A steroid-immunosuppressed rat model of invasive pulmonary aspergillosis was use to examine the usefulness of galactomannan enzyme immunoassay (GM) and quantitative real time PCR (RT-PCR) in evaluating the association between response and exposure after a high dose of prophylactic posaconazole.
MethodsTwo different strains of Aspergillus fumigatus with different in vitro posaconazole susceptibility were used.
ResultsSerum concentrations demonstrated similar posaconazole exposure for all treated animals. However, response to posaconazole relied on the in vitro susceptibility of the infecting strain. After prophylaxis, galactomannan index and fungal burden only decreased in those animals infected with the most susceptible strain.
ConclusionThis study demonstrated that both biomarkers may be useful tools for predicting efficacy of antifungal compounds in prophylaxis.
Se evaluaron en un modelo de aspergilosis pulmonar invasiva en rata inmunodeprimida la utilidad del galactomanano sérico y de la PCR cuantitativa en tiempo real y su relación con la respuesta y la exposición después de altas dosis profilácticas de posaconazol.
MétodosDiferentes grupos de animales se infectaron con una cepa sensible in vitro y otra resistente de Aspergillus fumigatus.
ResultadosDespués de la profilaxis, el índice de galactomanano y la carga fúngica disminuyó en los animales infectados con la cepa sensible. La exposición resultó similar en todos los animales tratados. Sin embargo, la respuesta a posaconazol medida como aumento de la carga fúngica y el índice de galactomanano fue peor en aquellos animales infectados con la cepa resistente.
ConclusiónEste estudio demuestra la utilidad de ambos biomarcadores en la evaluación de la respuesta a antifúngicos en profilaxis.
Invasive aspergillosis (IA) remains a leading cause of infectious disease related to deaths in patients with hematologic malignancies and undergoing hematopoietic stem cell transplantation despite advances in diagnostic tools and treatment options.1
Early diagnosis is critical for rapid treatment, but the lack of sensitive and specific clinical symptoms and radiological patterns is a hindrance. Traditional histopathological examination and fungal culture rely on invasive procedures, are relatively insensitive, and are not commonly used in clinical diagnosis owing to the challenges of sampling pulmonary fluids or tissues from critically ill patients.2 As a consequence of this, the high morbility and mortality still related to fungal infection has made the use of prophylaxis, or empiric treatment regimens, the main alternative for clinicians to better manage these patients.
Several antifungal drugs have been used for both regimens in recent years, basically conditioned by epidemiological reasons and availability of individual compounds, as well as cost, efficacy, drug-drug interaction and resistance prevalence.3 Before the incorporation of posaconazole to the antifungal therapeutic arsenal, itraconazole and fluconazole had been most frequently used to prevent fungal infections in at-risk populations.4 Itraconazole was associated with a lower incidence of invasive fungal infections in heart transplants, and fluconazole demonstrated efficacy in the prevention of invasive candidiasis.3,5
Posaconazole (PSC) is a broad-spectrum triazole with activity against many filamentous fungal pathogens, including mucorales. In the last few years, some reported cases confirmed the efficacy of posaconazole prophylaxis in clinical settings.6–8 The recommended dosage for prophylaxis is 600mg or 8.6mg/kg per day (200mg three times per day, t.i.d.). As with voriconazole and itraconazole, pharmacokinetic studies indicate marked interpatient variability in both healthy volunteers and patient populations,6 especially for digestive diseases (mucositis and diarrhea), resulting in lower posaconazole serum concentrations and loss of efficacy.9 In the context of antifungal prophylaxis, exposure–response relationships need to be clarified, although in the particular case of posaconazole, several studies facilitate the assessment of a relationship between blood concentration (exposure) and prophylactic regimen efficacy (response).10
Since the 1930s, experimental models of infection have allowed the in vivo evaluation of antimicrobial agents for the treatment of experimentally induced infection. In this context, experimental models of fungal infection have contributed to better explain exposure–response relationships after antifungal treatment, making them useful tools to evaluate whether clinical success is potentially limited to exposure to the drug or dependent on the susceptibility of the causative strain. Regarding posaconazole exposure and fungal infection, very little data has been published until now.10–15 In our case, we used a steroid-immunosuppressed rat model of invasive pulmonary aspergillosis to connect exposure and efficacy of posaconazole in a prophylaxis regimen. For this purpose, two different strains of Aspergillus fumigatus, differing in posaconazole in vitro susceptibility, were included. The evolution of infection and the effectiveness of prophylaxis were evaluated using two different methodologies, detection of fungal DNA by quantitative real-time PCR (RT-PCR) in conjunction with measurement of galactomannan antigen (GM). Serum levels of posaconazole were also determined and compared.
This work was presented in part at 23rd ECCMID: [E. Cendejas-Bueno et al.] Exposure–response relationship after prophylaxis with posaconazole in a rat model of invasive pulmonary aspergillosis. Abstract number P1085 in the 23rd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Berlin, Germany, 27–30 April 2013.
Materials and methodsIsolatesTwo A. fumigatus clinical strains differing in posaconazole susceptibility, were used in this work. Susceptibility testing was performed based on the European Committee on Antimicrobial Susceptibility Testing (EUCAST).16 They included a posaconazole susceptible strain (CNM CM-237, MICPSC=0.12mg/L; wild type cyp51A gene) and a clinical strain (CNM CM-2266), isolated from the sputum of a patient with aspergillosis, that showed a point mutation in the target cyp51A gene (MICPSC≥8mg/L; G54W).17
Animals and infectionThe study was carried out on an animal model of invasive pulmonary aspergillosis, described previously.18 All animal research procedures were approved by the Ethics Committee of Animal Research of the Vall d’Hebrón Hospital (exp. Number PI081206). Briefly, animals were immunosuppressed with subcutaneous cortisone acetate 125mg/kg (Sigma, St Louis, MO, USA) three times/week, starting 14 days before the fungal challenge and continuing to the end of the experiment. To avoid bacterial super infection during immunosuppression, rats were given colistine 2500IU/mL in drinking water and cefepime 150mg/kg twice-daily subcutaneously. After six doses of steroids (day 0), a fresh conidial suspension was prepared from a subculture of the A. fumigatus strain grown in Saboraud Dextrose Agar (SDA) plates. Conidia were harvested with a sterile pipette by flooding the plates with sterile saline containing Tween-20 0.025% (v/v). The resulting suspension was washed in sterile phosphate-buffered saline, counted in a haemocytometer, and adjusted in sterile saline to a final concentration of 8×106conidia/mL. Serial ten-fold dilutions were plated on SDA plates for assessment of purity, size and viability of the inoculum.18 At least 95% of conidia in the inoculum were viable as assessed by colony counts. Rats were then anesthetized with a combination of ketamine and xylazine (ketamine 100mg/kg plus xylazine 10mg/kg, intramuscularly). Three hundred microliters of the prepared inoculum (2–3×106 conidia) were then administered to each animal via tracheotomy.
Posaconazole dosagePosaconazole (Noxafil 40mg/ml, Merck Sharp & Dohme, Spain) was administered orally by gavage. Lower posaconazole efficacy has been detected in those isolates harboring cyp51A substitutions.19 This loss of efficacy was completely or partly compensated for by increasing the posaconazole dose. A high dose was then chosen to evaluate its efficacy against mutant strains.
A prophylactic dose of 24mg/kg (three times higher than recommended dosage for humans, 600mg/day – 8.6mg/kg day) was initiated by oral gavage beginning 3 days prior to inoculation (−3) and was subsequently administered at −2, −1, 0, 1, 2 and 3 days postchallenge (seven doses). The dose was administered once daily. Two control groups (including 6 untreated but infected rats) for both strains were included. Seven other groups of three treated and infected rats were sacrificed at prefixed time points (2, 6, 8, 24h after the fourth dose, coinciding with the challenge day; and 2, 6 and 8h after the seventh dose, three days post-challenge).
Posaconazole serum concentrationSerum samples were prepared for chromatographic analysis by the addition of 2.4μL of an extraction standard (ravuconazole, final concentration 2mg/L) in 77.6μL of rat serum sample. After mixing, 160μL of acetonitrile (1:3, v/v) were added and the final mixture was vortexed at maximum speed for 30s followed by centrifugation (13,800×g, 25°C, 15min). The supernatant was then filtered through 4mm PTFE 0.45μm filters (Waters Corporation, Massachusetts, USA) and transferred to glass vial to be directly injected into the HPLC instrument. The method was cross validated in all matrices studied prior to sample analysis. Within each analytical run, a blank sample, a zero sample (sample with extraction standard at 2mg/L) and a control sample (sample with extraction standard and posaconazole at 2mg/L) were analyzed. Rat serum samples used for these run controls were obtained from infected but untreated animals. Total posaconazole serum concentrations were measured using a validated high-performance liquid chromatographic method (HPLC-PDA).20 Some modifications from the original method were performed (70:30 acetonitrile/water instead of 60:40). A partial validation was made, including new linearity, accuracy and precision studies (data not shown). Posaconazole concentrations were expressed in mg/L. Data were processed using Empower Software (version 2.0, Waters Corporation, Massachusetts, USA). Several Pharmacokinetic parameters were evaluated and compared: the Area Under the concentration-time Curves from time zero (0h) to 24h (AUC0–24) and to the time of the endpoint (AUC0–80); maximum serum concentration of the antifungal agent (Cmax); and time to Cmax (Tmax). All calculations were performed with Microsoft (MS) Excel® spreadsheet, using the PK solver add-in program, which has demonstrated equivalence calculating PK/PD parameters compared to others specific pharmacokinetic programs.21
Estimation of fungal progressionAt pre-determined time points (see below) blood samples were obtained and rats were humanely sacrificed, and immediately dissected. Lungs were aseptically removed, weighed, and separated serum and lungs were frozen until processed.
Representative samples (lungs and serum) were collected after the fourth (2 and 24h post challenge) and seventh doses (8h, corresponding to the end of the experiment). To assess the evolution of Aspergillus infection and the effectiveness of prophylaxis, two different methodologies were performed.
RT-PCR assayFungal DNA was extracted from the lung homogenates using the Qiamp DNA mini kit (Qiagen, Izasa, Madrid, Spain), following manufacturer instructions. The description, development and validation of the RT-PCR assay were described previously with slight modifications. Briefly, a PCR assay was designed to detect Aspergillus fumigatus strains by using specific molecular beacon probes labeled with a fluorescent dye (FAM) and directed to the ITS1 region of ribosomal DNA.22,23 Results were expressed as pg/g of tissue.
Galactomannan quantificationPlatelia Aspergillus Galactomannan enzyme inmunoassay (GM EIA, BioRad) was used to quantify galactomannan index in serum samples in accordance with manufacturer instructions. Index higher than 0.5 was determined to be positive.
Statistical analysisStatistical analysis was performed using the Mann–Whitney U test (Graph Pad Software v 5.0, Inc., San Diego, CA). Statistical significance was defined by a P value of ≤0.05.
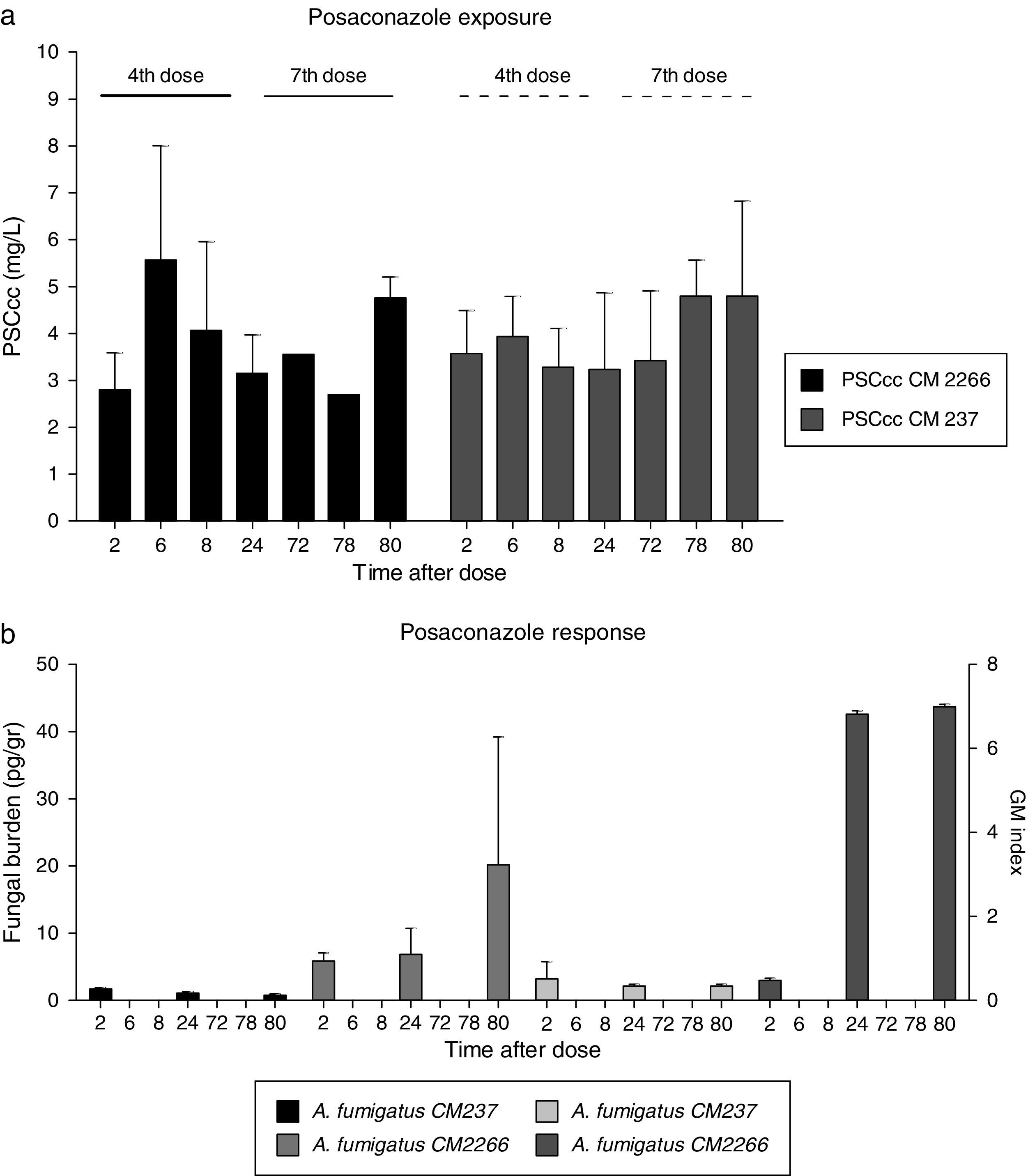
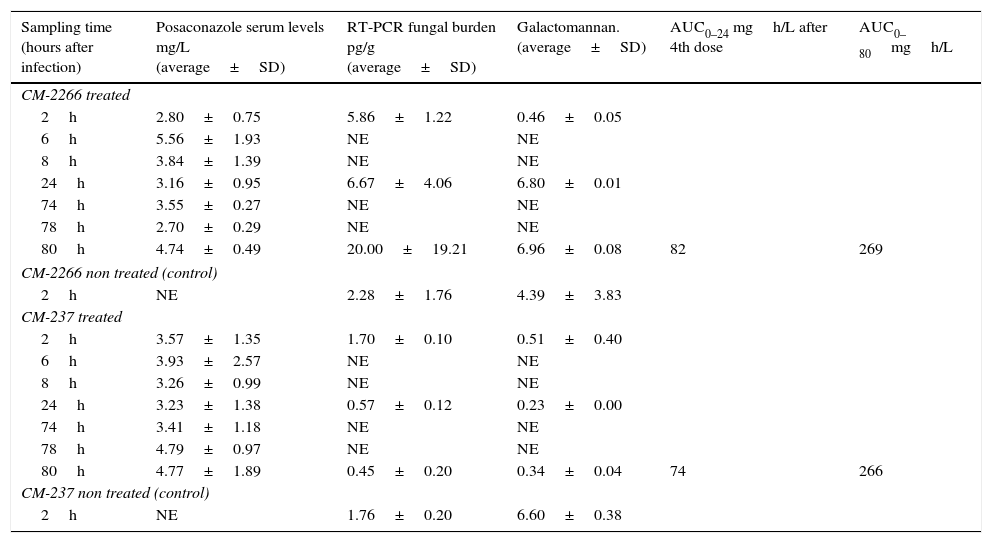
ResultsAntifungal prophylaxisPosaconazole exposure, expressed as AUC0–24h after the fourth dose, remained similar in all treated animals regardless of infecting strains (82 vs. 74mgh/L for CM-2266 and CM-237 respectively, Table 1). In addition Cmax (5.56mg/L vs. 4.79mg/L), average concentration (3.76mg/L vs. 3.85mg/L) values were similar in both groups. Tmax was 6h in both groups of animals (6h after the 4th dose and 6h after the 7th dose). Slightly higher serum concentrations were observed after the 7th dose of posaconazole (PSC average 4th dose, 3.66mg/L, PSC avg 7th dose, 3.99mg/L). AUC0–80h also remained equal in both groups (269 vs. 266mgh/L for CM-2266 and CM-237, Table 1). The AUC for each strain was used to calculate the AUC0–24/MIC which resulted in 5.13 for CM-2266 strain and 1235.16 for CM-237 strain. AUC0–80/MIC ratio was also calculated, resulting 16.86 for CM-2266 strain and 4437.50 for CM-237 strain.
Comparison of diagnostic markers at selected time points.
| Sampling time (hours after infection) | Posaconazole serum levels mg/L (average±SD) | RT-PCR fungal burden pg/g (average±SD) | Galactomannan. (average±SD) | AUC0–24 mgh/L after 4th dose | AUC0–80mgh/L |
|---|---|---|---|---|---|
| CM-2266 treated | |||||
| 2h | 2.80±0.75 | 5.86±1.22 | 0.46±0.05 | ||
| 6h | 5.56±1.93 | NE | NE | ||
| 8h | 3.84±1.39 | NE | NE | ||
| 24h | 3.16±0.95 | 6.67±4.06 | 6.80±0.01 | ||
| 74h | 3.55±0.27 | NE | NE | ||
| 78h | 2.70±0.29 | NE | NE | ||
| 80h | 4.74±0.49 | 20.00±19.21 | 6.96±0.08 | 82 | 269 |
| CM-2266 non treated (control) | |||||
| 2h | NE | 2.28±1.76 | 4.39±3.83 | ||
| CM-237 treated | |||||
| 2h | 3.57±1.35 | 1.70±0.10 | 0.51±0.40 | ||
| 6h | 3.93±2.57 | NE | NE | ||
| 8h | 3.26±0.99 | NE | NE | ||
| 24h | 3.23±1.38 | 0.57±0.12 | 0.23±0.00 | ||
| 74h | 3.41±1.18 | NE | NE | ||
| 78h | 4.79±0.97 | NE | NE | ||
| 80h | 4.77±1.89 | 0.45±0.20 | 0.34±0.04 | 74 | 266 |
| CM-237 non treated (control) | |||||
| 2h | NE | 1.76±0.20 | 6.60±0.38 | ||
SD: standard deviation; RT-PCR: real time quantitative PCR; h: time in hours; NE: not evaluated. ∼: not applicable.
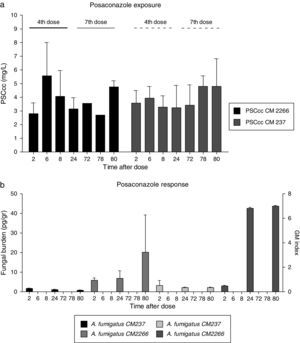
Fungal DNA was detected in all lung samples evaluated (Table 1 and Fig. 1b). Differences in fungal burden were observed between groups of animals.
Posaconazole exposure (a) and response (b) relationship measured by tissue fungal burden and by galactomannan index. (a) PSC cc: average posaconazole concentration at fourth and seventh dose in rats infected with two A. fumigatus strains differing in PSC susceptibility, CNM CM-237, MICPSC=0.125mg/L and CNM CM-2266, MICPSC≥8mg/L. (b) F Burden (pg/g) and galactomannan index by infecting specie.
Fungal burden tissue in treated rats infected with the susceptible strain (CM-237) decreased from 1.70pg/g to 0.45pg/g of lung tissue (P value 0.100). This lack of progression of infection was also indirectly demonstrated by galactomannan index comparison (Fig. 1b). This biomarker remained constant and negative (below 0.51) in these groups of animals.
Regarding treated but infected animals with less susceptible strain (CM-2266), tissue fungal burden showed a progressive increase from 5.87pg/g to 20.00pg/g of lung tissue. This progression of infection was also demonstrated by galactommanan index (Fig. 1b) which increase from 0.46 to 6.96 at the end of the experiment.
Fungal burden and GM values detected in treated animals infected with less susceptible strain were numerically higher than in those infected with susceptible strain throughout the period of study (average 20.00 vs 0.45pg/g and 6.96 vs. 0.34). These differences were not statistically significant (P>0.05), probably due to the low number of samples evaluated and the variability of reported data.
In the untreated control group, a higher index of galactomannan was observed at the first sampling time (2h post challenge, 4.39 and 6.60) compared to treated animals (0.46 and 0.51 at the same time point), suggesting a rapid release of antigens from fungal cells in the untreated animals (Table 1), as has been described for human patients.24,25
DiscussionAnimal model research has proven to be useful for designing optimal regimens and defining susceptibility or resistance in the context of antimicrobial treatment. For the particular case of filamentous fungi and posaconazole results from recent studies demonstrated that AUC0–24/MIC ratio provided a useful measure of exposure-response relationship. More posaconazole exposure was suggested for efficacy against organisms with reduced in vitro susceptibility. Although the strength of such a relationship has not been well elucidated yet, an AUC0–24/MIC ratio ranging from 167 to 178 was estimated recently as predictive of success associated with half-maximal efficacy or higher.19,26,27
In this study, a prophylactic dose of posaconazole showed potent in vivo antifungal activity against susceptible Aspergillus fumigatus strains (MIC<0.125mg/L) for which the AUC0–24/MIC was calculated to be 1235.16. Efficacy was demonstrated by decreasing fungal burden (76% reduction, from 1.70 to 0.45pg/g) and also GM index (34% reduction, from 0.51 to 0.34). Of note, in those animals infected with the “susceptible strain”, posaconazole blood concentration (average=3.76mg/L) was more than optimal as it was above the amount required for growth inhibition. The effect of such a drug environment could explain the negative results achieved for the two biomarkers. Several studies highlighted the ability of posaconazole to suppress serum galactomannan expression.28,29 Concentrations of the GM antigen and DNA have been reported to be affected by antifungal drugs that are active against molds, probably because of the failure of the fungal cells to grow and in consequence to release antigen into blood,30 confirming the treatment effect. The values of both biomarkers found in the group of rats infected with the susceptible strain and treated suggest the absence of infection progression. Therefore, they may be valid indicators of efficacy as no standard measure that best predicts the outcome has been defined yet. Of note, posaconazole concentration average was also higher than the MIC value of most of the A. fumigatus strains (PSC MIC90=0.125mg/L), and the PK/PD index (AUC/MIC ratio) associated with efficacy was in the range of previous studies.19 However, posaconazole showed poor prophylaxis efficacy with less susceptible strains of A. fumigatus (AUC0–24/MIC ratio=5.13).
Additionally, a new pharmacodynamics index AUC0–80/MIC, was calculated in the study, and showed great differences between susceptible and resistant strains (AUC0–80/MIC=4437.5 vs AUC0–80/MIC=16.86), demonstrating that PSC MICs affected exposure–response relationships. Those strains with higher MICs required higher exposure (AUC0–24 or AUC0–80) to achieve any effect.
One of the main challenges to evaluate drug effect is the way to assess progression of infection. Diagnostic tests such as galactomannan enzyme immunoassay and PCR, have been widely employed to assess infection progression. As previously published, these two biomarkers increase significantly during progression of infection,31 and combined have even better diagnostic applicability.
Our results showed a clear progression of infection in those groups of treated animals infected with the less susceptible strain compared to those animals infected with the susceptible Aspergillus strain. Bearing this data in mind, our study supports those previously described for other resistant molds.14,15
As shown in this study, quantification of fungal burden by RT-PCR and GM-EIA are both valid strategies for evaluating the progression of infection and the effectiveness of prophylaxis. Nevertheless, the lack of validated methods for PCR could raise doubts about the validity of this technique for evaluating the prophylaxis and treatment efficacy in animal models. GM-EIA assessment in serum remains the most practical and valuable method in the clinical setting and the obtained results certify that GM-EIA is a valid and reliable technique for evaluating efficacy of posaconazole prophylaxis in animal models, although because of its high cost we can consider their use only in some specific cases, as was previously published.13,31 A combination of both biomarkers has been considered a good alternative for a better monitoring of fungal infection.
A drawback of this experiment was the limited number of animals used per time point and the evaluation of only one time matched point in non-treated controls. Worth of mention is the limited number of A. fumigatus evaluated and the lack of isolates with other cyp51A mutations different from the G54. Further studies are warranted including more strains differing in PSC susceptibility profile. Nevertheless, the results obtained by both methodologies confirmed the efficacy of the prophylactic regimen in animals infected with the susceptible strain and the failure in those infected with the resistant strain. These results also corroborate the utility of this experimental model to evaluate novel diagnostic tools such as biomarkers to assess efficacy of antifungal compounds and exposure–response relationships.
Conflict of interest statementThe authors declare no conflict of interest.
We thank Merck Sharp & Dohme by posaconazole powder supply. This study has been financed by the Research Project from the Fondo de Investigaciones Sanitarias, FIS_ISCIII (Exp number PI09/0624). E. Cendejas-Bueno had a predoctoral fund from Instituto de Salud Carlos III (Grant AFTDOC 11/02, Spain). Currently E. Cendejas has a Juan Rodés Contract from Instituto de Salud Carlos III. A. Forastiero is founded by a fellowship from Agencia Española de Cooperación Internacional para el Desarrollo (MAEC-AECID, Convocatoria 2011–2012, 0000557290).