To investigate mortality risk factors in patients with zygomycosis.
Patients and MethodsRetrospective case history review of patients diagnosed with proven zygomicosis in 17 centres in Spain. We compared demographics and risk factors in patients who survived, and in those who died.
ResultsWe identified twenty-five patients with proven zygomycosis. The primary site of infection was rhino-orbito-cerebral (28%) and disseminated (20%) or cutaneous/soft infections (20%) of the patients. Eleven patients (44%) received preemptive or empirical antifungal treatment; of these patients, 4 received liposomal amphotericin B, 1 received amphotericin B lipid complex, and 6 received other antifungals. The overall mortality rate was 72%. In the univariate analysis factors associated with an increased risk of death were the presence of a haematological malignancy (P=.03), neutropenia (P=.03) and monocytopenia (P=.008).
ConclusionOur study supports previous research that has documented a high mortality rate among patients with invasive zygomycosis, especially among those with an underlying haematological malignancy, and the need for a rapid initiation of an effective antifungal treatment.
Investigar los factores de riesgo de mortalidad en pacientes con zigomicosis.
Pacientes & MétodosRevisión retrospectiva de pacientes diagnosticados de zigomicosis documentada en 17 centros en España, comparando los datos demográficos y los factores de riesgo entre los pacientes que sobrevivieron y aquellos que fallecieron.
ResultadosSe identificaron 25 pacientes con zigomicosis probada. El lugar primario de la infección fue rino-órbito-cerebral (28%) e infecciones diseminadas o cutáneas / de tejidos blandos en el 20% de los pacientes cada una. Once pacientes (44%) recibieron tratamiento antifúngico anticipado o empírico; de estos pacientes, cuatro de ellos recibieron anfotericina B liposomal, un paciente recibió anfotericina B complejo lipídico y 6 pacientes recibieron otros antifúngicos. La tasa de mortalidad global fue del 72%. En el análisis univariado, los factores asociados a un mayor riesgo de muerte fueron la presencia de enfermedad hematológica maligna (p=0,03), neutropenia (p=0,03) y monocitopenia (p=0,008).
ConclusiónLos datos de nuestro estudio concuerdan con los de trabajos previos que habían documentado una elevada tasa de mortalidad en pacientes con zigomicosis invasiva, especialmente en aquellos con enfermedad hematológica maligna subyacente, y la necesidad de instaurar rápidamente un tratamiento antifúngico eficaz.
Zygomycosis is a rare but devastating invasive fungal infection (IFI) caused by the agents of Zygomycetes, most commonly by those of the order Mucorales.1 Zygomycosis is becoming increasingly important among certain populations, such as recipients of haematopoietic stem cell or solid-organ transplants and among patients with haematological malignancies.1,2 In these populations, reports of zygomycosis have been increasing since the 1990s,2,3 and zygomycosis has become the third leading cause of IFI (following invasive aspergillosis and invasive candidiasis).1,4 Although it has been proposed that this increase in the frequency of zygomycosis might be due to the introduction of voriconazole, an antifungal not active against Zygomycetes, for the prophylaxis of opportunistic fungal infections,5,6 this association is not yet well-established.3
The clinical manifestations of invasive zygomycosis include tissue necrosis from invasion of blood vessels and subsequent thrombosis, and the most common clinical presentations are a rhinocerebral syndrome and pulmonary infection.1 A definitive diagnosis of zygomycosis can be difficult to reach and requires histopathological confirmation.1 The adequate management of zygomycosis requires a multifaceted approach that includes the control or reversal of the underlying risk factors (such as diabetic ketoacidosis or immunosuppression), surgical debridement, and the use of systemic antifungal therapy.1,7 Amphotericin B, particularly the liposomal formulation, is the mainstay for the treatment of this IFI.7-9 More recently, posaconazole has also been shown to be active against zygomycosis,10 with some evidence of its clinical efficacy as a salvage therapy11,12 but without the same spectrum of coverage as amphotericin B.
Zygomycosis has a poor prognosis. Despite the introduction of amphotericin B, mortality in patients with zygomycosis is high and has remained almost unchanged in the last four decades.2 According to data from the largest series of patients with zygomycosis,2 the current mortality rate is close to 40%, and it can be as high as 76% in cases of pulmonary zygomycosis.2 However, a smaller study examined 16 cases of proven zygomycosis and found an overall mortality rate of only 25%.13 These different mortality rates may be due to differences in the clinical characteristics of the cases or in their management. To better manage this serious infection, it is important to know what factors are associated with a greater risk of mortality.
Our study was aimed at investigating risk factors for mortality in a retrospective case series of patients with proven zygomycosis.
Patients and methodsWe performed a retrospective review of the clinical history of all patients with zygomycosis who were seen in seventeen health centres in Spain between January 1, 2007 and June 30, 2008. Patients were included in the study only if they had no previous history of zygomycosis and had been diagnosed with proven zygomycosis according to the EORTC/MSG criteria.14
A standardised case report form was used to extract information from the medical records. Demographic information, including age and sex, and clinical data were recorded. The clinical information collected included (1) clinical outcome and, in cases of death, the relationship between this outcome and the IFI; (2) site of the infection (categorized as sinusitis, sinopulmonary, pneumonia, rhino-orbito-cerebral, disseminated, and cutaneous/soft-tissue infection); (3) underlying conditions and APACHE II at the time of diagnosis; (4) risk factors for zygomycosis within one month of the diagnosis (including type of bone marrow transplantation (BMT), graft-versus-host disease grade, use of immunosuppressive therapy, cytomegalovirus infection, diagnosis of diabetes mellitus, hyperglycaemia defined as a glucose level equal to or greater than 200g/dL, neutropenia defined as a neutrophil count equal to or lower than 500 cells/mm3, lymphopenia, monocytopenia, acidosis defined as a pH value lower than 7.35, malnutrition defined as a serum albumin level equal to or lower than 3g/dL, use of corticosteroids, use of tumour necrosis factor inhibitors, serum iron level, serum ferritin level, and use of deferoxamine therapy); (5) use of an antifungal agent within three months of the diagnosis as either prophylactic, pre-emptive, empirical, or targeted therapy; and (6) whether or not the patient underwent surgical debridement.
Demographic and clinical characteristics were described using the mean, median and standard deviation for continuous measures and the frequency and percentage for categorical variables. The demographic information and risk factors of patients who survived were compared with the information of those who died in a univariate analysis using the Student's t-test, Chi-squared test, or Fisher's exact test where appropriate. In addition, a stepwise logistic regression analysis was planned to explore variables independently associated with mortality. All statistical analyses were performed using SPSS version 15.0 (SPSS Inc, Chicago, Illinois). All analyses were two-tailed and were considered significant if P<.05.
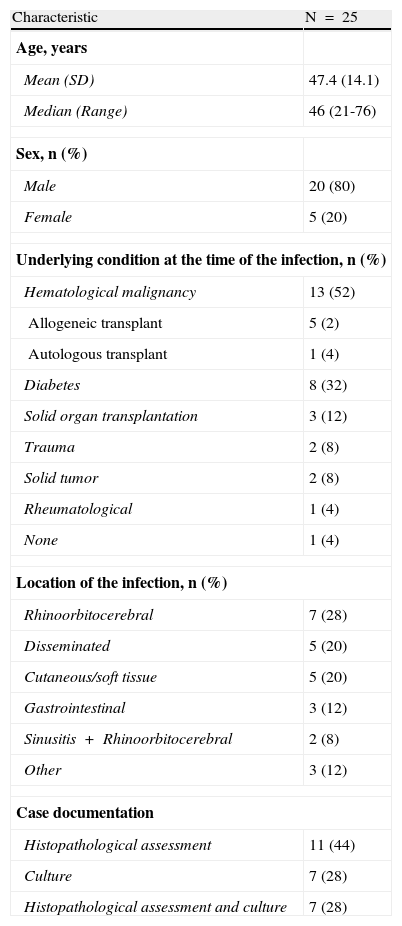
ResultsThroughout the 18-months study period, we identified twenty-five patients with proven zygomycosis. Patients were predominantly male (80%) with a median age of 46 years. The most common underlying conditions were haematological malignancies (52%) and diabetes mellitus (32%). At the time of diagnosis, nearly one third of the patients presented with rhino-orbito-cerebral infections; 20% of the patients presented with disseminated infections and 20% presented with cutaneous/soft-tissue infections. The median of the follow-up duration was 2 months (interquartile range 0.9 to 5.5) and the mean (SD) was 5.2 (7.1) months. Mean time between the beginning of the symptoms and the diagnosis, independently of patient outcome, was 8 days. In all cases, targeted antifungal treatment was started in the first 24h after a confirmatory diagnosis. Patient demographics, underlying conditions, infection locations and case history are presented in Table 1.
Demographic and clinical characteristics.
| Characteristic | N=25 |
| Age, years | |
| Mean (SD) | 47.4 (14.1) |
| Median (Range) | 46 (21-76) |
| Sex, n (%) | |
| Male | 20 (80) |
| Female | 5 (20) |
| Underlying condition at the time of the infection, n (%) | |
| Hematological malignancy | 13 (52) |
| Allogeneic transplant | 5 (2) |
| Autologous transplant | 1 (4) |
| Diabetes | 8 (32) |
| Solid organ transplantation | 3 (12) |
| Trauma | 2 (8) |
| Solid tumor | 2 (8) |
| Rheumatological | 1 (4) |
| None | 1 (4) |
| Location of the infection, n (%) | |
| Rhinoorbitocerebral | 7 (28) |
| Disseminated | 5 (20) |
| Cutaneous/soft tissue | 5 (20) |
| Gastrointestinal | 3 (12) |
| Sinusitis+Rhinoorbitocerebral | 2 (8) |
| Other | 3 (12) |
| Case documentation | |
| Histopathological assessment | 11 (44) |
| Culture | 7 (28) |
| Histopathological assessment and culture | 7 (28) |
SD: standard deviation.
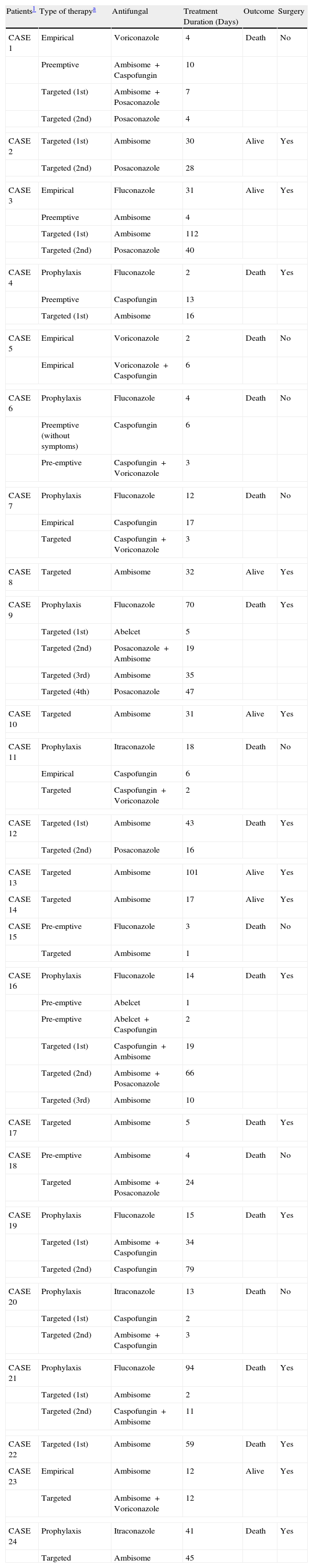
Eleven patients (44%) received pre-emptive or empirical antifungal treatment; of these, 4 (16%) received liposomal amphotericin B (in one case, this treatment was combined with caspofungin), 1 (4%) received amphotericin B lipid complex, and 6 (24%) received other antifungals (Table 2). These other antifungals were not effective against zygomycosis. The rationale to start empirical or pre-emptive antifungal treatment was based on clinical suspicion by the clinician in charge of the patient. One patient did not receive any antifungal treatment, and the remaining thirteen patients (52%) commenced antifungal treatment after they were diagnosed with zygomycosis. The most common antifungals, used either alone or in combination as targeted treatment, were liposomal amphotericin B (n=19, 76%), posaconazole (n=7, 28%), caspofungin (n=6, 24%), voriconazole (n=3, 12%), and amphotericin B lipid complex (n=1, 4%). Table 2 shows the antifungals received by each patient during the entire follow-up period. Seventeen patients (68%) underwent surgical debridement.
Use of antifungals in 25 cases of definite or probable zygomycosis.
| Patients1 | Type of therapya | Antifungal | Treatment Duration (Days) | Outcome | Surgery |
| CASE 1 | Empirical | Voriconazole | 4 | Death | No |
| Preemptive | Ambisome+Caspofungin | 10 | |||
| Targeted (1st) | Ambisome+Posaconazole | 7 | |||
| Targeted (2nd) | Posaconazole | 4 | |||
| CASE 2 | Targeted (1st) | Ambisome | 30 | Alive | Yes |
| Targeted (2nd) | Posaconazole | 28 | |||
| CASE 3 | Empirical | Fluconazole | 31 | Alive | Yes |
| Preemptive | Ambisome | 4 | |||
| Targeted (1st) | Ambisome | 112 | |||
| Targeted (2nd) | Posaconazole | 40 | |||
| CASE 4 | Prophylaxis | Fluconazole | 2 | Death | Yes |
| Preemptive | Caspofungin | 13 | |||
| Targeted (1st) | Ambisome | 16 | |||
| CASE 5 | Empirical | Voriconazole | 2 | Death | No |
| Empirical | Voriconazole+Caspofungin | 6 | |||
| CASE 6 | Prophylaxis | Fluconazole | 4 | Death | No |
| Preemptive (without symptoms) | Caspofungin | 6 | |||
| Pre-emptive | Caspofungin+Voriconazole | 3 | |||
| CASE 7 | Prophylaxis | Fluconazole | 12 | Death | No |
| Empirical | Caspofungin | 17 | |||
| Targeted | Caspofungin+Voriconazole | 3 | |||
| CASE 8 | Targeted | Ambisome | 32 | Alive | Yes |
| CASE 9 | Prophylaxis | Fluconazole | 70 | Death | Yes |
| Targeted (1st) | Abelcet | 5 | |||
| Targeted (2nd) | Posaconazole+Ambisome | 19 | |||
| Targeted (3rd) | Ambisome | 35 | |||
| Targeted (4th) | Posaconazole | 47 | |||
| CASE 10 | Targeted | Ambisome | 31 | Alive | Yes |
| CASE 11 | Prophylaxis | Itraconazole | 18 | Death | No |
| Empirical | Caspofungin | 6 | |||
| Targeted | Caspofungin+Voriconazole | 2 | |||
| CASE 12 | Targeted (1st) | Ambisome | 43 | Death | Yes |
| Targeted (2nd) | Posaconazole | 16 | |||
| CASE 13 | Targeted | Ambisome | 101 | Alive | Yes |
| CASE 14 | Targeted | Ambisome | 17 | Alive | Yes |
| CASE 15 | Pre-emptive | Fluconazole | 3 | Death | No |
| Targeted | Ambisome | 1 | |||
| CASE 16 | Prophylaxis | Fluconazole | 14 | Death | Yes |
| Pre-emptive | Abelcet | 1 | |||
| Pre-emptive | Abelcet+Caspofungin | 2 | |||
| Targeted (1st) | Caspofungin+Ambisome | 19 | |||
| Targeted (2nd) | Ambisome+Posaconazole | 66 | |||
| Targeted (3rd) | Ambisome | 10 | |||
| CASE 17 | Targeted | Ambisome | 5 | Death | Yes |
| CASE 18 | Pre-emptive | Ambisome | 4 | Death | No |
| Targeted | Ambisome+Posaconazole | 24 | |||
| CASE 19 | Prophylaxis | Fluconazole | 15 | Death | Yes |
| Targeted (1st) | Ambisome+Caspofungin | 34 | |||
| Targeted (2nd) | Caspofungin | 79 | |||
| CASE 20 | Prophylaxis | Itraconazole | 13 | Death | No |
| Targeted (1st) | Caspofungin | 2 | |||
| Targeted (2nd) | Ambisome+Caspofungin | 3 | |||
| CASE 21 | Prophylaxis | Fluconazole | 94 | Death | Yes |
| Targeted (1st) | Ambisome | 2 | |||
| Targeted (2nd) | Caspofungin+Ambisome | 11 | |||
| CASE 22 | Targeted (1st) | Ambisome | 59 | Death | Yes |
| CASE 23 | Empirical | Ambisome | 12 | Alive | Yes |
| Targeted | Ambisome+Voriconazole | 12 | |||
| CASE 24 | Prophylaxis | Itraconazole | 41 | Death | Yes |
| Targeted | Ambisome | 45 | |||
NOTE. One patient did not receive any antifungal agent.
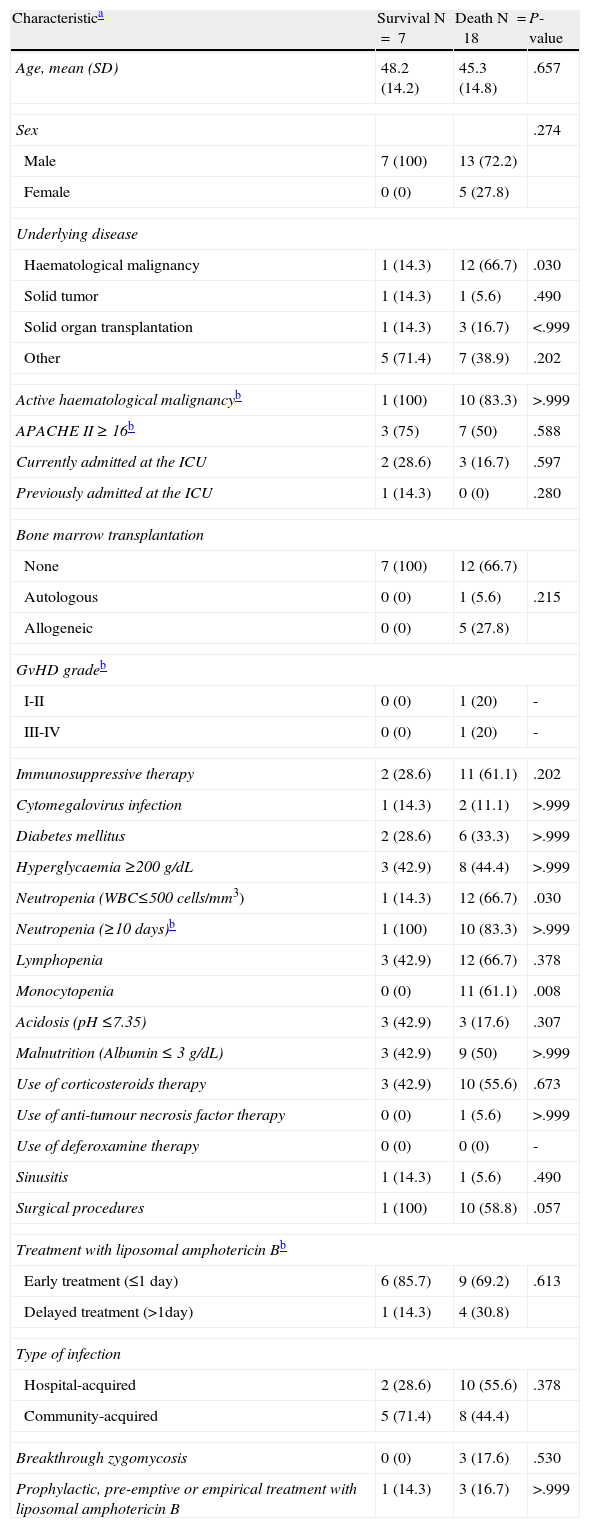
The overall mortality rate in our cohort was 72% (18 of 25 patients). Mortality was high between patients treated empirically with an ineffective drug (80%). The only case treated empirically with an active antifungal against zygomycosis survived. The mortality rate was similar between patients treated empirically (67%) and patients who received a targeted treatment (70%). Zygomycosis was the cause of death in 13 of the 18 patients who died (52%) and contributed to the death of four other patients (16%). In the univariate analysis (Table 3) the factors associated with an increased risk of death were the presence of an underlying haematological malignancy (P=.03), neutropenia (P=.03) and monocytopenia (P=.008). We did not run a multivariate analysis since the same patients had both haematological malignancies and neutropenia, and the monocytopenia variable had a zero cell count.
Univariate analysis of risk factors for mortality among 25 patients with proven or probable zygomycosis.
| Characteristica | Survival N=7 | Death N=18 | P-value |
| Age, mean (SD) | 48.2 (14.2) | 45.3 (14.8) | .657 |
| Sex | .274 | ||
| Male | 7 (100) | 13 (72.2) | |
| Female | 0 (0) | 5 (27.8) | |
| Underlying disease | |||
| Haematological malignancy | 1 (14.3) | 12 (66.7) | .030 |
| Solid tumor | 1 (14.3) | 1 (5.6) | .490 |
| Solid organ transplantation | 1 (14.3) | 3 (16.7) | <.999 |
| Other | 5 (71.4) | 7 (38.9) | .202 |
| Active haematological malignancyb | 1 (100) | 10 (83.3) | >.999 |
| APACHE II ≥ 16b | 3 (75) | 7 (50) | .588 |
| Currently admitted at the ICU | 2 (28.6) | 3 (16.7) | .597 |
| Previously admitted at the ICU | 1 (14.3) | 0 (0) | .280 |
| Bone marrow transplantation | |||
| None | 7 (100) | 12 (66.7) | |
| Autologous | 0 (0) | 1 (5.6) | .215 |
| Allogeneic | 0 (0) | 5 (27.8) | |
| GvHD gradeb | |||
| I-II | 0 (0) | 1 (20) | - |
| III-IV | 0 (0) | 1 (20) | - |
| Immunosuppressive therapy | 2 (28.6) | 11 (61.1) | .202 |
| Cytomegalovirus infection | 1 (14.3) | 2 (11.1) | >.999 |
| Diabetes mellitus | 2 (28.6) | 6 (33.3) | >.999 |
| Hyperglycaemia ≥200 g/dL | 3 (42.9) | 8 (44.4) | >.999 |
| Neutropenia (WBC≤500 cells/mm3) | 1 (14.3) | 12 (66.7) | .030 |
| Neutropenia (≥10 days)b | 1 (100) | 10 (83.3) | >.999 |
| Lymphopenia | 3 (42.9) | 12 (66.7) | .378 |
| Monocytopenia | 0 (0) | 11 (61.1) | .008 |
| Acidosis (pH ≤7.35) | 3 (42.9) | 3 (17.6) | .307 |
| Malnutrition (Albumin ≤ 3 g/dL) | 3 (42.9) | 9 (50) | >.999 |
| Use of corticosteroids therapy | 3 (42.9) | 10 (55.6) | .673 |
| Use of anti-tumour necrosis factor therapy | 0 (0) | 1 (5.6) | >.999 |
| Use of deferoxamine therapy | 0 (0) | 0 (0) | - |
| Sinusitis | 1 (14.3) | 1 (5.6) | .490 |
| Surgical procedures | 1 (100) | 10 (58.8) | .057 |
| Treatment with liposomal amphotericin Bb | |||
| Early treatment (≤1 day) | 6 (85.7) | 9 (69.2) | .613 |
| Delayed treatment (>1day) | 1 (14.3) | 4 (30.8) | |
| Type of infection | |||
| Hospital-acquired | 2 (28.6) | 10 (55.6) | .378 |
| Community-acquired | 5 (71.4) | 8 (44.4) | |
| Breakthrough zygomycosis | 0 (0) | 3 (17.6) | .530 |
| Prophylactic, pre-emptive or empirical treatment with liposomal amphotericin B | 1 (14.3) | 3 (16.7) | >.999 |
GvHD: graft-versus-host-disease; ICU: intensive care unit; SD: standard deviation.
In our cohort of 25 patients with proven zygomycosis the overall mortality rate was 72%. This rate is much higher than the 40% mortality rate found by Roden et al.2 in the largest review of reported zygomycosis cases over the last several decades. However, the primary sites of infection among patients in our cohort differed from those in the cases reported by Roden et al. In their review, Roden et al. found that 58% of 929 patients presented with either sinus (39%) or cutaneous (19%) infections; these locations were associated with the lowest mortality rates (46% and 31%, respectively).2 In our sample, two-thirds of the patients had rhino-orbito-cerebral, disseminated, or gastrointestinal infection; Rhoden et al. found that infections in these locations were associated with higher mortality rates (from 62% in the case of rhinocerebral infection to 85% in the case of gastrointestinal infection.2 The most common underlying disease in our study was haematological malignancy, which was present in over half of the patients. Mortality rates reported in the literature in patients with zygomycosis and underlying haematological malignancies are similar to those seen in our study.15,16 In a retrospective study of 59 patients with haematological malignancies and proven or probable zygomycosis, the overall mortality rate was 79.7%; in 41 of the cases (69.5%) the death was attributed to the IFI.15 In another review of 120 cases of zygomycosis with an underlying haematological or oncological disorder, the overall mortality rate was 61.6%; 68 of the patients (56.7%) died from either zygomycosis or a combination of zygomycosis and an additional factor.16 However, the presence of a haematological malignancy as underlying disease cannot entirely explain the high mortality observed in our study. In a recently reported survey which included 60 cases of zygomycosis, 37 (60%) of them having a haematological malignancy, Pagano et al. reported an attributable mortality rate of 32%, a rate that is substantially lower than the 52% we have found in our study.17
We identified several mortality risk factors in the univariate analysis: the presence of an underlying haematological malignancy, neutropenia, and monocytopenia. These risk factors for mortality are consistent with those reported in previous studies of patients with zygomycosis and other IFIs. As mentioned earlier, zygomycosis in patients with haematological malignancies is associated with a high mortality rate.15,16 Moreover, in a multivariate analysis of 70 patients with zygomycosis and haematological malignancies, Chamilos et al. reported that the presence of an active haematological malignancy was an independent predictor of a poorer outcome.18 Neutropenia has been associated with a higher risk of mortality in patients with haematological malignancies and other IFIs such as Fusarium sp.19 or invasive aspergillosis.20 However, in two different series of patients with haematological malignancies and zygomycosis, neutropenia was not associated with a higher risk of mortality in either univariate or multivariate analysis.15,18 One of those studies, however, found that monocytopenia was an independent risk factor for a poorer outcome18 and both studies found that neutrophil recovery was associated with a protective effect.15,18 Like Chamilos,18 we think that it is likely that monocytopenia reflects the immunosuppression state of those patients, and the neutrophil recovery reflects the reversal of the predisposing underlying condition.
In our analysis the use of amphotericin B was not identified as a protective factor; this may be due to the small study sample size. However, the use of amphotericin B has been associated with a positive outcome in patients who have zygomycosis and several underlying conditions, such as patients with underlying haematological malignancies,15,18 patients with haematological or oncological disorders16 and solid organ transplant recipients.21 In the multivariate analysis by Chamilos et al, a delay in treatment with an amphotericin-based regimen was an independent predictor of mortality (OR 8.1, 95% CI 1.7-38.2).14 Similarly, a multivariate analysis of 59 patients with zygomycosis and underlying haematological malignancies found that treatment with liposomal amphotericin B was the only factor that predicted recovery from the IFI (Relative risk 0.5, 95% CI 0.3-0.8).12 It is noteworthy that only 5 patients (20%) in our study received an amphotericin B-based treatment as a pre-emptive or empirical treatment. This fact alone could explain the high mortality rate observed in our study, and it illustrates the need for a rapid initiation of an effective antifungal treatment. Early diagnosis and treatment, including surgical debridement, reversal of underlying risk factors, and rapid initiation of an amphotericin B-based treatment, are the mainstays of management of zygomycosis.1,7 Moreover, since zygomycosis is difficult to diagnose, some authors have proposed the use of pre-emptive amphotericin B-based regimens in patients who are at high risk for zygomycosis and who are receiving other antifungal agents that have little or no activity against Zygomycetes.18 With the data obtained and according to the published literature, when a zygomycosis infection is suspected, treatment with effective in vitro antifungal drugs should be started as soon as possible.
Despite the limitations of our retrospective design and the small sample size, our study supports previous research that has documented a high mortality rate among patients with invasive zygomycosis, especially among those with an underlying haematological malignancy, and the need for a rapid initiation of an effective antifungal treatment and the correction of underlying predisposing factors.
FundingThis study was partially funded by a research grant from Gilead Sciences S.L.
Biometría Médica provided assistance in preparing the manuscript
Conflict of interestThe authors have no conflict of interest to declare.
We would like to thank following authors for taking part on this study:
Llorente A, Hospital Joan XXIII, Tarragona.
García E, Clínica Universitaria de Navarra, Navarra.
Heras I, Hospital General Universitario Morales Messeguer, Murcia.
Fraile V, Hospital Universitario “Río Hortega”, Valladolid.
García P, Hospital Clínico San Carlos, Madrid.
López J, Hospital Universitario Ramón y Cajal, Spain
Salavert M, Hospital Universitario La Fe, Valencia.
Pérez I, Hospital Universitario La Paz, Madrid.
Bobillo F, Hospital Clínico Universitario de Valladolid, Valladolid.
Cantalapiedra A, Hospital Universitario “Río Hortega”, Valladolid.
Gómez J, Hospital Central de Asturias, Oviedo.
Andreu MAJ, Hospital de Mostoles, Madrid.
Fortún J, Hospital Ramón y Cajal, Madrid.
Díaz MA, Hospital de Mérida, Badajoz.
Heredia M, Hospital Clínico Universitario de Valladolid, Valladolid.
Martín P, Complejo Hospitalario Carlos Haya, Málaga.
Martínez P, Hospital Universitario Puerto Real, Cádiz.
Sanduende Y, Hospital Montecelo, Pontevedra.
Sarriá C, Hospital Universitario La Princesa, Madrid.
Bermúdez A, Hospital Universitario Marqués de Valdecilla, Cantabria.