Approximately 4% to 8% of patients with HIV-1 treated with abacavir present a hypersensitivity reaction (HSR). Various studies have shown a direct association between human leukocyte antigen (HLA)-B*5701 and HSR to abacavir. The objective of this study was to analyze whether systematic HLA-B*5701 testing to prevent HSR in patients treated with abacavir is a cost-effective option for the Spanish National Health System.
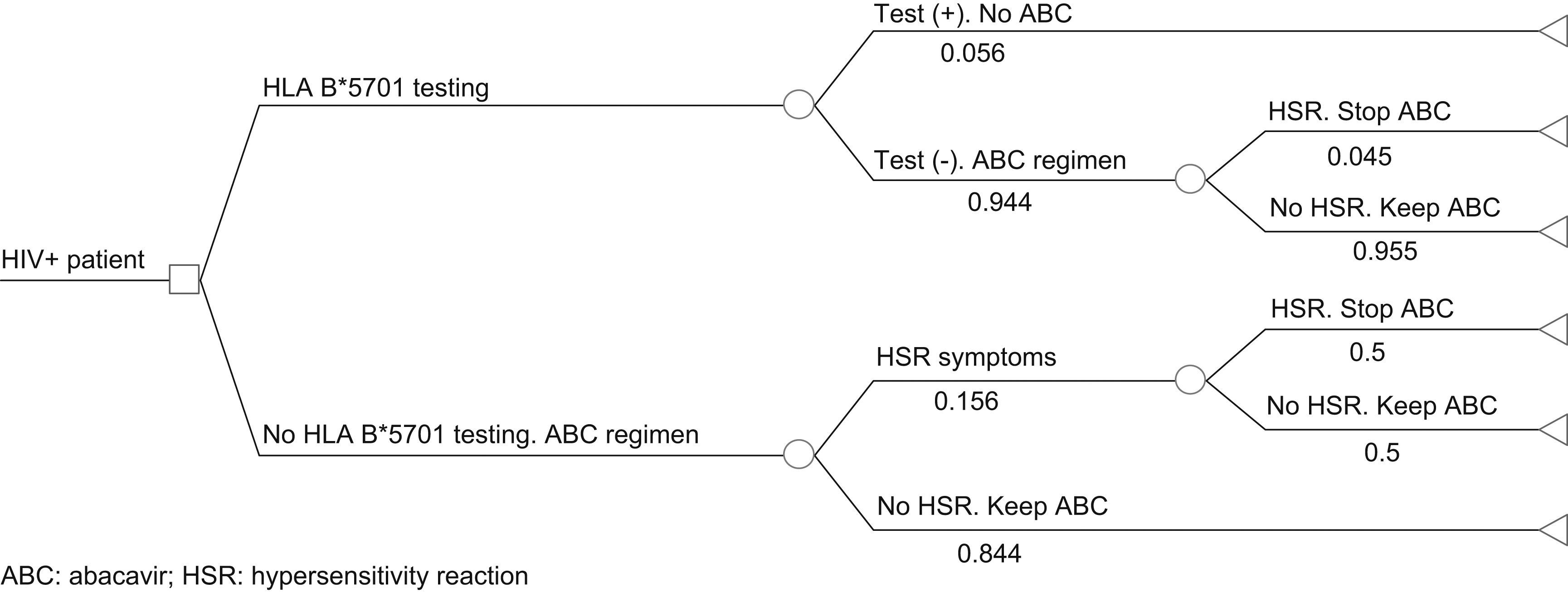
MethodsAn analytical decision-making model was constructed as a decision tree model for a simulated cohort of 1000 HIV patients to evaluate whether HLA-B*5701 testing to prevent HSR to abacavir was cost effective compared with not performing the test. The parameters included in the model and the use of healthcare resources should the patient develop HSR were taken from the PREDICT-1 study and the opinion of clinical experts. The principal result obtained was the incremental cost per HSR avoided. The time horizon of the analysis was 6 months. All costs were expressed in 2008 Euros.
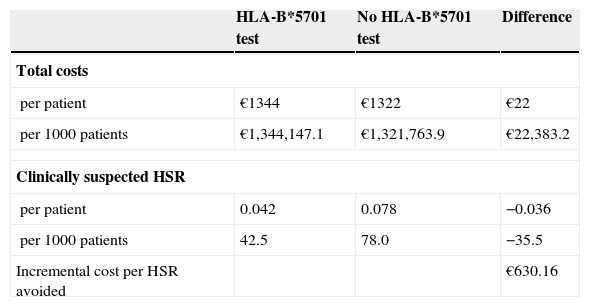
ResultsThe analysis showed that the total direct healthcare costs per patient were €1344 and €1322 with and without HLA-B*5701 testing respectively, and that 36 cases of HSR were prevented per 1000 screened patients. These results yielded a cost per HSR avoided of €630. The sensitivity analysis showed that the results were sensitive to the cost of the test, with an economic saving of €102 or a cost-effectiveness ratio of €4234.
ConclusionsThe model predicts that generalized use of the HLA-B*5701 test before prescribing abacavir in HIV+ patients could represent an economic saving or a limited additional cost for the National Health System which may be counterbalanced by the benefits in terms of a lower incidence of HSR.
Aproximadamente el 4–8% de los pacientes con VIH-1 tratados con abacavir presentan una reacción de hipersensibilidad (RHS). Diversos estudios han mostrado que existe una asociación directa entre el antígeno leucocitario humano (HLA)-B*5701 y la RHS a abacavir. El objetivo del presente estudio ha sido analizar si la realización sistemática del test HLA-B*5701 para prevenir la RHS en los pacientes tratados con abacavir es una opción coste-efectiva para el Sistema Nacional de Salud (SNS) español.
MétodosSe realizó un modelo analítico de decisiones mediante un modelo de árbol de decisión para simular una cohorte de 1.000 pacientes con VIH en el que se comparó si la realización del test HLA-B*5701 para prevenir la RHS al tratamiento con abacavir era una opción coste-efectiva versus no realizar el test. Los parámetros introducidos en el modelo así como el uso de recursos sanitarios en caso de que el paciente desarrollase una RHS provenían del estudio PREDICT-1 y de la opinión de expertos clínicos. El resultado principal del studio fue el coste incremental por RHS evitada. El horizonte temporal del análisis fue de 6 meses. Todos los costes se expresaron en euros del año 2008.
ResultadosEl análisis demostró que los costes sanitarios directos totales por paciente fueron 1.344 € y 1.322 € al realizar o no el test HLA-B*5701, respectivamente, evitando unos 36 casos de RHS por cada 1.000 pacientes cribados. Estos resultados dieron lugar a una razón de coste por RHS evitada de 630 €. El análisis de sensibilidad mostró que los resultados fueron sensibles al coste del test produciendo desde un ahorro económico de 102 € hasta una razón coste-efectividad de 4.234 €.
ConclusionesEl modelo predice que la generalización del uso del test HLA-B*5701 previamente a la prescripción de abacavir en los pacientes HIV+ podría suponer un ahorro económico o un coste adicional limitado para el SNS que puede verse compensado por los beneficios en términos de menor incidencia de RHS.
Human immunodeficiency virus type 1 (HIV-1) infection has become one of the world's greatest public health problems. However, the use of highly active antiretroviral therapy (HAART) has decreased the morbidity and mortality associated with HIV infection.1 HAART typically includes a regimen combining nucleoside reverse transcriptase inhibitors (NRTIs) with protease inhibitors or non-nucleoside reverse transcriptase inhibitors (NNRTIs).2 Abacavir is a NRTI that has shown efficacy, few drug interactions, and a favorable long-term toxicity profile.3,4 The most important adverse effect of abacavir that limits its use in therapy is that approximately 4% to 8%5–7 of patients treated with abacavir present hypersensitivity to the drug, with an idiosyncratic systemic reaction including fever, rash, fatigue, and gastrointestinal and respiratory symptoms.6 This hypersensitivity reaction (HSR) usually appears within 6 weeks of starting treatment,6 its severity varies, and in 0.03% of patients it leads to death.6 Therefore, abacavir is contraindicated in patients who develop a HSR. Although the precise mechanisms that produce the HSR are unknown, it has been suggested that genetic, immunological, and metabolic factors are involved.8,9 Various studies have shown a direct association between human leukocyte antigen (HLA)-B*5701 and HSR to abacavir, as patients who present the HLA-B*5701 allele have a 100-fold greater risk of experiencing HSR when exposed to abacavir.8,10–14 Systematic HLA-B*5701 typing before prescribing abacavir would therefore enable clinicians to determine susceptibility to HSR, thus increasing safety in the management of HIV+ patients.
HIV is the world's main infectious cause of death and produces a substantial economic burden for society;15 thus, economic evaluations are useful to make appropriate treatment decisions based on clinical and financial grounds. Several studies have evaluated the cost-effectiveness of different HAART regimens previously.16–18 Because abacavir is one of the NRTI included in HAART regimens recommended by Spanish HIV+ treatment guidelines, the present work aims to analyze whether systematic HLA-B*5701 testing to prevent HSR in patients who are candidates for abacavir treatment is a cost-effective option for the Spanish National Health System (NHS).
MethodsType of analysisA cost-effectiveness analysis was performed to compare the healthcare costs associated with performing the HLA-B*5701 test or not in HIV+ patients before they receive antiretroviral combinations containing abacavir, with the clinical benefit in terms of HSR avoided with the test. The study was based on an analytical decision-making model in which the results were expressed in relation to the incremental cost-effectiveness ratio of screening, as cost per HSR avoided.
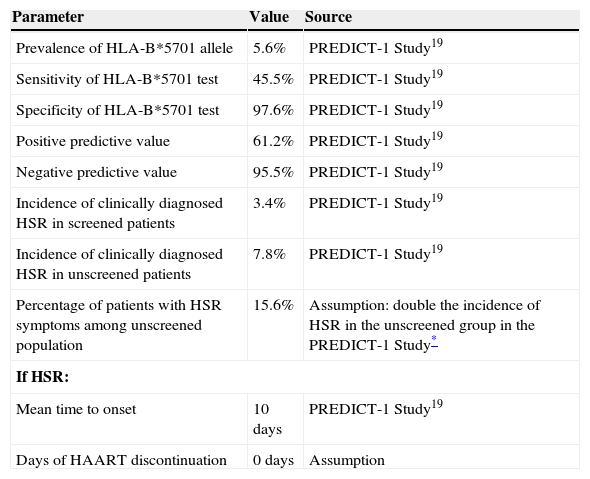
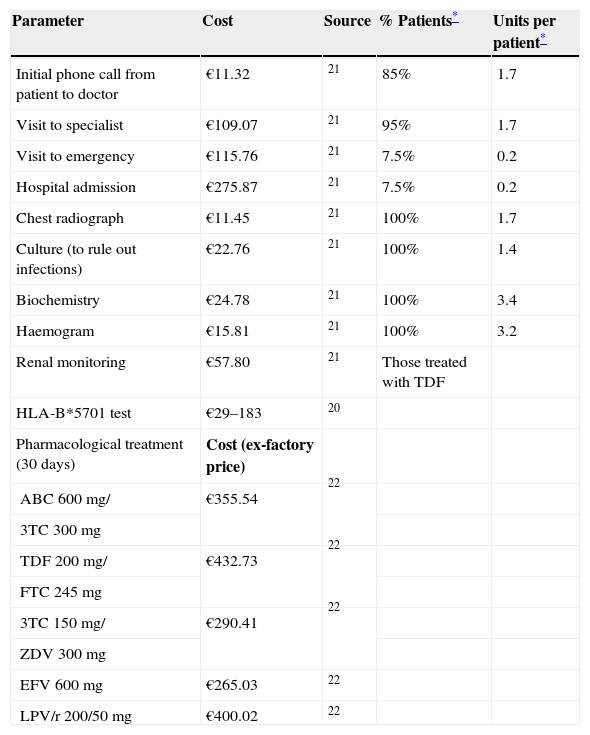
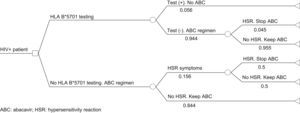
Pharmacoeconomic modelA simulated cohort of HIV+ patients was built with a decision tree model to represent clinical practice, depending on whether the patients’ susceptibility to treatment with abacavir was known or not. The model assumed that patients presenting a positive HLA-B*5701 test result would receive a HAART regimen that did not contain abacavir, whereas patients presenting a negative HLA-B*5701 test would receive HAART containing abacavir, and even then, could still present HSR (Fig. 1). The parameters included in the model and the use of healthcare resources should the patient develop HSR were taken from current literature19,20 and the opinion of 3 clinical experts, obtained with a specific questionnaire. The first part of the questionnaire showed the assumptions of the cost-effectiveness model and clinical parameters used, and the experts answered whether they agreed with the proposed value. The second part of the questionnaire evaluated healthcare resource use and the units used per patient, and the questions were open to any proposed answer. The clinical experts answered the questionnaire individually, and the mean of the percentage of patients using each healthcare resource and the units used per patient were then calculated. The answer variability was evaluated in the sensitivity analysis. The model enabled us to estimate the direct healthcare costs and incidence of HSR associated with the comparative options from data found in the current literature and explicit assumptions. Table 1 shows the parameters included in the model, which come from the PREDICT-1 study19 and were validated by clinical experts. The use of healthcare resources should a patient develop HSR to abacavir, obtained from the opinion of the 3 clinical experts, is shown in Table 2. The model considered the cost of the test, the cost of treating HSR and the cost and selection of alternative antiretroviral therapeutic combinations. Unit costs were obtained from the Spanish Healthcare Costs Database e-Salud21 and the Spanish Database of Medicines.22 The principal result of the study was the incremental cost per HSR avoided, which was calculated by dividing the cost difference between performing the test or not and between the difference in HSR incidence when the test is performed or not. The study was conducted from the perspective of the National Health System. All costs were expressed in 2008 Euros and no discount rate was applied because of the short time horizon of the analysis (6 months).
Clinical parameters of the model
| Parameter | Value | Source |
| Prevalence of HLA-B*5701 allele | 5.6% | PREDICT-1 Study19 |
| Sensitivity of HLA-B*5701 test | 45.5% | PREDICT-1 Study19 |
| Specificity of HLA-B*5701 test | 97.6% | PREDICT-1 Study19 |
| Positive predictive value | 61.2% | PREDICT-1 Study19 |
| Negative predictive value | 95.5% | PREDICT-1 Study19 |
| Incidence of clinically diagnosed HSR in screened patients | 3.4% | PREDICT-1 Study19 |
| Incidence of clinically diagnosed HSR in unscreened patients | 7.8% | PREDICT-1 Study19 |
| Percentage of patients with HSR symptoms among unscreened population | 15.6% | Assumption: double the incidence of HSR in the unscreened group in the PREDICT-1 Study* |
| If HSR: | ||
| Mean time to onset | 10 days | PREDICT-1 Study19 |
| Days of HAART discontinuation | 0 days | Assumption |
HSR, hypersensitivity reaction; HAART, highly active antiretroviral therapy.
Use of healthcare resources and unit costs used in the model
| Parameter | Cost | Source | % Patients* | Units per patient* |
| Initial phone call from patient to doctor | €11.32 | 21 | 85% | 1.7 |
| Visit to specialist | €109.07 | 21 | 95% | 1.7 |
| Visit to emergency | €115.76 | 21 | 7.5% | 0.2 |
| Hospital admission | €275.87 | 21 | 7.5% | 0.2 |
| Chest radiograph | €11.45 | 21 | 100% | 1.7 |
| Culture (to rule out infections) | €22.76 | 21 | 100% | 1.4 |
| Biochemistry | €24.78 | 21 | 100% | 3.4 |
| Haemogram | €15.81 | 21 | 100% | 3.2 |
| Renal monitoring | €57.80 | 21 | Those treated with TDF | |
| HLA-B*5701 test | €29–183 | 20 | ||
| Pharmacological treatment (30 days) | Cost (ex-factory price) | |||
| ABC 600mg/ | €355.54 | 22 | ||
| 3TC 300mg | ||||
| TDF 200mg/ | €432.73 | 22 | ||
| FTC 245mg | ||||
| 3TC 150mg/ | €290.41 | 22 | ||
| ZDV 300mg | ||||
| EFV 600mg | €265.03 | 22 | ||
| LPV/r 200/50mg | €400.02 | 22 |
3TC, lamivudine; ABC, abacavir; EFV, efavirenz; FTC, emtricitabine; LPV/r, lopinavir/ritonavir; TDF, tenofovir; ZDV, zidovudine.
To evaluate the impact of the uncertainty of the parameters included in the study results and to validate their robustness, successive univariate sensitivity analyses were performed with the model's key parameters, such as the cost and diagnostic precision of the HLA-B*5701 test, the prevalence of the HLA-B*5701 allele and the incidence of HSR. A bivariate analysis was also performed using different combinations of regularly prescribed antiretroviral drugs, together with the parameter with the greatest impact on the analytical results (the cost of the test).
We performed a probabilistic sensitivity analysis using a second-order Monte Carlo simulation, replicating the cost-effectiveness results 1000 times. To conduct the probabilistic sensitivity analysis, we selected some fixed distributions, as well as estimating the parameters of each distribution as a function of the primary data gathered. Thus, we used a log-normal distribution for the costs and a beta distribution for the model probabilities.23,24
ResultsIn the pharmacoeconomic analysis, the total direct healthcare costs included pharmacological costs, the cost of the test, and the cost of HSR management. The pharmacological costs varied according to the drug combination chosen, based on whether the patient had undergone the HLA-B*5701 test and whether the test result was positive or negative. The base case used the median cost of the test (€55) reported in a Spanish study.20 The total cost of managing each diagnosed HSR was estimated at €395, with €209 per suspected HSR, based on the use of resources provided by clinical experts and the costs described in Table 2. Considering these parameters, the results showed that the total direct healthcare costs were €1344 and €1322 with or without the HLA-B*5701 test, respectively (additional cost of €22 per patient) (Table 3). When the HLA-B*5701 was performed, 42 cases of HSR appeared per 1000 screened patients. When patients were treated without the test, there were 78 cases of HSR per 1000 patients. Systematic HLA-B*5701 testing therefore avoided 36 cases of HSR/1000 patients (Table 3). These results gave rise to a cost ratio per HSR avoided of €630 (Table 3).
Cost-effectiveness analysis
| HLA-B*5701 test | No HLA-B*5701 test | Difference | |
| Total costs | |||
| per patient | €1344 | €1322 | €22 |
| per 1000 patients | €1,344,147.1 | €1,321,763.9 | €22,383.2 |
| Clinically suspected HSR | |||
| per patient | 0.042 | 0.078 | −0.036 |
| per 1000 patients | 42.5 | 78.0 | −35.5 |
| Incremental cost per HSR avoided | €630.16 | ||
HSR, hypersensitivity reaction.
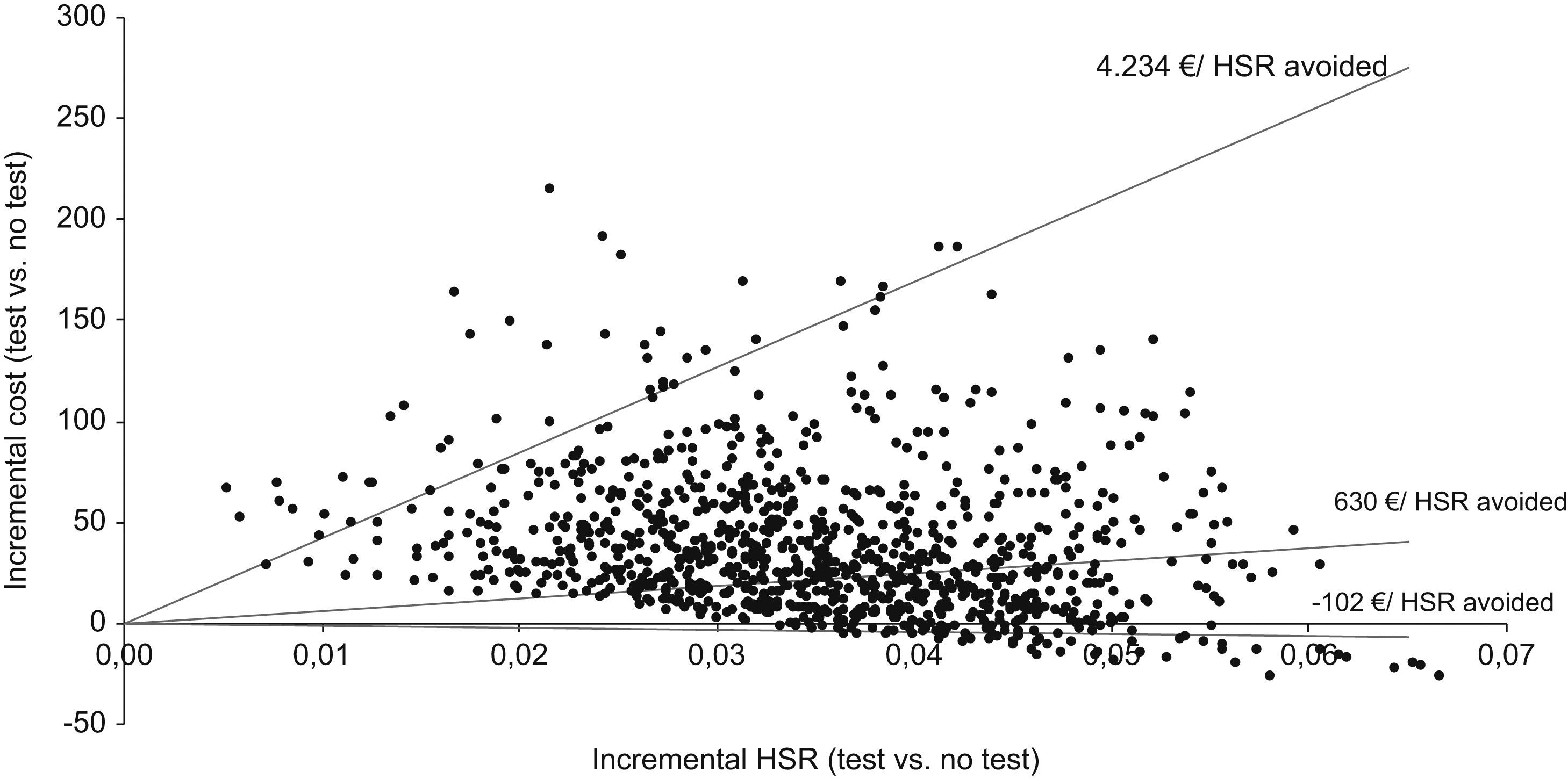
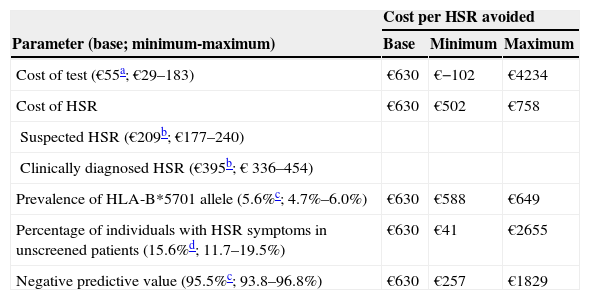
The univariate sensitivity analysis showed that the results did not vary significantly with changes in the antiretroviral combination, in the cost of HSR or in the prevalence of the HLA-B*5701 allele. However, they were sensitive to the cost of the test, the percentage of individuals with HSR symptoms among unscreened patients, and the negative predictive value of the test (Table 4): with the cost of the test ranging from €29–€183, there was an economic saving of €102 (if the cost of the test was €29) or a maximum additional cost of €4234 (if the cost of the test was €183) per HSR avoided; with the percentage of individuals with HSR symptoms among unscreened patients ranging from 11.7–19.5, there was an additional €41 to €2655 per HSR avoided; with the predictive value of the test ranging from 93.8% to 96.8%, there was an additional cost of €257 to €1829 per HSR avoided.
Univariate sensitivity analysis
| Cost per HSR avoided | |||
| Parameter (base; minimum-maximum) | Base | Minimum | Maximum |
| Cost of test (€55a; €29–183) | €630 | €−102 | €4234 |
| Cost of HSR | €630 | €502 | €758 |
| Suspected HSR (€209b; €177–240) | |||
| Clinically diagnosed HSR (€395b; € 336–454) | |||
| Prevalence of HLA-B*5701 allele (5.6%c; 4.7%–6.0%) | €630 | €588 | €649 |
| Percentage of individuals with HSR symptoms in unscreened patients (15.6%d; 11.7–19.5%) | €630 | €41 | €2655 |
| Negative predictive value (95.5%c; 93.8–96.8%) | €630 | €257 | €1829 |
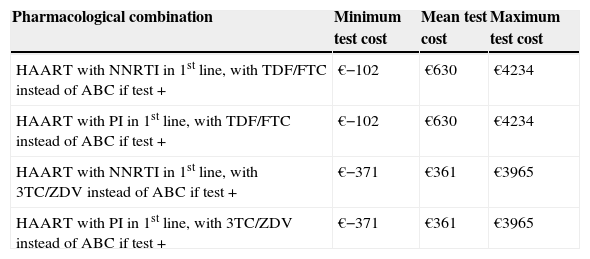
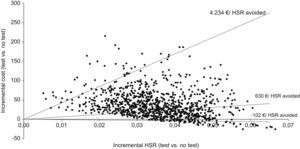
In the bivariate analysis, we observed that when different antiretroviral combinations and HLA-B*5701 test costs were used, the incremental cost per HSR avoided could range from an economic saving of €371 (if the cost of the test was €29) to €4234 (if the cost of the test was €183) (Table 5). For a test cost of €33, the incremental cost per HSR avoided would be zero. The results of the probabilistic analysis (using a log-normal distribution for the costs and a beta distribution for the model probabilities) demonstrated that even taking into account the uncertainty in the main variables of the model, systematic HLA-B*5701 testing could be a cost-effective option (Fig. 2). Thus, the evaluation of 1000 different scenarios allowed us to estimate the results for all the possible healthcare situations. Taking all the results together, it can be confirmed that HLA-B*5701 testing could produce a range from economic savings to a limited additional cost.
Bivariate sensitivity analysis-therapeutic regimens
| Pharmacological combination | Minimum test cost | Mean test cost | Maximum test cost |
| HAART with NNRTI in 1st line, with TDF/FTC instead of ABC if test + | €−102 | €630 | €4234 |
| HAART with PI in 1st line, with TDF/FTC instead of ABC if test + | €−102 | €630 | €4234 |
| HAART with NNRTI in 1st line, with 3TC/ZDV instead of ABC if test + | €−371 | €361 | €3965 |
| HAART with PI in 1st line, with 3TC/ZDV instead of ABC if test + | €−371 | €361 | €3965 |
3TC, lamivudine; ABC, abacavir; FTC, emtricitabine; HAART, highly active antiretroviral therapy; HSR, hypersensitivity reaction; NNRTI, non-nucleoside analogue reverse transcriptase inhibitor; PI, protease inhibitor; TDF, tenofovir; ZDV, zidovudine.
Several HAART regimens that do not contain abacavir are currently available on the market. Therefore, studies that provide data on the cost-effectiveness of systematic typing of the HLA-B*5701 allele to inform treatment decision-making processes are important. Systematic HLA-B*5701 typing is fundamental for preventing HSR to abacavir which, although uncommon (4%–8%) can be serious.5–7 Genetic screening is particularly appropriate because of its negative predictive value (nearly 100%), which enables abacavir prescription to be ruled out in patients who test positive to HLA-B57.19 The AIDS Study Group and the Spanish National AIDS Plan recently published an update of HAART recommendations for adult HIV-infected patients that advise HLA-B*5701 detection prior to starting a treatment containing abacavir.2 Based on the treatment record and resistance trials, these recommendations also suggest that abacavir should only be administered to HLA-B*5701-positive patients if no other therapeutic option is available.
Pharmacoeconomic studies analyzing whether pharmacogenetic tests enabling prevention of toxicity due to certain drugs are cost-effective are currently on the increase.25 This study has shown that systematic HLA-B*5701 testing before prescribing abacavir could be the dominant option (less costly and more effective) compared to not performing the test, depending on the cost of the test itself. In a similar study conducted in the United Kingdom, Hughes et al.26 found that generalized use of the test is cost-effective in nearly all scenarios and that the type of alternative HAART and the prevalence of HSR to abacavir were the parameters that most affected the results, due to their high variability. In our study, however, the cost of the test had the greatest impact on the results of the analysis (essentially because of its high variability in our country), together with the percentage of individuals with HSR symptoms among unscreened patients and the negative predictive value of the test, whereas the model was not very sensitive to changes in pharmacological combinations, the cost of HSR, or the prevalence of the HLA-B*5701 allele. Schackman et al.27 recently analyzed whether HLA-B*5701 testing in HIV+ patients to be treated with abacavir is cost-effective in the long term in the USA. The results showed that HLA-B*5701 typing increases 0.04 months of quality-adjusted life with an incremental cost of $110 compared with not performing the test, giving rise to a cost-effectiveness ratio of $36,700/year of quality-adjusted life years gained, which is less than the accepted threshold in the USA (approximately $50,000).28 As in our study, these results were sensitive to the cost of the test ($68–341), but also varied depending on the prevalence of HLA-B*5701. Our study used the prevalence of HLA-B*5701 found in the PREDICT-1 study, which is within the observed range in the Spanish population (4.6%–6%).20,29 It is important to note that the prevalence of the HLA-B*5701 allele can vary depending on the racial characteristics of the study population, and this constraint should be considered in each specific pharmacoeconomic analysis.12 In Spain, a study with 63 HIV+ patients showed the same results as ours, as they obtained a cost of €630 per HSR avoided (considering a test cost of €30 and HSR cost of €1890).29
The model used in this study has some limitations. First, it considered that the adverse events associated with different therapeutic regimens are similar (except for the incidence of HSR). However, if the HAART included tenofovir, the renal monitoring recommended in the summary of product characteristics was accounted for.30 Another limitation is that it assumed that HAART would not be discontinued due to HSR, although there might be a change in the drugs used (for instance, substituting abacavir with another agent, such as tenofovir or emtricitabine). It also considered that adherence to treatment was 100%, although it is usually lower in clinical practice. The fixed-dose combination of efavirenz, tenofovir, and emtricitabine was recently approved in Spain, but it was not included in the model because the cost of the triple combination is similar to the sum of the costs of the three drugs separately; hence the study results would not be significantly altered. It is interesting to note that this study included lost medication. That is, when HSR develops, the drug combination is changed (maintaining the protease inhibitor and non-nucleoside analogue reverse transcriptase inhibitor components of HAART) and the pack containing abacavir has to be eliminated, thus losing the remaining product. In addition, the model did not include the cardiovascular risk or risk of death associated with abacavir treatment.2,31
In summary, this study is especially relevant because it determines whether HLA-B*5701 testing is a cost-effective option specifically in the Spanish Health System, enabling more informed treatment decisions to be taken.
ConclusionThis study shows that generalized use of HLA-B*5701 testing before administering abacavir in HIV+ patients could represent an economic saving or a limited additional cost for the Spanish NHS, which could be compensated by its benefits in terms of a lower incidence of HSR. The cost of the test varies considerably in Spain, and this factor determines whether systematic typing of the HLA-B*5701 allele is cost-effective.
Conflict of interestsLaura García-Bujalance and Isabel Pérez-Escolano work for GlaxoSmithKline, the company sponsoring this study; Óscar de la Calle, José A. Iribarren and Antonio Rivero participated in the preparation of the study as clinical experts; Diana Nieves and Max Brosa received a grant from GlaxoSmithKline to conduct the research.