A correct diagnosis of the type of diabetes mellitus (DM) is crucial to provide patients with the most appropriate treatment and follow-up.
Here we present the case of a 15-year-old female patient sent to our Diabetes Unit due to DM onset. During an episode of fever, a fasting plasma glucose level of 173mg/dL was detected, and treatment with diet and physical exercise was started. The first blood test result in our service was: fasting plasma glucose of 137, glycated hemoglobin (HbA1c) of 7.6%, negative pancreatic autoimmunity, and non-stimulated C-peptide of 17.46ng/mL. Regarding the family history, her grandmother had type 2 DM (DM2). With regards to the patient's history, low birthweight, failure to thrive, and delayed tooth eruption were the most remarkable findings. Her age at menarche was 14 years, and, afterwards, she developed secondary amenorrhea. The physical examination revealed 145cm of height (below the 3rd percentile), 37.9kg of weight, and a body mass index of 18kg/m2. Additional features were syndromic facies, with a broad forehead and mid-facial hypoplasia and prominent ears. She also had a high-pitched voice, facial and thoracic lipodystrophy, mild acanthosis nigricans on the axillae, complete pubertal development, and no signs of hyperandrogenism. In the complementary examinations due to low height and secondary amenorrhea, a female 46,XX karyotype was confirmed, as well as, negative celiac disease autoimmunity, and normal values of prolactin, estradiol, thyrotropin, follicle-stimulating hormone, luteinizing hormone, testosterone, 17-hydroyprogesterone, dehydroepiandrosterone sulfate, and insulin-like growth factor-1. Moreover, ultrasound detected polycystic ovaries (thus, she met the criteria for polycystic ovary syndrome, given the previously mentioned data). She also displayed a normal bone age (bone/chronological age: 17/16 years). Treatment with metformin 850mg/12h was started, with the patient recovering her menstrual cycles after 5 months and maintaining HbA1c<7%.
The syndromic phenotype led us to conduct a genetic study through next generation sequencing (NGS) for monogenic diabetes, including genes associated with insulin resistance. A heterozygous, de novo (neither the patient's parents nor siblings were affected), pathogenic variant was identified in exon X of PIK3R1. This defect is associated with SHORT syndrome, which accounts for less than 60 cases worldwide.1,2 c.1945C>T is the most frequently described pathogenic variant. PIK3R1 encodes the regulatory subunit (p85α) of the PI3K holoenzyme. The subunit p85α stabilizes the catalytic subunit (p110α) that regulates the AKT/mTOR pathway. This pathway is critical for proper cell proliferation and growth. When p85α binds to p110α, phosphatidylinositol3,4 bisphosphate is converted to phosphoinositol3–5 triphosphate, which recruits AKT and initiates the downstream effectors of cellular growth. In SHORT syndrome, there is a diminished capacity to activate the AKT pathway and downstream targets. Reduced p85α results in p110α not being available for downstream effects.3 SHORT syndrome is inherited in an autosomal dominant manner, although de novo pathogenic variant cases appear significant. The acronym refers to: Short stature; Hyperextensibility, Ocular depression, Rieger anomaly (a congenital ocular defect caused by anterior segment dysgenesis), and Teething delay. The features most consistently observed are mild intrauterine growth restriction, short stature, partial lipodystrophy, a characteristic facial gestalt (prominent forehead and deep-set eyes, relatively small middle and lower thirds of the face, prominent ears, etc.), insulin resistance and DM. Other frequent features include Axenfeld-Rieger anomaly or related ocular anterior chamber dysgenesis, delayed dentition and other dental issues, and sensorineural hearing loss.3 These and other manifestations are present with variable frequency in subjects with this syndrome. In addition to the findings already mentioned in the description of our case, she also had mild impaired hearing, with 8000Hz. As observed in the present case, not all manifestations considered in the syndrome acronym occur always, and others not mentioned may appear. SHORT syndrome is diagnosed in a proband with compatible clinical features (with emphasis on the facial gestalt) and a heterozygous pathogenic variant in PIK3R1 identified by molecular genetic testing. Management consists of treating the manifestations and monitoring those that may appear.
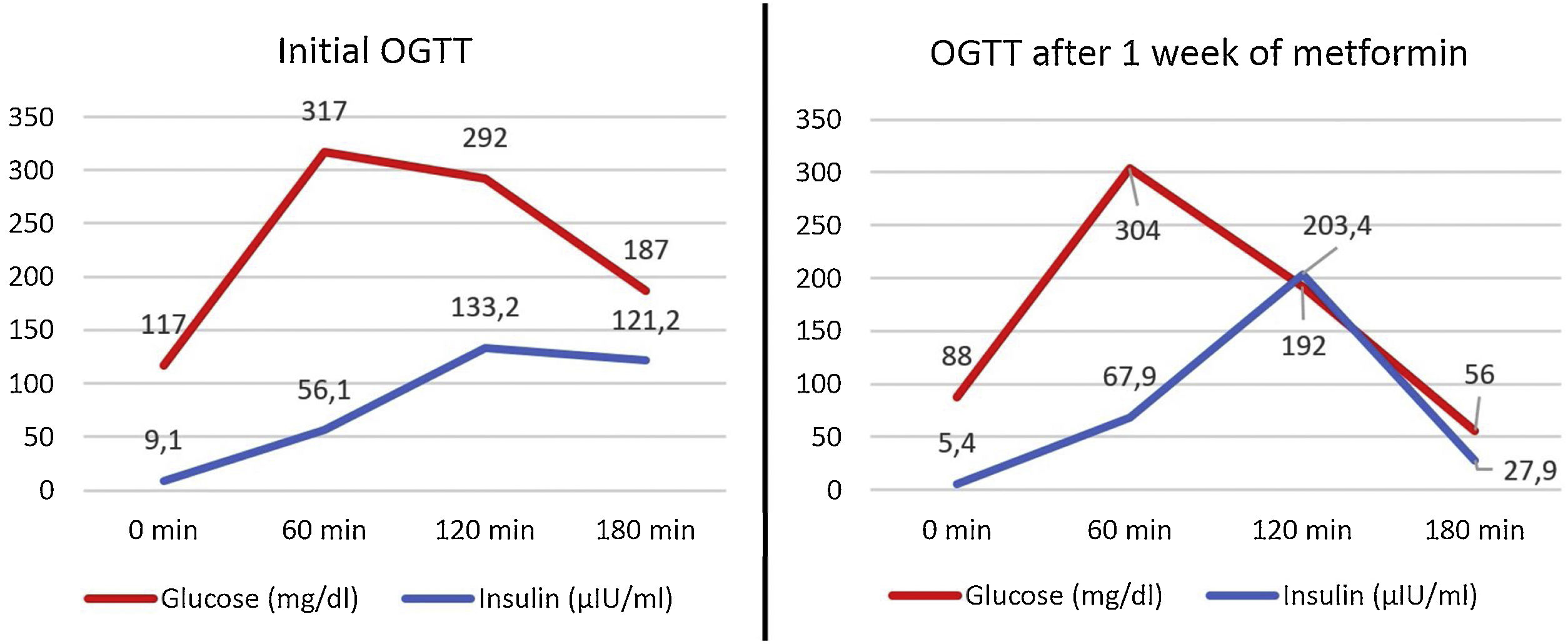
Lewandowski KC et al. reported the case of an individual with an identical pathogenic variant who, after four days of treatment with metformin, showed worse glycemia levels and an increase in insulin resistance, identified through a 75g oral glucose tolerance test (OGTT).4 Our patient underwent the same test before starting treatment and after one week on 850mg/12h of metformin, which showed a good response to metformin (Fig. 1) and an improvement in homeostasis model assessment (HOMA) of insulin resistance, which changed from 2.6 (score associated with suspected insulin resistance) to 1.08 (no insulin resistance),5 and in HOMA β cell function, which changed from 60.86 to 78.26. In Lewandowski KC et al. case, it was hypothesized that the worsening found after starting metformin could be since the drug partially inhibited the PI3K signaling pathway, a pathway that affects very immediate post-receptor steps of insulin signaling and that is mutated in SHORT syndrome. Nevertheless, the main mechanism by which metformin lowers blood glucose is the inhibition of hepatic gluconeogenesis. Hypoglycemic effects by action on muscle or intestine are considered less important.6 Therefore, the possible affectation of the PI3K signaling pathway by metformin would not influence the main hypoglycemic mechanism of the drug, and the decrease in pancreatic glucotoxicity could explain the improvement in the insulin response to the OGTT.
This case demonstrates the relevance of achieving an accurate DM diagnosis. Indeed, the syndrome was diagnosed due to the assessment of the type of DM. NGS can be useful in patients with DM and a characteristic phenotype or several, apparently non-related, defects. PIK3R1 gene should be incorporated into the genetic panels for the study of monogenic diabetes.
AuthorshipAll authors had access to the data and a role in writing this manuscript.
FundingIgnacio Ruiz-García is the recipient of a Río Hortega grant CM20/00225 from the Institute de Salud Carlos III and co-funded by Fondo Social Europeo 2014–2020. Laura Saso-Jimenez is the recipient of a predoctoral grant from the Education Department of the Basque GovernmentPRE_2020_1_0282). This work was partially supported by a grant from the Department of Health of the Basque Country Government (2019111064).
Conflict of interestThe authors have no conflicts of interest to declare for this work.