number of samples |
| Respiratory tract | 202 | 31 | 40 | 273 |
| Otic exudate | 0 | 33 | 29 | 62 |
| Skin/nails scales | 2 | 1 | 3 | 6 |
| Bone biopsy | 0 | 1 | 0 | 1 |
| Conjunctival exudate | 0 | 1 | 0 | 1 |
| Skin burn | 0 | 1 | 0 | 1 |
| Peritoneal drainage | 0 | 1 | 0 | 1 |
| Pharyngeal exudate | 3 | 0 | 0 | 3 |
| Pleural fluid | 0 | 0 | 1 | 1 |
| Vitreous humor | 1 | 0 | 0 | 1 |
| Wound exudate | 0 | 0 | 1 | 1 |
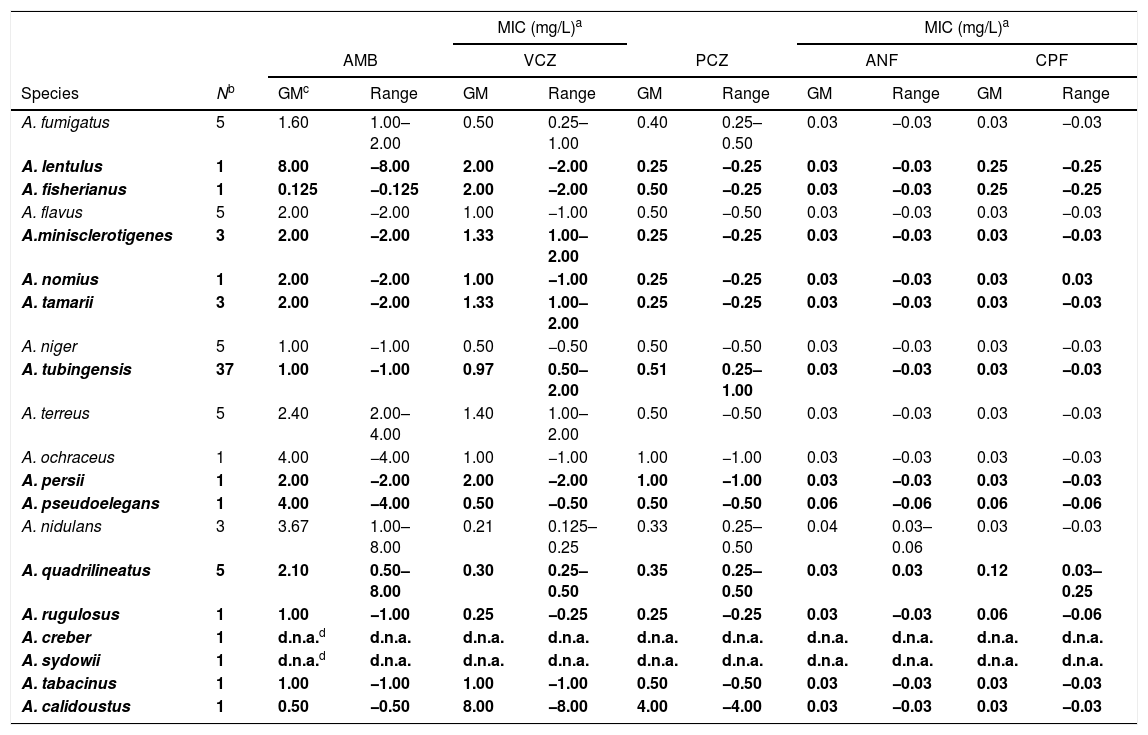
The results of the susceptibility testing of the strains are shown in Table 3. The highest MIC of 8.0mg/L of amphotericin B was found against A. lentulus, A. nidulans and A. quadrilineatus, followed by MICs of 4.0mg/L against A. terreus and in two representatives of the section Circumdati (A. ochraceus and A. pseudoelegans). The remaining species had MICs of 2.0mg/L. Aspergillus calidoustus was the species with the highest MIC values to triazoles tested with a MIC of 8.0mg/L for voriconazole and 4.0mg/L for posaconazole, while the remaining cryptic species showed MIC ranges of 0.125–2.0mg/L and 0.25–1.0mg/L for voriconazole and posaconazole, respectively. The echinocandins were the most active drugs with a MIC of anidulafungin of 0.06mg/L in A. nidulans and A. pseudoelegans; and 0.25mg/L of caspofungin in A. lentulus, A. quadrilineatus and A. fisherianus. Aspergillus creber and A. sydowii, from the Versicolores section, could not be tested due to the non-viability of the strains at the time of carrying out this part of the study (after the identification phase).
Antifungal susceptibility of Aspergillus spp. recovered in this study.
| MIC (mg/L)a | MIC (mg/L)a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMB | VCZ | PCZ | ANF | CPF | |||||||
| Species | Nb | GMc | Range | GM | Range | GM | Range | GM | Range | GM | Range |
| A. fumigatus | 5 | 1.60 | 1.00–2.00 | 0.50 | 0.25–1.00 | 0.40 | 0.25–0.50 | 0.03 | −0.03 | 0.03 | −0.03 |
| A. lentulus | 1 | 8.00 | −8.00 | 2.00 | −2.00 | 0.25 | −0.25 | 0.03 | −0.03 | 0.25 | −0.25 |
| A. fisherianus | 1 | 0.125 | −0.125 | 2.00 | −2.00 | 0.50 | −0.25 | 0.03 | −0.03 | 0.25 | −0.25 |
| A. flavus | 5 | 2.00 | −2.00 | 1.00 | −1.00 | 0.50 | −0.50 | 0.03 | −0.03 | 0.03 | −0.03 |
| A.minisclerotigenes | 3 | 2.00 | −2.00 | 1.33 | 1.00–2.00 | 0.25 | −0.25 | 0.03 | −0.03 | 0.03 | −0.03 |
| A. nomius | 1 | 2.00 | −2.00 | 1.00 | −1.00 | 0.25 | −0.25 | 0.03 | −0.03 | 0.03 | 0.03 |
| A. tamarii | 3 | 2.00 | −2.00 | 1.33 | 1.00–2.00 | 0.25 | −0.25 | 0.03 | −0.03 | 0.03 | −0.03 |
| A. niger | 5 | 1.00 | −1.00 | 0.50 | −0.50 | 0.50 | −0.50 | 0.03 | −0.03 | 0.03 | −0.03 |
| A. tubingensis | 37 | 1.00 | −1.00 | 0.97 | 0.50–2.00 | 0.51 | 0.25–1.00 | 0.03 | −0.03 | 0.03 | −0.03 |
| A. terreus | 5 | 2.40 | 2.00–4.00 | 1.40 | 1.00–2.00 | 0.50 | −0.50 | 0.03 | −0.03 | 0.03 | −0.03 |
| A. ochraceus | 1 | 4.00 | −4.00 | 1.00 | −1.00 | 1.00 | −1.00 | 0.03 | −0.03 | 0.03 | −0.03 |
| A. persii | 1 | 2.00 | −2.00 | 2.00 | −2.00 | 1.00 | −1.00 | 0.03 | −0.03 | 0.03 | −0.03 |
| A. pseudoelegans | 1 | 4.00 | −4.00 | 0.50 | −0.50 | 0.50 | −0.50 | 0.06 | −0.06 | 0.06 | −0.06 |
| A. nidulans | 3 | 3.67 | 1.00–8.00 | 0.21 | 0.125–0.25 | 0.33 | 0.25–0.50 | 0.04 | 0.03–0.06 | 0.03 | −0.03 |
| A. quadrilineatus | 5 | 2.10 | 0.50–8.00 | 0.30 | 0.25–0.50 | 0.35 | 0.25–0.50 | 0.03 | 0.03 | 0.12 | 0.03–0.25 |
| A. rugulosus | 1 | 1.00 | −1.00 | 0.25 | −0.25 | 0.25 | −0.25 | 0.03 | −0.03 | 0.06 | −0.06 |
| A. creber | 1 | d.n.a.d | d.n.a. | d.n.a. | d.n.a. | d.n.a. | d.n.a. | d.n.a. | d.n.a. | d.n.a. | d.n.a. |
| A. sydowii | 1 | d.n.a.d | d.n.a. | d.n.a. | d.n.a. | d.n.a. | d.n.a. | d.n.a. | d.n.a. | d.n.a. | d.n.a. |
| A. tabacinus | 1 | 1.00 | −1.00 | 1.00 | −1.00 | 0.50 | −0.50 | 0.03 | −0.03 | 0.03 | −0.03 |
| A. calidoustus | 1 | 0.50 | −0.50 | 8.00 | −8.00 | 4.00 | −4.00 | 0.03 | −0.03 | 0.03 | −0.03 |
The taxonomy of microorganisms has changed extensively due to advances in the identification tools. Among these, filamentous fungi have also undergone changes in their classification.1 In the case of the genus Aspergillus, numerous novel species have been recently described, a fact that has caught the attention of the medical community, since these new species have been isolated in cases of invasive infections.14–17 These species have been called cryptic species and they are worth of investigation because some have diminished profiles of antifungal sensitivity.1,18 In addition, the described cases of resistant strains are increasing. All this leads to the need to perform a correct identification at the species level and antifungal susceptibility testing.16,19,20
Diseases caused by Aspergillus spp. are associated with a spectrum of immunity disorders8,21 and invasive aspergillosis (IA) is an important cause of death in these patients. The incidence of IA in different studies ranges from <1% to 30% depending on the patient population.2 However, despite obtaining Aspergillus spp. isolates from immunosuppressed patients, there were no cases of IA recorded in our series.
Most of Aspergillus spp. isolates (77.8%) in our study grew in respiratory tract samples. Invasive pulmonary aspergillosis is the most common manifestation of aspergillosis.22 However, the isolation of Aspergillus spp. in respiratory tract samples is unspecific in immunocompetent patients because of the difficulty of distinguishing between disease and colonization.23 The isolate specificity increases when the patient's immunocompetence decreases. In our series, only 11.3% of the respiratory isolates were considered clinically relevant.
In the multicentre FILPOP study,13 carried out with clinical samples from 29 Spanish hospitals, and where the aim was to investigate the epidemiology and antifungal resistance of filamentous fungi isolated from deep tissues samples, blood cultures and respiratory samples; A. fumigatus was the most frequent species among the 278 strains of Aspergillus spp. isolated. The same happened in our study, reporting a similar prevalence (48.5% versus 44.1%). Similar results were obtained in both studies with respect to A. niger (6.5% versus 5.8%), whereas it was higher for A. flavus (8.4% versus 18.5%) and A. terrreus (8.1% versus 15.3%) in our study.
We have characterized a large number of cryptic species belonging to the sections Fumigati, Flavi, Nigri, Circumdati, Nidulantes and Usti in clinical samples, representing a prevalence of 15.3%. These results are similar to those already published in other studies conducted in Europe and in the United States where the prevalence ranged from 10% to 15%.13,18,24Aspergillus tubingensis was the cryptic species most commonly found, being the number of isolates of this species slightly higher than that of its type species A. niger. These data are similar to those published in other studies, in which both species are found in similar proportions.19,25 In addition to the section Nigri, in Flavi and Nidulantes sections a high number of cryptic species was found.
The antifungal susceptibility testing shows that the most common species are not usually resistant, as opposed to cryptic species that exhibit low susceptibility to multiple antifungals in vitro. Aspergillus calidoustus was the only species of section Usti and coincidental with previously reports, it showed reduced in vitro susceptibility to triazole drugs.7,26Aspergillus lentulus showed decreased susceptibility to azoles and amphotericin B, as described before.27 Coincidental with Alcazar-Fuoli et al.,20 we found higher MIC values of triazoles in A. fisherianus compared to A. fumigatus, but similar values for the rest of the drugs tested. Aspergillus niger showed a twofold higher in vitro susceptibility to voriconazole and posaconazole than A. tubingensis, in accordance with the results reported by Hendrickx et al.19 Amphotericin B resistance was found in some isolates of A. terreus, as previously reported.28 Among the species of the section Nidulantes highlights its different in vitro susceptibility to amphotericin B, with MICs of up to 8.0mg/L for A. nidulans and A. quadrilineatus, compared to 1.0mg/L for A. rugulosus. Unlike the results obtained by Verweij et al.,29 the strains of A. quadrilineatus did not have lower MICs than A. nidulans to triazoles than A. nidulans. Finally, in section Circumdati we stress the raised MIC to amphotericin B of A. ochraceus and A. pseudoelegans, and the higher MIC values to triazoles of A. persii.30
The results of this study emphasize the need to achieve a correct identification at the species level within the genus Aspergillus, due to the different profiles of antifungal sensitivity among species of different sections and even among different species of the same section. The results obtained could influence the choice of the most appropriate empirical treatment and, therefore, in the patient's prognosis. As an example, it is not the same for a patient to isolate in a bronchoalveolar lavege A. calidoustus (with intrinsic resistance to azoles) or A. ustus: a correct identification would lead to choose amphotericin B as the treatment of choice instead of voriconazole. More investigations of this type are needed to know the local prevalence of the most common fungal agents, which will help to guide the empirical treatment of the different types of fungal infections, and will adjust the first-line antifungal treatments in each hospital center.
Conflicts of interestThe authors declare no conflicts of interest.