Due to the emergence of drug-resistant pneumococcal isolates, new fluoroquinolones have been recommended for the treatment of pneumococcal infections. The purpose of this study was to establish surveillance, and to conduct molecular characterization, of fluoroquinolone-resistant Streptococcus pneumoniae in Seville.
MethodNorfloxacin-resistant S. pneumoniae isolates were characterized by quinolone resistance-determining region (QRDR) substitutions, reserpine-sensitive efflux, serotype and by pulsed-field gel electrophoresis (PFGE) patterns.
ResultsFourteen isolates (5.1%) showed an MIC>16μg/ml to norfloxacin. Eight of 10 adult isolates were susceptible to levofloxacin. The 4 infant isolates with norfloxacin MIC>16μg/ml were susceptible to levofloxacin. Seven of these 12 low-level-resistant isolates had mutations in ParC, while mutations both in ParC and GyrA genes were only detected in one of the two high-level-resistant isolates. All the isolates without QRDR substitutions that remained norfloxacin-resistant were positive for reserpine-inhibited efflux. The serotyping and PFGE revealed significant heterogeneity. We obtained 9 different profiles, 3 of which had two isolates each. Two of the isolates with the same pulsotype were from the same patient. The first isolate showed a mutation in the QRDR of ParC, and the second one had an additional GyrA mutation.
ConclusionIn our study a levofloxacin resistance rate of 0.7% was found among invasive isolates. Although resistance level is low, surveillance is necessary, especially to prevent cases of in vivo resistance development as reported.
En los últimos años se ha detectado un aumento de resistencias a fluorquinolonas entre las cepas de Streptococcus pneumoniae. El objetivo de este estudio es realizar la caracterización molecular y vigilancia de la resistencia a fluorquinolonas en aislamientos de Streptococcus pneumoniae en Sevilla.
MétodosLa caracterización de las cepas resistentes a norfloxacina se realizó mediante el estudio de las mutaciones en los genes QRDR, estudio de bombas de reflujo con reserpina, serotipado y patrones de electroforesis en campo pulsante (ECP).
ResultadosCatorce (5,1%) aislamientos mostraron una CMI a norfloxacino >16μg/ml. De los 10 aislamientos de adultos, 8 fueron sensibles a levofloxacina. Los 4 aislamientos pediátricos resistentes a norfloxacina fueron sensibles a levofloxacina. Siete de los 12 aislamientos con bajo nivel de resistencia mostraron una mutación en parC, mientras que sólo en una de las dos cepas con resistencia de alto nivel encontramos mutaciones en los genes ParC y GyrA. Los aislamientos que no mostraron tener mutaciones, pero eran resistentes a la norfloxacina, fueron positivos para la reserpina. El serotipado y los patrones de ECP revelaron una considerable heterogeneidad. Obtuvimos 9 perfiles diferentes, 3 de los cuales contenían 2 aislamientos cada uno. Dos de los aislamientos con el mismo pulsotipo resultaron ser del mismo paciente. El primer aislamiento mostró una mutación en el parC y el segundo, una mutación adicional en el gyrA.
ConclusionesAunque la tasa de resistencia a levofloxacina obtenida fue baja (0,7%), es necesaria una vigilancia para prevenir el desarrollo de resistencias in vivo como la que detectamos.
Streptococcus pneumoniae is the main cause of community-acquired pneumonia and an important agent of meningitis and bacteraemia. Due to the emergence of drug-resistant pneumococcal isolates and the clinical failures reported with older fluoroquinolones (FQs), newer fluoroquinolones have been recommended for the treatment of pneumococcal infections.1
The 2001 Spanish surveillance study2 found a 7% prevalence of ciprofloxacin resistance among S. pneumoniae isolates. A recent nationwide study, SAUCE, observed a decreasing trend in the FQ resistance rates in Spain, with ciprofloxacin and levofloxacin (LVX) resistance rates of 2.2% and 0.5%, respectively, in 2006–2007.3
The mechanisms of resistance to FQs include DNA gyrase and topoisomerase IV mutations and active efflux of agents from the cell.4,5 When the mutation affects only one of both targets, we have a first-step mutant. Second-step mutants result from mutations in DNA gyrase and topoisomerase IV.6 Decreased susceptibility to FQs is primarily due to amino acid substitutions in the ParC subunit of topoisomerase IV or in the GyrA subunit of DNA gyrase and less in ParE and GyrB.7–9 The active efflux is also cause of low-level or first-step resistance. This mechanism mostly affects hydrophilic quinolones like norfloxacin (NFX) and ciprofloxacin.5
In order to detect low-level-resistant pneumococci and prevent in vivo selection of resistant mutants or therapeutic failures, the French Microbiology Society suggests a screening method with NFX.10
The aim of this study was to establish surveillance and conduct molecular characterization, of fluoroquinolone-resistant S. pneumoniae in order to monitor the increase of fluoroquinolone resistance in Seville. NFX-resistant S. pneumoniae isolates were characterized by QRDR substitutions, reserpine-sensitive efflux, serotype and pulsed-field gel electrophoresis (PFGE) patterns.
Materials and methodsS. pneumoniae isolatesWe recovered 274 invasive isolates (49%) from the 560 obtained from 2002 to 2008 in Seville. These included 114 paediatric isolates (96 previously typed by serological determination of capsular type) and 160 adult isolates, representing a subset of all invasive isolates. All isolates were recovered from sterile fluids: blood (232 isolates), cerebrospinal fluid (30 isolates), pleural fluid (10 isolates), and synovial fluid (2 isolates). The samples were cultured on Columbia Blood agar in 5% CO2 at 37°C, and the α-haemolytic colonies were tested for optochin susceptibility and bile solubility to differentiate S. pneumoniae from other α-haemolytic streptococci. Pneumococcal isolates were stored at −70°C in skimmed milk.
SerotypingIsolates were serotyped using the published multiplex PCR protocol with 7 sequential reactions.11 Each of the seven reactions included four serotype-specific primer pairs and a conserved region of the cps operon as internal positive control. We modified the order of the primers proposed to improve the efficiency of the original scheme for surveying our geographic area.12 The multiplex assay was supplemented by sequencing and a PCR assay to differentiate between serotypes 6A, 6B and 6C.13
PFGEGenetic similarity of the isolates was tested by PFGE. S. pneumoniae DNA was embedded in agarose blocks and digested with SmaI restriction enzyme. PFGE was performed in 1.2% agarose gel for 21h at 11°C in 0.5× TBE, at 6V/cm, with pulse times of 1–30s and an angle of 60°. The gel was stained with 1mg/mL of ethidium bromide for 30min.
PFGE profiles were analysed optically and using Bionumerics software. We have defined a “clone” as isolates that differed by not more than 3 bands, or 90%.
Antimicrobial susceptibilitiesSusceptibility to NFX and LVX was determined by E-test diffusion and 5μg disc diffusion (only NFX) on Müller-Hinton Blood (bioMerieux) with 0.5 McFarland standard densities.10 When the inhibition zone around a disk of NFX was <10mm or the MIC was >16μg/ml, we considered it a low-level fluoroquinolone resistant pneumococcus.8,10 When the NFX MIC was >16μg/ml and the LVX MIC was ≥8mg/mL we considered it a high-level fluoroquinolone resistant pneumococcus.10,14
Sequencing and identification of mutationsWe identified the mutated genes through PCR and reverse hybridization with specific probes for ParC and GyrA sequence (CAP-Resistenz, GenID® GmbH, Straßberg) in all the strains with an NFX MIC>16μg/ml. The GyrA, GyrB, ParC, and ParE genes from S. pneumoniae were also amplified by PCR and sequenced to detect QRDR mutations as described.15
Active effluxAll strains with NFX MICs>16mg/ml were grown with and without reserpine (10μg/ml) in Müller-Hinton medium containing 5% sheep blood with doubling dilutions of NFX. Strains with a fourfold or greater reduction in NFX MIC in the presence of reserpine16 were considered positive for reserpine-sensitive efflux.
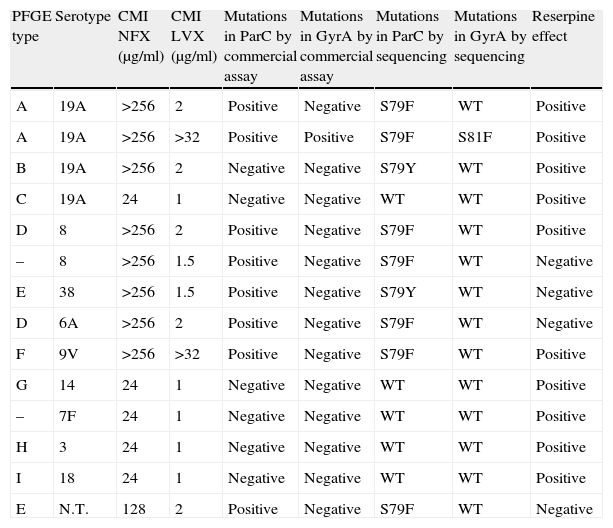
ResultsFourteen isolates (5.1%) showed an inhibition zone of >9mm around a disk containing 5μg of NFX and a MIC>16μg/ml to NFX by the E-test diffusion method (Table 1).
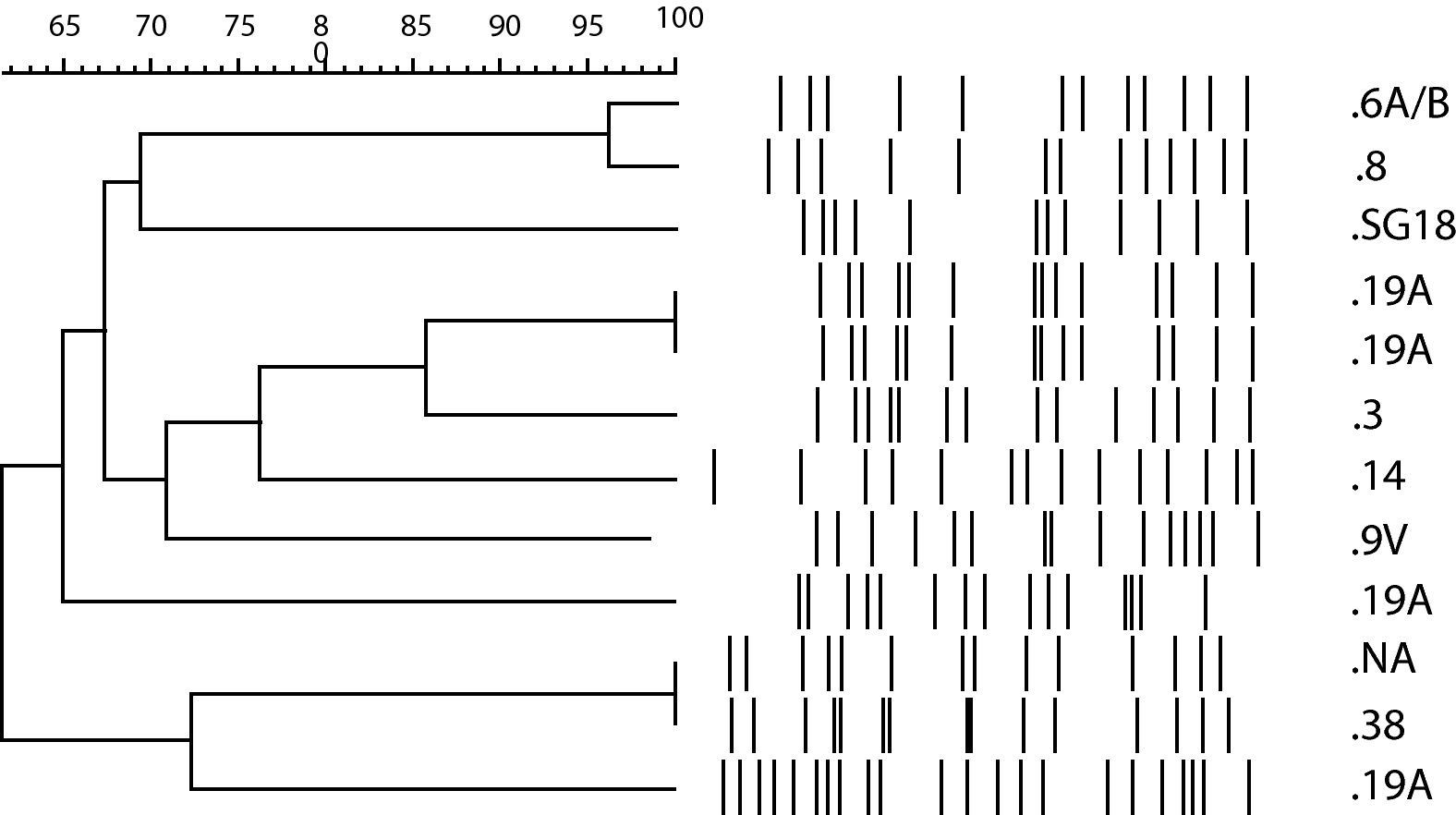
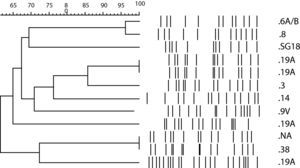
Molecular characteristics of NFX-resistant S. pneumoniae isolates.
| PFGE type | Serotype | CMI NFX (μg/ml) | CMI LVX (μg/ml) | Mutations in ParC by commercial assay | Mutations in GyrA by commercial assay | Mutations in ParC by sequencing | Mutations in GyrA by sequencing | Reserpine effect |
| A | 19A | >256 | 2 | Positive | Negative | S79F | WT | Positive |
| A | 19A | >256 | >32 | Positive | Positive | S79F | S81F | Positive |
| B | 19A | >256 | 2 | Negative | Negative | S79Y | WT | Positive |
| C | 19A | 24 | 1 | Negative | Negative | WT | WT | Positive |
| D | 8 | >256 | 2 | Positive | Negative | S79F | WT | Positive |
| – | 8 | >256 | 1.5 | Positive | Negative | S79F | WT | Negative |
| E | 38 | >256 | 1.5 | Positive | Negative | S79Y | WT | Negative |
| D | 6A | >256 | 2 | Positive | Negative | S79F | WT | Negative |
| F | 9V | >256 | >32 | Positive | Negative | S79F | WT | Positive |
| G | 14 | 24 | 1 | Negative | Negative | WT | WT | Positive |
| – | 7F | 24 | 1 | Negative | Negative | WT | WT | Positive |
| H | 3 | 24 | 1 | Negative | Negative | WT | WT | Positive |
| I | 18 | 24 | 1 | Negative | Negative | WT | WT | Positive |
| E | N.T. | 128 | 2 | Positive | Negative | S79F | WT | Negative |
N.T.: non-typeable.
Ten (6.3%) strains were isolated from adult patients and four (3.5%) more from infants. Eight of 10 adult isolates were susceptible to LVX and the remaining two had a MIC>32μg/ml, and the four infant isolates with NFX MIC>16μg/ml were all susceptible to LVX. These 4 infant isolates presented low non-susceptible MICs to NFX (24μg/ml).
Two out of 274 isolates (0.7%) were resistant to LVX, both of them with a MIC>32μg/ml. Those isolates susceptible to LVX, but resistant to NFX could be low-level-resistant mutants and the two resistant isolates to both FQs could be high-level-resistant mutants.
A probe hybridization test was performed on the 14 NFX resistant isolates, and mutations in ParC were detected in 6 of 12 low-level-resistant mutants. The remaining 6 isolates hybridized with wild sequences of both genes. One of the two possible high-level-resistant mutants showed mutations in ParC and GyrA sequences, while the other one only in ParC sequence.
All strains with mutations in the ParC by the probe hybridization test were confirmed by sequencing, and the most common genotype observed was S79F (ParC) substitution. Additionally, sequencing detected one more resistant strain with the S79Y mutation. Seven of the 12 low-level-resistant isolates (58.3%) had mutations in ParC. Only in one of the two high-level-resistant isolates were mutations detected in ParC and GyrA genes. The other LVX resistant isolates showed a mutation in ParC. All substitutions observed in the resistant isolates are shown in Table 1. No mutation was found in the ParE and GyrB genes.
Overall, 10 (71.4%) isolates were positive for reserpine-inhibited efflux. All the five isolates without QRDR substitutions though NFX-resistant were positive for reserpine-inhibited efflux. The isolate with high-level resistance that only showed ParC mutation was also positive for reserpine-inhibited efflux.
The 14 NFX-resistant S. pneumoniae isolates studied belonged to 9 different serotypes, 19A (4 isolates), 8 (2), 6A (1), 14 (1), 18 (1), 38 (1), 3 (1), 9V (1) and 7F (1) and one isolate could not be amplified.
PFGE revealed significant heterogeneity among the NFX-resistant isolates, and most of them were genetically unrelated (Fig. 1). Nine different profiles were found, and 3 of these contained two isolates each. Two of the strains with the same genotype were serotype 19A, and were isolated from the same patient. The isolates were obtained in two different episodes and hospital admissions, separated by 4 months. The patient was a 28-year-old man with Job syndrome, characterized by recurrent skin abscesses, atopic dermatitis, pneumonia and high serum levels of IgE. The patient was first hospitalized diagnosed with a community-acquired pneumonia (CAP). Sputum and blood samples were taken and LVX empirical treatment was started. S. pneumoniae susceptible to penicillin (E-test MIC of 0.016μg/ml) and LVX (E-test MIC of 2μg/ml) was grown in both samples. Two months later, the patient was hospitalized again with a new diagnosis of CAP. Isolates were not obtained, but he was again treated with LVX. Four months after the first hospitalization, he returned to the hospital with a S. pneumoniae bacteraemia. The S. pneumoniae isolated from blood cultures was susceptible to penicillin (E-test MIC of 0.006μg/ml), but resistant to LVX (E-test MIC of >32μg/ml). The first isolate carried a mutation in the QRDR of ParC (S79F) and the second one had an additional GyrA mutation (S81F).
DiscussionAlthough antimicrobial resistance rates in S. pneumoniae continue being a worldwide problem, the prevalence of FQ-resistant strains remains low (1–7%).17–19 A 1998–2002 United States surveillance study found a higher prevalence of LVX resistance among lower respiratory tract isolates compared to blood isolates.20
In the 1998 Active Bacterial Core Surveillance program, the Centers for Disease Control and Prevention reported a LVX resistance rate of 0.2% among 3475 invasive isolates.21 In our study, a higher LVX resistance rate (0.7%) was found among invasive isolates. Although the resistance level is low, surveillance is necessary, especially to prevent cases of in vivo resistance development as reported. We performed the screening test proposed by CA-SFM to detect low-level-resistant isolates, as failing to detect them may lead to treatment failure.22–24 In Spain, a fatal LVX treatment failure was reported in a patient with a pneumococcal pneumonia who developed in vivo resistance,25 and in this work, we described a new case that developed in vivo resistance.
Ten (6.3%) adult isolates and 4 (3.5%) infant isolates showed a MIC>16μg/ml to NFX by the E-test diffusion method, but only 2 (1.3%) adult isolates, and none of the infant isolates were LVX-resistant. Moreover, 4 infant isolates presented low non-susceptible MICs to NFX (24μg/ml) and the only resistance mechanism detected was the active efflux, suggesting that these infant strains had probably not been exposed to FQ.
We have found mutations in the Ser-79 of ParC and Ser-81 of GyrA. ParC substitutions at Ser-79 were to either Phe or Tyr, but the S79F substitution was more prevalent. We detected mutations exclusively in ParC sequence in 8 isolates (57.1%), and 7 of them were susceptible to LVX (first-step resistance). There was one isolate that only had ParC mutation and was resistant to LVX. We found other polymorphisms not related to fluoroquinolone resistance in this isolate, ParC (K137N) and ParE (I460V). It was also positive to reserpine effect. This resistance pattern is unusual, because the isolate is high-level-resistant in a first-step mutation. The clinical impact of this pattern is very important, and the isolate should be studied more thoroughly. The 5 isolates with low-level resistance without mutations in the QRDR genes were positive to reserpine effect. As expected, the isolate with ParC and GyrA mutations (Ser-81) was not susceptible to NFX and LVX (high-level resistance).
Although penicillin- or macrolide-resistant isolates have spread clonally, the clonal relationship of fluoroquinolone-resistant isolates remains unclear.26 In the present study we did not find any clonal and serotype relationship, probably due to the low number of resistant strains. However, clonal diversity in LVX-resistant isolates has been reported in other large surveillance studies.27 Among the 3 pairs of isolates with the same PFGE profile, a different serotype was observed in one case, another pair had one non-typeable isolate, and the third pair was the strain that developed resistance in vivo.
When we performed the assay to estimate the efflux, the MICs obtained for NFX by microdilution without reserpine varied in one or two dilutions, but remained in the same category, except in two infant isolates. These two isolates had an MIC of 24μg/ml by E-test and 16μg/ml by microdilution. Mutations in the QRDR genes were not detected. According to CA-SFM recommendations, by E-test, these could be low-level fluoroquinolone-resistant pneumococci.
Active efflux mechanism was found in 10 of 14 NFX-resistant isolates, being associated with other resistance mechanisms in 5 isolates. The other 5 isolates that only presented the efflux mechanism showed lower MICs to NFX (24μg/ml) and the isolates that presented a ParC mutation showed higher MICs to NFX regardless of having the efflux mechanism or not.
The screening method that we have used, using only NFX and LVX E-test, is the simplest alternative according to Varon et al. This option does not discriminate between increased efflux and altered topoisomerase IV, but detects the most frequent low-level-resistant mutants.8
Conflict of interestThe authors have no conflict of interest to declare.