TSH is the parameter most widely accepted to assess thyroid function, especially in pregnant women. The aim of this current study was to analyze intra-individual changes in TSH during the first half of pregnancy in women with TSH levels higher than 2.5mIU/L in early pregnancy.
MethodsAn observational, prospective study was conducted on 243 healthy pregnant women in the first trimester of pregnancy. Thyroid function was assessed by testing TSH and free T4 levels. A subgroup of women with TSH levels >2.5mIU/L underwent additional tests (TSH, free T4, peroxidase antibodies). Information on dietary iodine intake and/or iodine supplements was also recorded.
ResultsMean TSH level was 1.89mIU/L (range 0.024–6.48mIU/L), and mean FT4 level was 1.19ng/dL (range 0.80–1.90ng/dL). Fifty-eight women (23.8%) had TSH levels>2.5mIU/L in the first trimester of pregnancy, and additional thyroid function tests were performed in 27 women. TSH levels significantly decreased from the first to the second test (3.59±0.92mIU/L vs 2.81±1.06mIU/L respectively; p<0.01), and the decrease was significantly greater in pregnant women who used iodized salt as compared to those who did not (1.16±0.65mIU/L vs 0.19±0.93mIUI/L respectively; p<0.01). A positive correlation was found between the time elapsed to the second measurement (24.3±17.2 days; range 8–58) and the decrease in TSH levels (r=0.40; p=0.038).
ConclusionTSH levels showed a continuous, uniform decrease during the first half of pregnancy in women with values slightly above the normal range. Pregnant women who used iodized salt were more likely to have decreased TSH levels in a second test.
La TSH es el parámetro más aceptado para evaluar la función tiroidea, especialmente en mujeres embarazadas. El objetivo del presente estudio fue analizar los cambios intraindividuales de TSH durante la primera mitad de la gestación, en aquellos casos en los que la TSH en las primeras etapas de la gestación fue superior a 2,5 mUI/L.
MétodosEstudio observacional prospectivo que incluyó a 243 mujeres embarazadas sanas en el primer trimestre de gestación. Se estudió función tiroidea mediante TSH y T4 libre. Un subgrupo de mujeres con TSH> 2,5 mUI/L fueron sometidas a un segundo análisis (TSH, T4 libre, anticuerpos antiperoxidasa). También se registró información sobre la ingesta de yodo con la dieta y/o suplementos.
ResultadosLa TSH media fue de 1,89 mUI/L (rango 0,024-6,48 mUI/L), y la T4 libre media fue de 1,19 ng/dL (rango 0,80-1,90ng/dL). El 23,8% (58 mujeres) presentaron TSH> 2,5 mUI/L en el primer trimestre de gestación, realizándose una segunda valoración en 27 pacientes. La TSH disminuyó significativamente del primer al segundo análisis (3,59±0,92 mUI/L vs. 2,81±1,06 mUI/L respectivamente, p <0,01). La TSH disminuyó significativamente más en aquellas mujeres embarazadas que consumieron sal yodada que en aquellas que no lo hicieron (1,16±0,65 mUI/L vs. 0,19±0,93 mUI/L respectivamente, p<0,01). Hubo una correlación positiva entre el tiempo transcurrido para una segunda determinación (24,3±17,2 días, rango 8-58 días), y la reducción en los niveles de TSH (r= 0,40; p=0,038).
ConclusiónLa disminución de los niveles de TSH con la edad gestacional es uniformemente continua a lo largo de la primera mitad de la gestación en aquellos casos con TSH ligeramente por encima del rango sugerido de normalidad. Las mujeres embarazadas que consumían sal yodada tenían más probabilidades de reducir los niveles de TSH en un segundo análisis.
Thyroid hormones (TH) are involved in essential functions such as somatic growth, metabolic regulation and neurodevelopment, so they play a pivotal role during gestation.1–3 The developing embryo/fetus is extremely dependent on maternal supply of TH, particularly in the first trimester of pregnancy, since its thyroid gland will be non-functional until mid gestation.4
A growing body of evidence supports the association of maternal thyroid dysfunction (even mild to moderate dysfunctions) in early stages of fetal development and the incidence of obstetric and/or perinatal complications.5 This concern has led scientific societies to develop several clinical guidelines over the last few years.6–8
Although the dilemma between a universal thyroid screening or a case-finding strategy for pregnant women remains controversial9 and there is no firm consensus on how thyroid dysfunction should be diagnosed, the main interest has focused on establishing reliable, trimester-specific cut-off values of thyroid function parameters which allow to identify the potential risk of maternal and/or neonatal adverse outcomes.10
It has been recommended to define population-based trimester-specific reference intervals; however, only iodine sufficient women without anti-thyroid peroxidase (anti-TPO) antibodies should be included in the standardizing population,11 and many commercial laboratories still do not provide these reference ranges.12
In 2011 the American Thyroid Association (ATA) proposed, in absence of specific reference intervals, a 2.5mUI/L cut-off value for the first-trimester.6 Since then, different studies worldwide have demonstrated that TSH and FT4 levels in several settings are outside the normal trimester-specific reference ranges used for United States population,13 resulting in potential overdiagnosis of hypothyroidism in those populations when the cut-off values proposed by the ATA are used.14
During the first trimester of gestation, the TSH levels are partially suppressed due to the stimulatory effect of human chorionic gonadotrophin (hCG) on the thyroid gland. The suppressive effect of hCG on TSH in the first trimester can be modulated by different factors such as thyroid autoimmunity, body mass index, smoking habit, age or parity.15,16
At the same time, a recent study has shown how widely TSH reference limits differ within the first trimester of pregnancy.17 While the lower TSH in weeks 9–12 of pregnancy are evidently explained by the high hCG production, considerably higher TSH values were observed earlier than 6 weeks of gestation, which are similar to non-pregnancy reference limits.
On the other hand, some studies have demonstrated that TSH levels show high within-person consistency between trimesters, though individual trends for each woman should be taken into account.18,19
Given this background, our aim was to evaluate the intra-individual variation of TSH during the first half of gestation in those cases of “potentially high” TSH in the first trimester.
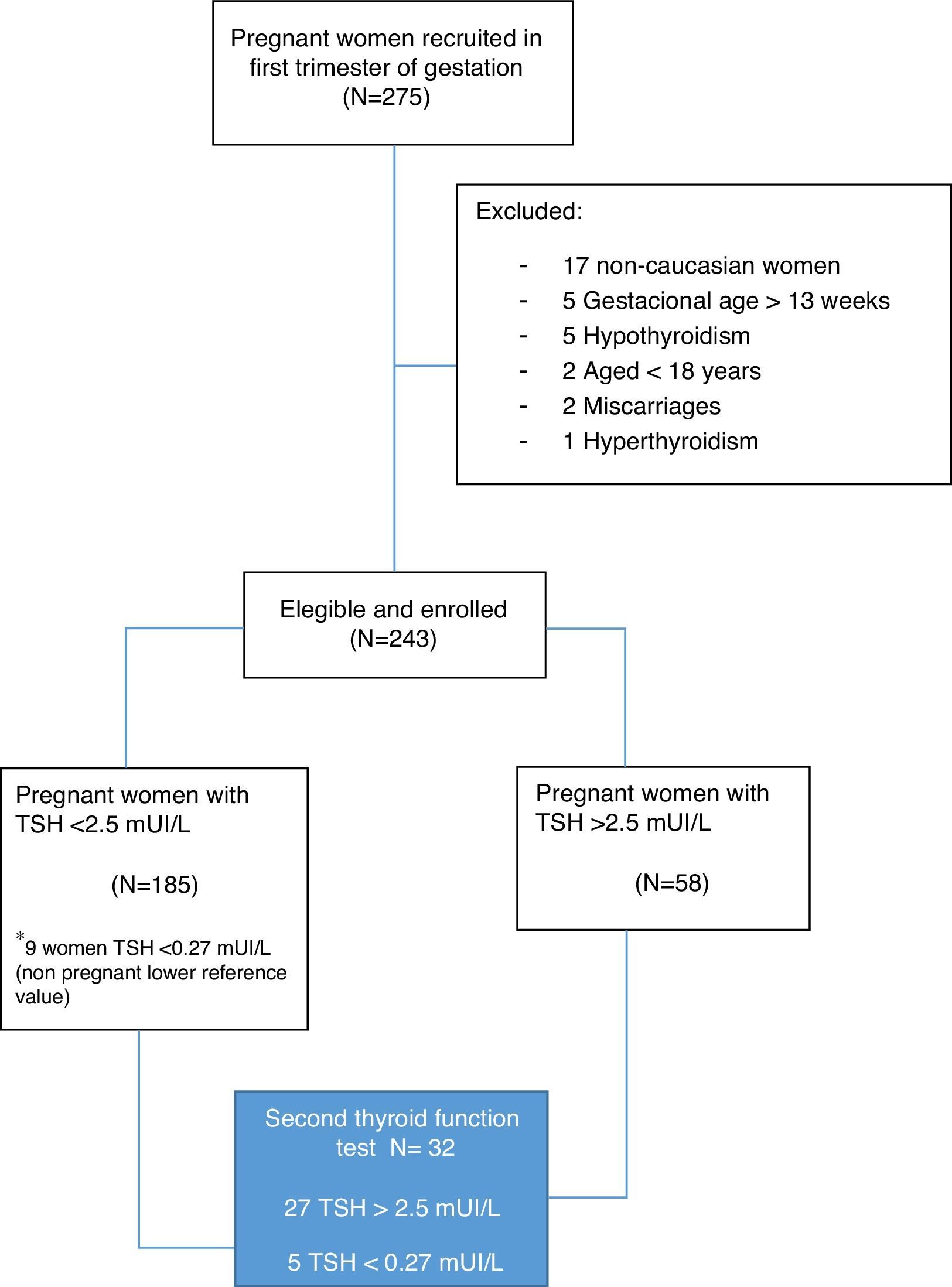
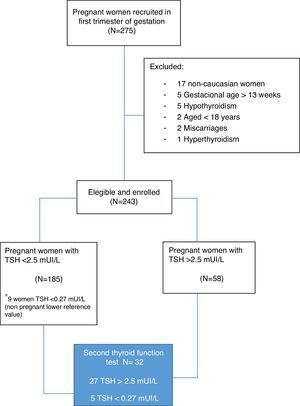
Subjects and methodsStudy subjectsWe carried out a longitudinal study on 243 healthy Caucasian women at the Hospital Universitario de La Ribera (HULR), Alzira (Valencia, Spain), at their first antenatal visit (gestational weeks 5–13), from September 2014 to January 2015. Inclusion criteria were age 18 or older, caucasian ethnicity, and gestational age up to and including week 13 of amenorrhea. All participants underwent an obstetric examination to exclude maternal and/or fetal risk factors.
Refusal to participate, non-caucasian ethnic groups, gestational age out of first trimester, high-risk pregnancies, personal history of thyroid or any other endocrine disorders and/or use of drugs which interfere with iodine metabolism were considered as exclusion criteria.
For those pregnant women who had a TSH>2.5mUI/L at the first visit (23.8%), a subsequent follow-up and a second thyroid function test were performed in 27 patients (Fig. 1). The second test included the study of anti-thyroid peroxidase (anti-TPO) antibodies.
Women enrolled were asked specifically about their use of supplements containing potassium iodide (KI), iron, folic acid and/or multivitamins during the pregnancy. The consumption of iodine-rich foods was assessed through a food frequency questionnaire (FFQ) previously validated in Spanish population.20
All pregnant women provided fasting peripheral venous blood samples from an antecubital vein early in the morning. Samples were centrifuged and serum was stored at −40°C until the analysis.
The study was approved by the Ethics and Clinical Research Committee of the Hospital Universitario de La Ribera and written informed consent was obtained from all the participants. This work complies with the principles laid down in the Declaration of Helsinki.
Laboratory proceduresThe TSH and Free T4 (FT4) were measured by chemiluminescence through the ADVIA Centaur immunoassay system (Siemens Healthcare Diagnostics, Germany). For TSH, the analytical measurement range given by the manufacturer was 0.27–5mUI/L, and the low, medium and high coefficient of variation (CV) was 4.45%, 3.77% and 5.17% respectively. For FT4, the analytical measurement range given by the manufacturer was 0.9–1.7ng/dL, and the low, medium and high coefficient of variation (CV) was 4.16%, 4.58% and 3.44% respectively. Anti-thyroid peroxidase (anti-TPO) was measured by a radioimmunometric assay (Immulite 2000; Siemens Healthcare Diagnostics, Germany). Anti-TPO were considered positive if the titer was >35mIU/L.
Statistical analysisThe quantitative variables were measured as the mean±standard deviation or mean (range) and the qualitative variables as percentages. The contrast hypothesis for two samples was evaluated with the Student's t-test for quantitative variables and Chi-squared test in cases of categorical ones. In the event that variables did not adjust to normality, a Kruskall–Wallis test was done. For the adjustment of the model for other variables, two- or multi-way analysis of the variance (ANOVA) were designed, introducing the continuous variables as covariables. The correlation between variables was determined using the Spearman test, designing multiple regression models in those cases where it was desired to predict the variance adjusted for other variables, besides the main variable. In all cases the rejection level for a null hypothesis was α<0.05.
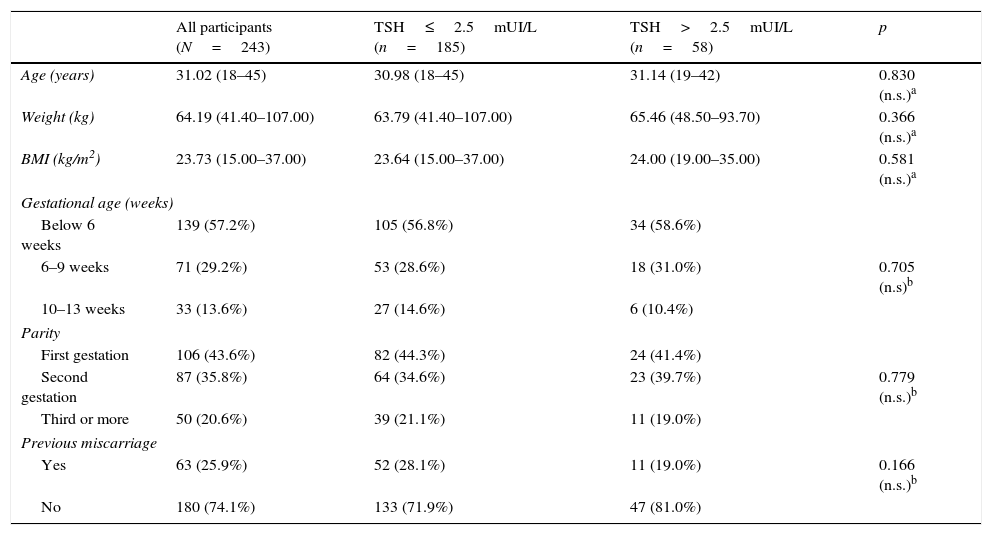
ResultsThe clinical characteristics of the pregnant women included in the study are shown in Table 1. All women who were eventually included had low risk gestations as assessed by obstetrical and ultrasound scan at the enrollment time. We found no differences in clinical variables of the pregnant women according to the initial level of TSH (up to 2.5mUI/L or above this level).
Main characteristics of participants and comparison between the two groups (according to TSH levels).
| All participants (N=243) | TSH≤2.5mUI/L (n=185) | TSH>2.5mUI/L (n=58) | p | |
|---|---|---|---|---|
| Age (years) | 31.02 (18–45) | 30.98 (18–45) | 31.14 (19–42) | 0.830 (n.s.)a |
| Weight (kg) | 64.19 (41.40–107.00) | 63.79 (41.40–107.00) | 65.46 (48.50–93.70) | 0.366 (n.s.)a |
| BMI (kg/m2) | 23.73 (15.00–37.00) | 23.64 (15.00–37.00) | 24.00 (19.00–35.00) | 0.581 (n.s.)a |
| Gestational age (weeks) | ||||
| Below 6 weeks | 139 (57.2%) | 105 (56.8%) | 34 (58.6%) | |
| 6–9 weeks | 71 (29.2%) | 53 (28.6%) | 18 (31.0%) | 0.705 (n.s)b |
| 10–13 weeks | 33 (13.6%) | 27 (14.6%) | 6 (10.4%) | |
| Parity | ||||
| First gestation | 106 (43.6%) | 82 (44.3%) | 24 (41.4%) | |
| Second gestation | 87 (35.8%) | 64 (34.6%) | 23 (39.7%) | 0.779 (n.s.)b |
| Third or more | 50 (20.6%) | 39 (21.1%) | 11 (19.0%) | |
| Previous miscarriage | ||||
| Yes | 63 (25.9%) | 52 (28.1%) | 11 (19.0%) | 0.166 (n.s.)b |
| No | 180 (74.1%) | 133 (71.9%) | 47 (81.0%) | |
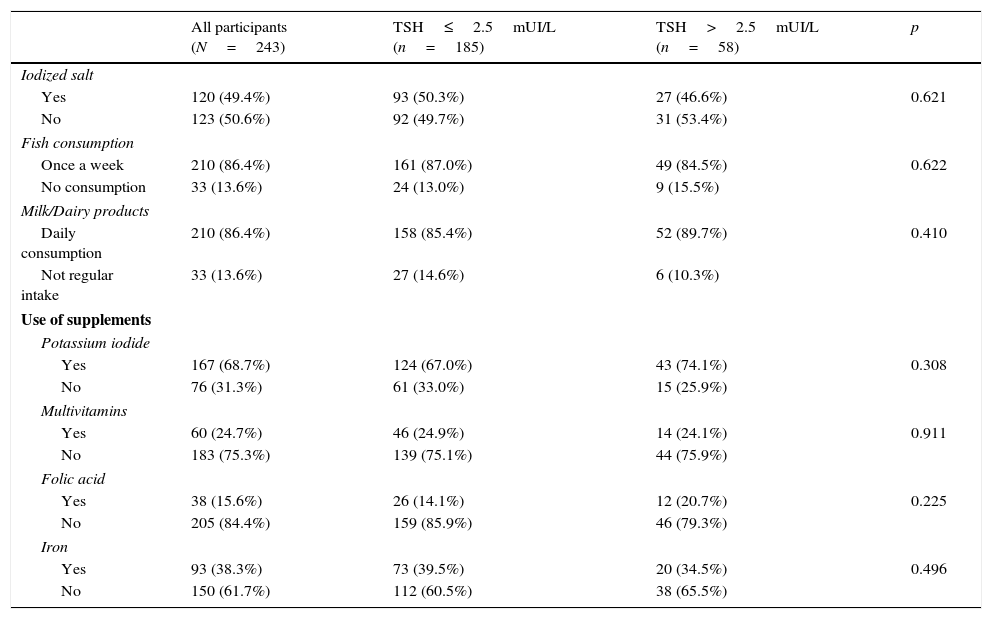
In order to find out the iodine intake, all pregnant women were asked about the consumption of iodine-rich foods through a FFQ. They also declared their use of supplements during pregnancy (KI, folic acid, multivitamins and/or iron). No differences were found in the consumption of iodized salt, fish or dairy products between pregnant women with normal TSH and those who had TSH >2.5mUI/L (Table 2).
Iodine intake, use of supplements and comparison between the two groups (according to TSH levels).
| All participants (N=243) | TSH≤2.5mUI/L (n=185) | TSH>2.5mUI/L (n=58) | p | |
|---|---|---|---|---|
| Iodized salt | ||||
| Yes | 120 (49.4%) | 93 (50.3%) | 27 (46.6%) | 0.621 |
| No | 123 (50.6%) | 92 (49.7%) | 31 (53.4%) | |
| Fish consumption | ||||
| Once a week | 210 (86.4%) | 161 (87.0%) | 49 (84.5%) | 0.622 |
| No consumption | 33 (13.6%) | 24 (13.0%) | 9 (15.5%) | |
| Milk/Dairy products | ||||
| Daily consumption | 210 (86.4%) | 158 (85.4%) | 52 (89.7%) | 0.410 |
| Not regular intake | 33 (13.6%) | 27 (14.6%) | 6 (10.3%) | |
| Use of supplements | ||||
| Potassium iodide | ||||
| Yes | 167 (68.7%) | 124 (67.0%) | 43 (74.1%) | 0.308 |
| No | 76 (31.3%) | 61 (33.0%) | 15 (25.9%) | |
| Multivitamins | ||||
| Yes | 60 (24.7%) | 46 (24.9%) | 14 (24.1%) | 0.911 |
| No | 183 (75.3%) | 139 (75.1%) | 44 (75.9%) | |
| Folic acid | ||||
| Yes | 38 (15.6%) | 26 (14.1%) | 12 (20.7%) | 0.225 |
| No | 205 (84.4%) | 159 (85.9%) | 46 (79.3%) | |
| Iron | ||||
| Yes | 93 (38.3%) | 73 (39.5%) | 20 (34.5%) | 0.496 |
| No | 150 (61.7%) | 112 (60.5%) | 38 (65.5%) | |
Mean TSH was 1.89mUI/L (range 0.024–6.48mUI/L). From 243 women, 185 had normal values of TSH and 58 had values >2.5mUI/L (1.44±0.58mUI/L vs 3.36±0.85mUI/L respectively, p<0.01). Mean FT4 was 1.19ng/dL (range 0.80–1.90ng/dL). No significant differences were found in FT4 levels between women with normal TSH and those who had TSH>2.5mUI/L (1.19±0.16ng/dL vs 1.17±0.18ng/dL respectively; p=0.58).
There was no difference in means of TSH according to the intake of iodized salt, fish or dairy products (data not shown). The consumption of KI, multivitamins, folic acid or iron did not show significant differences in TSH and FT4 levels (data not shown).
TSH showed a weak correlation with body mass index (r=0.12; p=0.04) but not with other variables such as maternal age, weight, parity or gestational age. FT4 did not show any correlation with other variables.
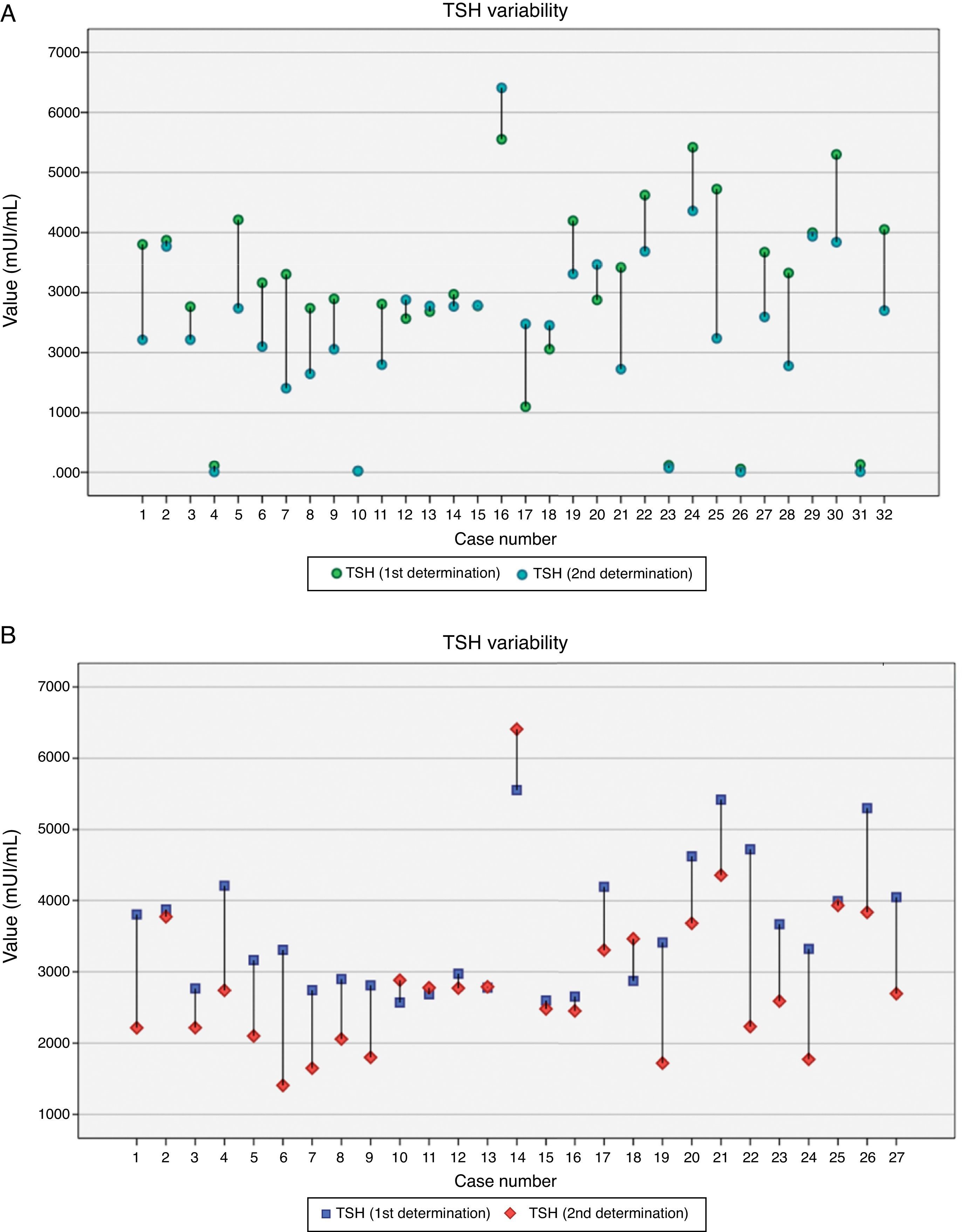
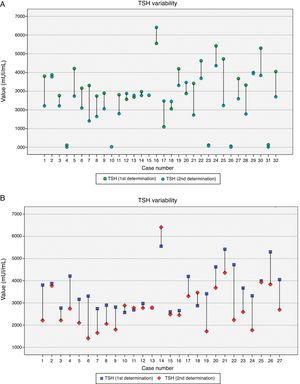
On 27 women who showed TSH >2.5mUI/L, a second test was performed which included TSH, FT4 and anti-TPO antibodies. Only 2 cases of anti-TPO >35UI were found. TSH decreased significantly from the first to the second analysis (3.59±0.92mUI/L vs 2.81±1.06mUI/L respectively; p<0.01) but there was a moderate correlation between both figures (r=0.66; p<0.01). Fig. 2 shows the variability of TSH within each participant. FT4 levels remained steady (1.16±0.16ng/dL vs 1.07±0.29ng/dL respectively; p=0.21) and no correlation between both measurements was found. Elapsed time between the first and the second analysis ranged from 8 to 58 days (24.3±17.2, median 16 days), and it was individualized taking into account previous TSH level. There was a positive correlation between the elapsed time and the reduction in TSH levels (r=0.40; p=0.038). The regression model shows how TSH decreased 0.022mUI/L per day elapsed from the first to the second analysis.
The mean difference between first and second TSH was −0.69mUI/L (ranged from −2.49 to 1.38mUI/L). No significant differences in TSH decrease were found according to gestational age at the first analysis (below 6 weeks, 6–9 weeks and 10–13 weeks). TSH dropped more significantly in those pregnant women who consumed iodized salt than in those ones who did not (1.16±0.65mUI/L vs 0.19±0.93mUI/L respectively; p<0.01), but the difference was not significant with regard to iodine supplements (data not shown). After a second thyroid test, levothyroxine treatment was introduced in 18.5% (5/27) of pregnant women.
DiscussionThis is the first study that analyzes the evolution and intra-individual variability of TSH along the first half of gestation when it is >2.5mUI/L in healthy pregnant women. Our main findings are as follows: (a) TSH levels uniformly decrease along the first trimester of gestation in cases slightly above the normal range, and (b) pregnant women who consumed iodized salt were more likely to reduce TSH levels in a second analysis.
The study of thyroid function in first trimester of pregnancy has received special attention in the last decade, in order to prevent fetal and maternal complications and the adverse effects on neurocognitive development of offspring.21 Nevertheless, the growing understanding of thyroid function in early stages of gestation highlight the practicalities of the thyroid function tests in pregnancy.22
During the first trimester, human chorionic gonadotropin (hCG) induces a transient lowering of TSH concentrations. However, recent studies have shown how hCG levels can be significantly influenced by maternal smoking, BMI, parity, ethnicity, fetal gender, placental weight and/or hyperemesis gravidarum symptoms.23 The practical implications of these observations are that TSH concentrations can consequently vary according to these factors, even within the same population.
Furthermore, gestational age has a crucial effect along the first trimester: while hCG reaches a peak at 9–11 weeks coincident with a fall in TSH, TSH concentration at 4–6 weeks of gestation is the same as in non-pregnant women.13,17 In our study, half of the pregnant women who had TSH >2.5mUI/L were tested before 6 weeks of gestation.
Different studies24,25 have demonstrated that TSH concentrations decrease significantly from the seventh week of pregnancy to their lowest point between gestational weeks 10 and 11. The mean time elapsed between first and second TSH determination in our work was 24.3 days, presumably coinciding with the decrease in TSH to its lowest point to the end of the first trimester. In this regard, our results are consistent with previous studies and also support the conclusion that the use of uniform limits of TSH for the entire first trimester may lead to frequent misclassification17 and, what is more serious, the indication of inappropriate treatment with thyroxine.26
TSH local reference values for first trimester of gestation (0.128–4.455mUI/L) were obtained in parallel with the last period of this study, therefore justifying the fact that in many cases TSH was not repeated.
On the other hand, our study shows that those pregnant women who consumed iodized salt had lower levels of TSH in their second analysis, but this effect did not appear in cases of supplementation with KI. In this regard, Moleti et al.27 compared thyroid function during pregnancy in three groups of pregnant women: women who had taken iodized salt at least for 2 years before becoming pregnant, others who took 150μg of KI and a third group who did not take iodized salt nor KI supplements. The lowest TSH concentrations were consistently observed in the iodized salt group. Santiago et al.28 also found lower TSH levels in the first trimester in women who were taking iodized salt compared to those who took KI supplements, although the difference was not significant (p=0.06).
The present study is constrained by some limitations: although we only studied the cases with TSH >2.5mUI/L, our results were consistent with previous studies with larger sample sizes. The lack of urinary iodine concentration (UIC) did not allow us to search for potential correlations between thyroid function and iodine status. However, the intake of iodized salt was associated with a significant effect on TSH decrease which has not been seen in cases of consumption of KI supplements.
To the best of our knowledge this is the first study to demonstrate that TSH declines uniformly along the first trimester, so exactly the same women can be classified in normal or pathological TSH values only depending on their gestational age at the time of thyroid test. This fact reinforces the need to establish a precise timing to study thyroid function in the first trimester of gestation, necessarily adjusted by gestational weeks, in order to avoid incorrect diagnosis and overtreatment.
Authors’ contributionsMTM, and CFM designed the study and MTM, RVC, and CFM and JGV conducted fieldwork. MTP, MPB and IV entered the data for analyses. MPB and IV conducted the statistical analyses. MTM, MPB, RVC, CFM and IV wrote the first draft of the manuscript and incorporated suggestions from all the coauthors. All the authors have read and approved the final version of this article.
FundingThis research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Conflict of interestThe authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
The authors are grateful to pregnant women who accepted to participate in the study. We are also grateful to Mario Ortuño, and the whole Midwifery team from the Obstetric and Gynecology Department, Hospital Universitario de la Ribera.