Bariatric surgery (BS) is an effective treatment. However, there have been concerns regarding the negative effect on the bone. The aim of this study was to assess changes in bone metabolism and the risk of fracture after biliopancreatic diversion (BPD).
Material and methodsA retrospective analysis of obese patients undergoing BPD between 1998 and 2017 was conducted, and patients with at least 1 year of follow-up were included. The incidence of fracture and of changes in bone metabolism was studied.
ResultsIn total, 216 patients were included (78.2% female), with a mean age of 42.5(10.6) years. The median follow-up was 6.8(IQR 10.2–3.2) years. The mean body mass index (BMI) was 49.7(6.3) kg/m2. 13.2% (n=29) suffered a bone fracture after surgery; the time until the first fracture was 7.9(3.8) years (55.2% secondary to a casual fall). The rate of fracture incidence was 19.6 per 1000 person-years (95%CI: 1.3–2.7), prevalence was 13.4% (95%CI: 8.9–18.0). The risk of bone fractures seems to increase with longer postoperative evolution time. PTH (pg/ml) levels were significantly higher in patients with fractures (1 year, 98.1 vs. 77.8; 5 years, 162.5 vs. 110.3 p<0.05, adjusted HR 1.10; 95%CI 1.01–1.11). Subjects with a higher %EWL had less risk of fractures after surgery (adjusted HR 0.97; 95%CI 0.94–0.99). Moreover, 25(OH)D levels were lower, and osteocalcin and β-Crosslaps levels were slightly higher (not significant) in patients with fractures.
ConclusionBPD is related to important changes in bone metabolism, which can lead to an increased risk of bone fractures. Assessing the risk of fractures should be part of BS patient care.
La cirugía bariátrica (CB) es un tratamiento eficaz. Sin embargo, sus efectos negativos sobre el metabolismo óseo son pocos conocidos. El objetivo del estudio fue evaluar los cambios del metabolismo óseo y el riesgo de fracturas tras derivación biliopancreática (DBP).
Material y métodosEstudio retrospectivo en obesos sometidos a DBP entre 1998-2017. Se incluyó apacientes con seguimiento mínimo de un año y se estudió el riesgo de fracturas y los cambios en el metabolismo óseo.
ResultadosSe estudió an 216 sujetos (78,2% femenino), edad media 42,5(10,6) años, mediana de seguimiento fue 6,8 (rango intercuartílico 10,2-3,2) años. Media inicial de índice de masa corporal fue 49,7 (6,3) kg/m2; el 13,2% (n=29) presentó alguna fractura ósea poscirugía, la media de tiempo hasta la primera fractura fue 7,9 (3,8) años (55,2% secundarias a caída casual). La tasa de incidencia de fracturas fue 19,6 por 1.000 personas-año (IC del 95%, 1,3-2,7), prevalencia 13,4% (IC del 95%, 8,9-18,0). El riesgo de fracturas óseas parece aumentar a mayor tiempo de evolución tras CB. Los niveles de parathormona (pg/ml) fueron mayores en los pacientes con fracturas (al año 98,1 vs. 77,8; 5 años 162,5 vs. 110,3, p<0,05) HR ajustada 1,10, IC del 95%, 1,01-1,11. Un mayor porcentaje de sobrepeso perdido implicó menos riesgo de fracturas (HR ajustada 0,97; IC del 95%, 0,94-0,99). Los sujetos con fractura poscirugía presentaron niveles de 25(OH)D más bajos y cifras de β-Crosslaps y osteocalcina más elevadas.
ConclusiónLa DBP asocia importantes cambios en el metabolismo óseo, con probable aumento del riesgo de fracturas. La evaluación del riesgo de fracturas debe formar parte de la atención al paciente con CB.
Obesity is a major global health problem, triplicating its prevalence as compared to 1975.1 According to the (World Health Organization) WHO2 in its 2018 report, 1.9 billion adults≥18 years were overweight, of which 650 million are obese. Also, 381 million children between the ages of 5 and 19 were overweight and/or obese. Currently, surgical treatment of obesity is the most effective for long-term sustained weight loss, accompanied by a reduction in mortality and associated comorbidities. The recommendations for its use are the following: body mass index (BMI)>40kg/m2 or between 35 and 40kg/m2 with associated comorbidities.3
Bariatric surgery (BS) procedures can be restrictive, mixed, and malabsorptive, and it is usually effective but not risk free.4,5 One of the least known complications is the alteration of the phospho-calcium metabolism, especially in predominantly malabsorptive techniques. The most recent studies have suggested a negative effect on bone health at the expense of increased bone resorption and consequently, a decrease in bone mineral density.6–11 The mechanisms by which bone health is affected after weight loss in patients undergoing BS are not entirely clear; multiple theories have been put forward in an attempt to explain this alteration, with malabsorption caused by these procedures being the most studied.10,12–16
Biliopancreatic diversion (BPD), being a technique with a greater malabsorptive component and in spite of the exogenous contribution of calcium and vitamin D, seems to have more influence on the elevation of the markers of bone turnover and a greater reduction of bone mineral density.7,10,17 Consequently, these patients may have greater bone fragility, which in the long term may lead to a greater number of bone fractures in areas typical or atypical of osteoporosis or secondary to osteomalacia. In recent years, several retrospective studies have been published on the incidence of bone fragility fractures in patients undergoing bariatric surgery, mostly with less than 5 years of follow-up and with controversial results.8,18 Subsequently, in other reviews published with longer-term follow-up (between 5 and 15 years after BS), the results coincide because there is an increased risk of osteoporotic fractures.9,10,19 In addition, in their meta-analysis published in 2018, Zhang et al.6 also concluded that BS increases the risk of bone fractures, especially in atypical areas of osteoporosis.
Given the disparity of results on this subject and the concern that this problem represents in the long term, we plan to carry out this research, with the aim of determining the incidence of bone fractures and the evolution of bone metabolism in patients who underwent BPD in the long term.
Material and methodsA retrospective observational study of morbidly obese patients undergoing BPD at Complejo Asistencial Universitario de León (CAULE) was performed to determine the incidence of postoperative fractures. The study period was from January 1998 to December 2017. The BS performance criteria included age between 18 and 60 years, BMI>40kg/m2, or 35–40kg/m2 with comorbidities. Included in the study were patients with a minimum follow-up of 1 year in the Clinical Nutrition and Dietetics Unit, who agreed to respond to the telephone survey.
The BS technique performed was BPD.20,21 Follow-up was performed: presurgery, postsurgery, every 3 months in the first year, every 6 months in the second year, and annually thereafter. Data were collected on associated comorbidities, anthropometric parameters of weight, height, BMI, percentage weight lost (%WL=(weight initial−current weight)/(weight initial)×100), and percentage of excess weight lost [(%WL=%EWL=weight initial−current weight/initial weight−ideal weight)×100], bone metabolism analysis, and data from before and after BS fractures. To measure body weight, an electric bioimpedance scale Tanita TBF-300A (TANITA Corporation, Japan) was used, and length was measured with an analog measuring rod. Since the ideal weight does not coincide with the “normal” average weight of the population, the Metropolitan Life Insurance22 ideal weight charts were used. All data were collected using structured questionnaires and subsequently registered in a database. Being a retrospective study and given the difficulty of obtaining each patient's informed consent, this was not performed. However, verbal consent was obtained during the telephone survey.
Blood testAll analytical determinations were performed in the central laboratory of CAULE, using fresh serum collected while fasting. Having attained the results, the clinical analysis specialist was responsible for validating them and comparing them with reference values by age and sex (the equipment used was Cobas 800 Roche® Omega 3000 program). Calcium (mg/dl) was determined using direct colorimetry (Cresolphthalein complexone). The total serum calcium was corrected for serum albumin (corrected calcium). Intact PTH (1–84), 25-hidroxy-vitamina D [25(OH)D], Osteocalcin, and β-CrossLaps (ng/ml): PTH (pg/ml), osteocalcin, and β-Crosslaps were analyzed from a plasma sample with EDTA by immunochemiluminescence. Also, 25(OH)D was determined using fresh serum and subsequently kept in a tube without anticoagulant. The normal ranges of each parameter are: corrected calcium (8.5–10.5mg/dL), PTH (15–65pg/mL), 25(OH)D (30–100ng/mL), β-Crosslaps (0.1–0.4ng/mL), and osteocalcin (<26ng/mL).
Bone fracture dataTo collect the fracture data, a structured questionnaire was carried out with the variables necessary to compile the history of fracture risk. Parameters were collected from various tools: Frax®, complementing the data with the QFracture® tool and the European guide on the diagnosis and treatment of osteoporosis. Patients who met the initial inclusion criteria were contacted by telephone, verbal consent was first requested before asking the questionnaire questions, and the data obtained from the hospital medical history record were subsequently confirmed.
Statistical analysisStatistical analysis was performed with SPSS v.20 for Windows 10 Pro. Kolmogorov–Smirnov tests were used to assess the adjustment to a normal distribution, and data are shown as the mean (standard deviation) in this case or median (interquartile range [IQR]). The significance value used was p<0.05. Student's t test was used to compare the independent mean variables, ANOVA was used to compare the means, and chi-square was used for proportions. For correlations, Pearson correlation and Spearman's Rho were used. Cox proportional hazards regression was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of fractures after bariatric surgery. Further assessed interactions by age, sex, comorbidities, anthropometric parameters and bone metabolism. To observe changes in fracture risk across the follow-up period, we also assessed the HRs of fracture in the following times: 1, 2, 3–5, 6–9, ≥10 years after the index date.
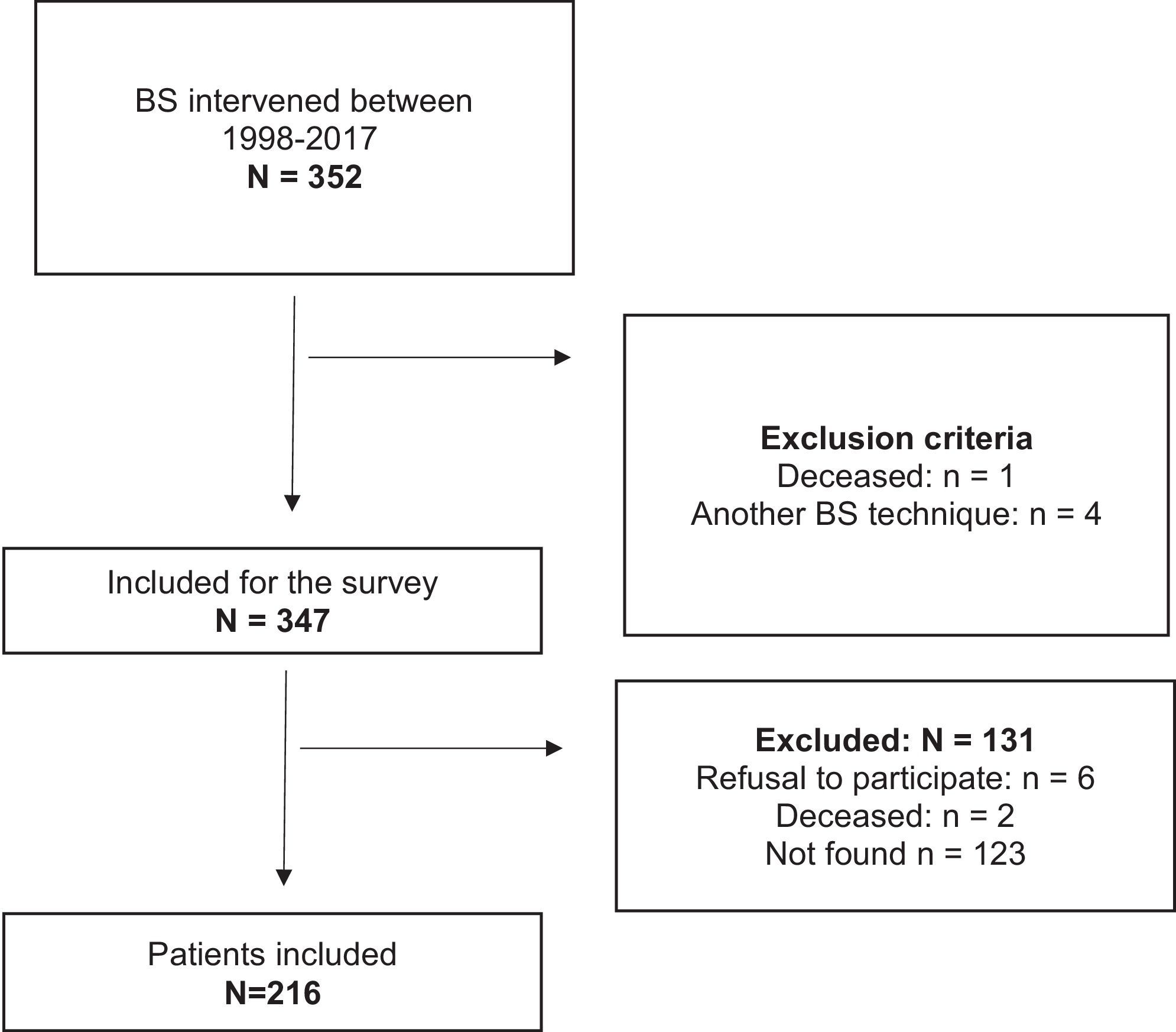
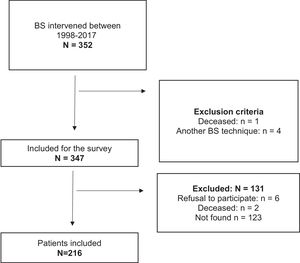
ResultsDuring the study period, 352 patients were intervened for BPD, and after applying the exclusion criteria, only 216 subjects were included (Fig. 1). Of this sample, 78.2% were female and the mean age was 42.5 (10.6) years, with a median follow-up of 6.8 (IQR 10.0–3.2) years. From all patients, 25.5% were smokers, and 71.3% (n=154) of individuals suffered from more than one associated comorbidity: 61% suffered from hypertension, 32.4% from type 2 diabetes (T2D), 30.1% from hypercholesterolemia, and 10.6% from chronic pulmonary disease. The mean initial BMI was 49.7 (6.3) kg/m2. Before surgery, 19.9% (n=43) had suffered some type of bone fracture during their lifetime, most of which occurred during childhood due to various traumatisms. Parameters of bone metabolism previous BS: calcium 9.1 (0.5) mg/dl, PTH 66.6 (29.5) pg/ml, osteocalcina 18.5 (8.7) ng/ml, β-Crosslaps 0.27 (0.14) ng/ml, 25(OH)D 20.6 (36.9–13.3) ng/ml. After BS, bone fractures occurred in 13.2% (n=29) of the patients: 82.8% were female, the mean age was 50.4 (12.5) years, and the mean time until the first fracture was 7.9 (3.8) years.
Fig. 2 describes the evolution of anthropometric data and analytical parameters of bone metabolism. The %WL ranged between 30 and 37% remained stable during the follow-up. A %EWL of more than 60% is objective, which remains stable throughout monitoring. PTH, osteocalcin, and β-Crosslaps show a tendency to increase from the first year after surgery, the latter two reaching their peak in the first year: osteocalcin: 47.3 vs. 18.5 (ng/ml) basal, β-Crosslaps 0.91 vs. 0.27 (ng/ml) basal. PTH reaches maximum levels at 8 years postsurgery: 129.1 (113.3) pg/ml, maintaining an inverse relationship to serum calcium corrected and 25(OH)D. Calcium: maximum association at the sixth year after surgery (Pearson correlation r=−0.49; p=0.000); 25(OH)D: maximum association at the fourth year after surgery (Rho Spearman −0.60; p=0.000). Levels of 25(OH)D were maintained in the deficit range (<30ng/ml) in all visits.
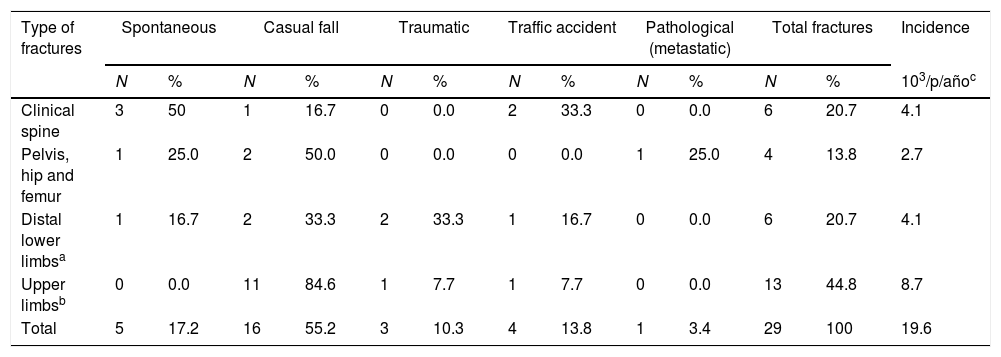
The incidence rate of postoperative bone fractures was 19.6 per 1000 person-years follow-up (95%CI: 1.3–2.7), with a prevalence of 13.4% (95%CI: 8.9–18.0). Of the sample, 10.4% (n=3) had more than one fracture episode during follow-up. The mean follow-up to the first fracture was at 7.9 years postsurgery. Most fractures occurred in the upper limbs 44.8% (n=13) and lower limbs 20.7% (n=6), in the spine 20.7% (n=6), and in the hip 13.8% (n=4). Amongst the causes of fractures, the most common was secondary to casual fall in 55.2% (n=16), followed by spontaneous in 17.2%, traffic accident in 13.8%, traumatic in 10.3%, and, finally, pathological or tumoral in 3.4%. See Table 1.
Description of the site and cause of bone fractures.
| Type of fractures | Spontaneous | Casual fall | Traumatic | Traffic accident | Pathological (metastatic) | Total fractures | Incidence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | 103/p/añoc | |
| Clinical spine | 3 | 50 | 1 | 16.7 | 0 | 0.0 | 2 | 33.3 | 0 | 0.0 | 6 | 20.7 | 4.1 |
| Pelvis, hip and femur | 1 | 25.0 | 2 | 50.0 | 0 | 0.0 | 0 | 0.0 | 1 | 25.0 | 4 | 13.8 | 2.7 |
| Distal lower limbsa | 1 | 16.7 | 2 | 33.3 | 2 | 33.3 | 1 | 16.7 | 0 | 0.0 | 6 | 20.7 | 4.1 |
| Upper limbsb | 0 | 0.0 | 11 | 84.6 | 1 | 7.7 | 1 | 7.7 | 0 | 0.0 | 13 | 44.8 | 8.7 |
| Total | 5 | 17.2 | 16 | 55.2 | 3 | 10.3 | 4 | 13.8 | 1 | 3.4 | 29 | 100 | 19.6 |
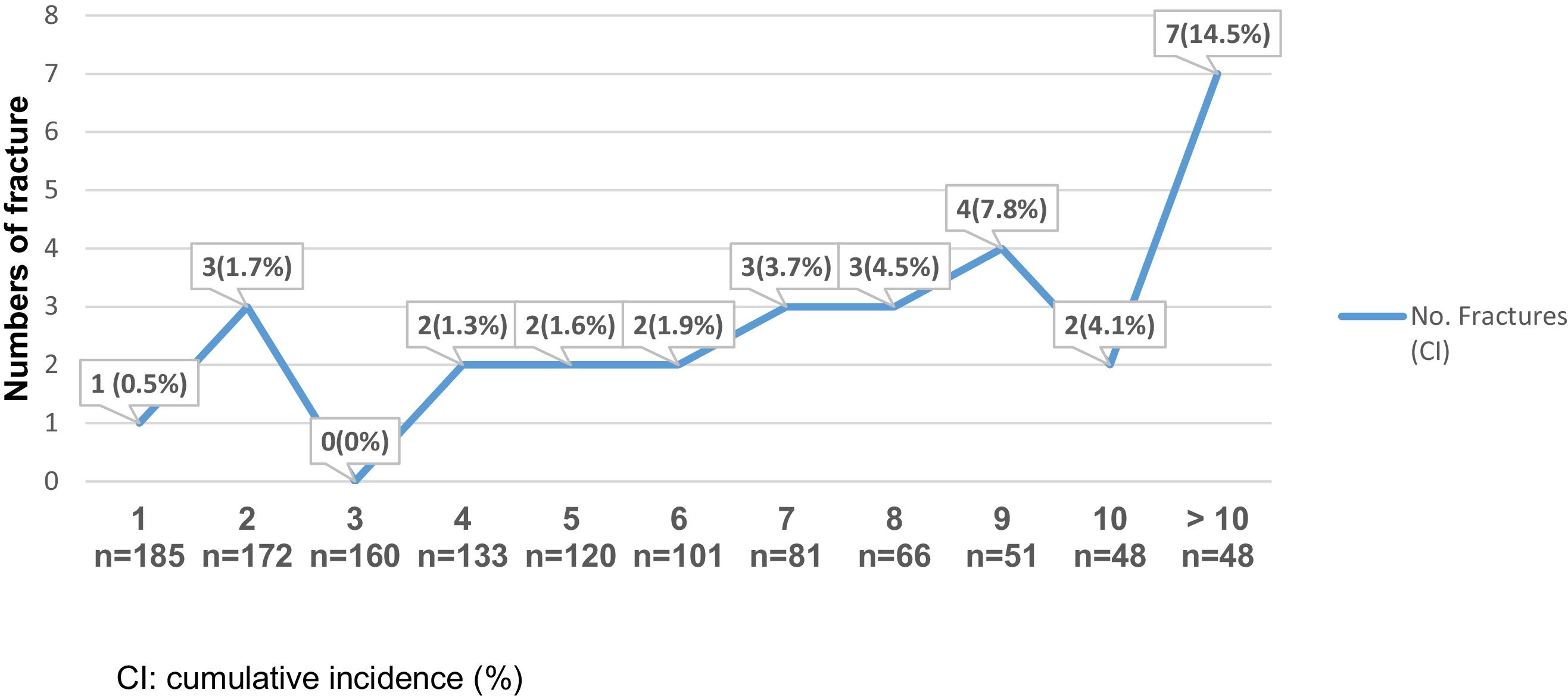
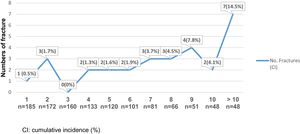
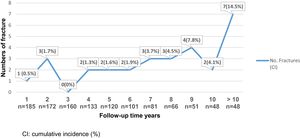
An increased risk of bone fractures to longer evolution time was observed, suffering from 0 to 3 fractures during the first 5 postoperative years (cumulative incidence 0–1.7%), between the fifth and tenth postoperative years, 2–4 new fractures episodes occurred each year. Also, for individuals with more than 10 postoperative years, 7 bone fracture events were reported (cumulative incidence 14.5%). See Fig. 3. The bivariate analysis did not show significant differences between age, sex and associated comorbidities in fractured versus unfractured patients.
Subjects who presented postsurgery bone fractures had a higher mean BMI (kg/m2): 35.2(5.2) vs. 32.2(5.1) p=0.03; 34.4(4.8) vs. 31.4(5.3) p=0.03) and lower %EWL (57.3% vs. 65.6% p=0.02; 59.5% vs. 67.6% p=0.04) than those unfractured patients in the first and second year after bariatric surgery. In terms of bone metabolism parameters, subjects with some type of postsurgery fracture had higher PTH (pg/ml) levels in the first years of follow-up. At 1 year: 98.1(50.1) vs. 77.8(32.8), p=0.04; 2 years: 130.2(115.6) vs. 92.8(51.5), p=0.03; 3 years: 142.8(128.7) vs. 98.9(57.8), p=0.07; 5 years: 162.5(106.4) vs. 110.3(76.0), p=0.03. 25(OH)D was lower in fractured patients, and the levels of β-Crosslaps and osteocalcin levels were slightly higher in comparison to the unfractured patients (without reaching statistical significance). %WL and corrected calcium levels do not differ in the groups.
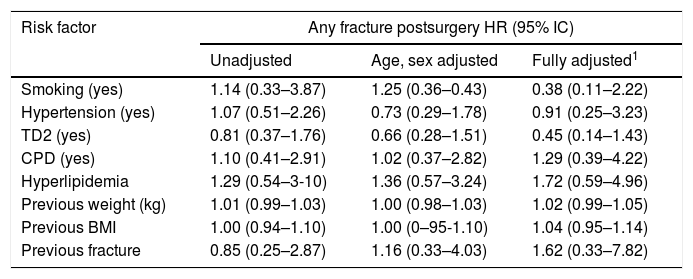
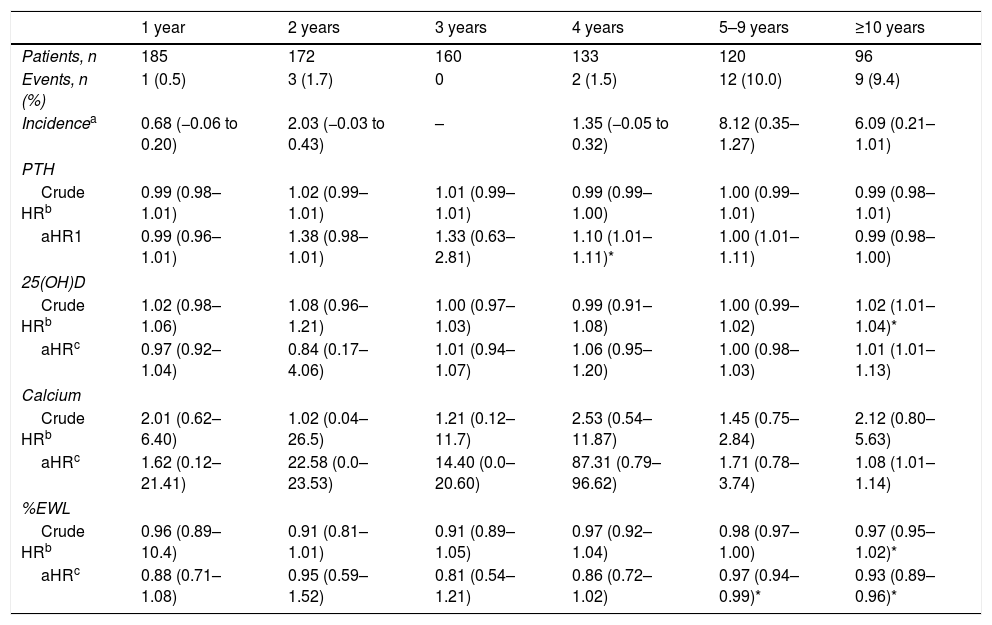
There was no association of fracture risk with sex, age, tobacco use, type 2 diabetes, hypertension, hyperlipidemia, chronic pulmonary disease, weight or BMI prior to surgery (unadjusted, sex, age adjusted, and fully adjusted). Likewise, the subjects with a history of fracture before undergoing bariatric surgery were not at significant risk of fracture after surgery (HR: 0.85, 95% CI: 0.25–2.87). See Table 2. In a univariate analysis, 25(OH)D was a risk factor for any fracture from the tenth year after surgery (Cox model, HR: 1.02; 95% CI: 1.01–1.04; p=0.01), and no significant interactions of adjusted HR were found. In a multivariate analysis, the risk of fractures was associated with increased levels of PTH at the fourth year after BS (adjusted HR: 1.10; 95% CI: 1.01–1.11; p=0.03). Subjects with a higher %EWL had less risk of fractures from the fifth year after surgery (adjusted Cox model HR 0.97; 95% CI 0.94–0.99; p=0.02). See Table 3.
Risk of any fracture in the patients receiving bariatric surgery.
| Risk factor | Any fracture postsurgery HR (95% IC) | ||
|---|---|---|---|
| Unadjusted | Age, sex adjusted | Fully adjusted1 | |
| Smoking (yes) | 1.14 (0.33–3.87) | 1.25 (0.36–0.43) | 0.38 (0.11–2.22) |
| Hypertension (yes) | 1.07 (0.51–2.26) | 0.73 (0.29–1.78) | 0.91 (0.25–3.23) |
| TD2 (yes) | 0.81 (0.37–1.76) | 0.66 (0.28–1.51) | 0.45 (0.14–1.43) |
| CPD (yes) | 1.10 (0.41–2.91) | 1.02 (0.37–2.82) | 1.29 (0.39–4.22) |
| Hyperlipidemia | 1.29 (0.54–3-10) | 1.36 (0.57–3.24) | 1.72 (0.59–4.96) |
| Previous weight (kg) | 1.01 (0.99–1.03) | 1.00 (0.98–1.03) | 1.02 (0.99–1.05) |
| Previous BMI | 1.00 (0.94–1.10) | 1.00 (0–95-1.10) | 1.04 (0.95–1.14) |
| Previous fracture | 0.85 (0.25–2.87) | 1.16 (0.33–4.03) | 1.62 (0.33–7.82) |
Events, n (%): 29 (13.2%), 19.6 per 1000 person years (95% CI 1.3–2.7) TD2: Type 2 Diabetes, CPD: Chronic Pulmonary disease, HR: hazard ratio, 1: adjusted for age, sex, smoking, hypertension, hyperlipidemia, type 2 diabetes, Chronic Pulmonary disease, previous weight, BMI and previous fracture.
Risk of any fracture in the patients receiving bariatric surgery by follow-up period.
| 1 year | 2 years | 3 years | 4 years | 5–9 years | ≥10 years | |
|---|---|---|---|---|---|---|
| Patients, n | 185 | 172 | 160 | 133 | 120 | 96 |
| Events, n (%) | 1 (0.5) | 3 (1.7) | 0 | 2 (1.5) | 12 (10.0) | 9 (9.4) |
| Incidencea | 0.68 (−0.06 to 0.20) | 2.03 (−0.03 to 0.43) | – | 1.35 (−0.05 to 0.32) | 8.12 (0.35–1.27) | 6.09 (0.21–1.01) |
| PTH | ||||||
| Crude HRb | 0.99 (0.98–1.01) | 1.02 (0.99–1.01) | 1.01 (0.99–1.01) | 0.99 (0.99–1.00) | 1.00 (0.99–1.01) | 0.99 (0.98–1.01) |
| aHR1 | 0.99 (0.96–1.01) | 1.38 (0.98–1.01) | 1.33 (0.63–2.81) | 1.10 (1.01–1.11)* | 1.00 (1.01–1.11) | 0.99 (0.98–1.00) |
| 25(OH)D | ||||||
| Crude HRb | 1.02 (0.98–1.06) | 1.08 (0.96–1.21) | 1.00 (0.97–1.03) | 0.99 (0.91–1.08) | 1.00 (0.99–1.02) | 1.02 (1.01–1.04)* |
| aHRc | 0.97 (0.92–1.04) | 0.84 (0.17–4.06) | 1.01 (0.94–1.07) | 1.06 (0.95–1.20) | 1.00 (0.98–1.03) | 1.01 (1.01–1.13) |
| Calcium | ||||||
| Crude HRb | 2.01 (0.62–6.40) | 1.02 (0.04–26.5) | 1.21 (0.12–11.7) | 2.53 (0.54–11.87) | 1.45 (0.75–2.84) | 2.12 (0.80–5.63) |
| aHRc | 1.62 (0.12–21.41) | 22.58 (0.0–23.53) | 14.40 (0.0–20.60) | 87.31 (0.79–96.62) | 1.71 (0.78–3.74) | 1.08 (1.01–1.14) |
| %EWL | ||||||
| Crude HRb | 0.96 (0.89–10.4) | 0.91 (0.81–1.01) | 0.91 (0.89–1.05) | 0.97 (0.92–1.04) | 0.98 (0.97–1.00) | 0.97 (0.95–1.02)* |
| aHRc | 0.88 (0.71–1.08) | 0.95 (0.59–1.52) | 0.81 (0.54–1.21) | 0.86 (0.72–1.02) | 0.97 (0.94–0.99)* | 0.93 (0.89–0.96)* |
%EWL: percentage of excess weight loss; PTH: parathyroid hormone, 25(OH)D: 25 hidroxi-vitaminD.
Currently, few studies have described the risk of fracture after BS and the results obtained to date are controversial. In this study we observed an incidence of 19.6 per 1000 person-years of follow-up (95%CI: 1.3–2.7) and a prevalence of 13.4% (95%CI 8.9–18.0). The risk of bone fractures increased to longer surgery evolution time. It should be noted that this study highlights a pattern of bone fractures in typical osteoporotic areas23 (greater frequency in the upper limbs, spine and hip, pelvis, and femur).
The Spanish population presents a medium risk of bone fractures due to fragility. The probability of presenting a hip fracture is lower than in the United States and Northern European countries.24 The study published by Alvarez-Nebreda et al.25 in 2008 estimated the incidence of hip fracture between 125.9–353.0 per 100,000 person-years over 65, and the incidence of hip fracture found in our series was similar with 2.7 per 1000 person-years (270.0 per 100,000 person-years). However, it should be noted that the mean age of the patients in this study was under 50 vs 65 years of age. As for vertebral fracture, it is usually infrequent in patients under 50 years of age, according to the European Prospective Osteoporosis Study (EPOS)26 and it presents an annual incidence of 1–3 in women and 5.7–6.8 in men per 1000 person-years over 65 years of age. If we compare our data with those of EPOS, it could be said that they are similar (incidence of 4.1 per 1000 person-years). However, the shorter longevity of this month should be highlighted again.
Comparison with other studiesNakamura et al.10 conducted a retrospective study (n=258) undergoing Roux-en-Y gastric bypass with a mean follow-up of 7.7 years, in which they studied the risk of fractures compared with the general U.S. population, described a history of fracture in 36% of presurgery, and 51% (n=132) of postsurgery patients, and the median time to first fracture was at 13 years of follow-up. They concluded that there is an early and sustained increase in the cumulative incidence of fractures in the BS group compared to what was expected (58 vs. 24%) respectively, with an increase in the overall fracture rate by more than double (RR 2.3; 95%CI 1.8–2.8). In our study, the mean time to the first fracture occurred much earlier (7.9 years postsurgery). This could be explained by the type of technique used (BPD), which involves a greater malabsorptive component and may cause more significant changes in bone metabolism. The percentage of patients with bone fractures was high but lower than that observed by Nakamura: 19.9% vs. 36% before surgery and 13.4% vs. 58% postsurgery.
In the United Kingdom, Lalmohamed et al.8 studied a total of 2079 obese patients undergoing BS (60% with adjustable gastric banding) to assess the risk of fracture compared with a control group during a mean of 2.2 years of follow-up, they concluded that there is no increased risk in the general population ratio of any type of fracture (8.8 vs 8.2 per 1000 person-years; RR 0.89 95%CI 0.6 to 1.3) compared to the control group. These results differ from those observed in this series, where we do find an increased risk of bone fractures. These differences may be caused by the type of technique used and the short follow-up time. Vertical gastrectomy carries less risk of malabsorption Regarding the short follow-up period, in this type of patient, 2.2 years is not enough time to assess the risk of fractures after BS. In our study, the increase in bone fractures was more evident after 10 years postsurgery.
Rousseau et al.9 developed a retrospective study of cases and controls to study the risk of bone fractures in patients undergoing BS compared with two groups of subjects (obese and non-obese). They studied a sample of n=12,676 (72,3 were women, and the mean age was 42.6 years), demonstrating that patients undergoing BS had a higher risk of fractures compared to the controls (RR 1.4 95%CI: 1.2–1.5), with a frequency of 4.1% of fractures and a mean time until the first fracture of 3.9 years, being higher in typical areas of osteoporosis (upper limbs, spine, and hip). In addition, this relationship was only observed in patients undergoing BPD; however, this was not conclusive in the remaining patients undergoing gastrectomy or gastric bypass due to the small number of cases and the short follow-up time. The fracture percentage was higher in our series 19.9% vs. 4.1% with a mean time until the first fracture of 7.9 years.
Regarding the anthropometric data from BMI and %EWL and their relationship to bone fractures, the data are more controversial. Subjects with some event of fractures during follow-up presented higher BMI and lower decrease in %EWL than patients who did not present any fracture: at one year: BMI 35.2 vs. 32.2kg/m2, p=0.03; %EWL 57.3% vs. 65.5%, p=0.03; 2 years postsurgery: BMI 34.4 vs. 31.5kg/m2, p=0.03; %EWL 59.5% vs. 67.6%. In addition, subjects with a higher %EWL had less risk of fractures from the fifth year after surgery (adjusted Cox model HR 0.97; 95% CI: 0.94–0.99; p=0.02). Some studies on the risk of fractures and BS have proposed a ratio of fracture protection to greater %EWL.27 The association of this study between lower loss of BMI and %EWL with increased risk of fractures may be related to poor adherence to lifestyle changes, calcium and vitamin D supplementation, which in a malabsorptive technique would be crucial to produce increased risk in the rate of bone fractures. Rousseau et al.9 also suggested that patients with higher BMI had more fracture episodes, hypothesizing that obesity may not be as protective for fractures as originally thought.
So far, most studies on bariatric surgery and its bone effects have been based on the evolution of resorption markers and bone mineral density, agreeing that BS caused negative changes in bone metabolism.15,17,28 In our series the levels of PTH, osteocalcin and β-Crosslaps increased gradually from the first year after surgery, maintaining an inverse relationship with 25(OH)D levels. However, only high levels of PTH (pg/ml) were significantly associated with patients with bone fractures, with higher levels than those not fractured during the first 5 years of follow-up: at 77.8 vs. 98.1and at 5 years: 110.3 vs. 162.5; p<0.05), adjusted Cox HR 1.10; 95%CI: 1.01–1.11; p=0.03). Other authors have agree with these results,7,11,15,17 highlighting the negative effect of bariatric surgery on bone metabolism using the BPD technique and the relationship of increased PTH, osteocalcin and β-Crosslaps with decreased bone mineral density. Therefore, it is essential to implement supplementation with calcium and vitamin D, and thus avoid or reduce the persistent secondary hyperparathyroidism that may occur in these patients, which causes an increase in the fracture risk.
Strengths and limitationsThe main strength of our study is the long follow-up time (>10 years), as when the postsurgery evolution time, is longer, the probability of bone fracture risk is greater. Another strength is our description of a cohort with a good number of patients from a single center undergoing malabsorptive surgery, which can cause greater problems in bone metabolism. We applied an equal follow-up protocol for all patients and performed blood tests in the same hospital.
With respect to limitations, the study's retrospective design leads to the incomplete follow-up of some subjects and the lack of a control group: in addition, collecting data on calcium and vitamin D supplementation plays an important role in bone metabolism. Also, we did not evaluate several determinants of bone health, such as exposure to estrogens in women, taking drugs that may cause bone damage or other causes of primary and secondary bone diseases. Another limitation was that we had no information on bone mineral density, which would have helped us in determining the underlying biological mechanism in the association between BS and fractures.
ConclusionDespite the limitations, we estimated the incidence of fractures over a long follow-up period in women and men undergoing biliopancreatic diversion. A specific analysis of the fracture site, objectified a greater frequency in the typical sites of osteoporotic fractures, mainly in the spine and upper limbs, and observed a positive relationship between the increase in PTH, β-Crosslaps, and the risk of fracture. To minimize the effect of BS especially in malabsorptive techniques such as BPD on bone health, the importance of adherence to calcium and vitamin D supplements should be reinforced. Finally, more studies should be conducted on the mechanisms involved in increasing the risk of fractures after bariatric surgery, given the paucity of evidence in this area.
Authors’ contributionMirian Alejo Ramos. Conception and design of the study, data acquisition, analysis and interpretation of the data, preparation of the article, final approval of the version presented.
María D. Ballesteros Pomar. Conception and design of the study, data acquisition, analysis and interpretation of the data, final approval of the version presented
Ana Urioste Fondo. Data acquisition, critical review of the article.
Luis Gonzalez Herráez. Critical review of the article.
Tomas González de Francisco. Critical review of the article.
Matilde Sierra Vega. Critical review of the article.
Isidoro M. Cano Rodríguez. Critical review of the article and final approval of the version presented.
Informed consentInformed consent was not needed in this study. However, an informed verbal consent was requested to complete the telephone survey.
Funding sourcesNo funding sources were required in the study.
Conflict of interestThe authors declare no conflicts of interest.
The authors thank the clinical analysis team of CAULE for their collaboration and for providing the analytical data.