The purpose of this paper is to compare the effect of vaginal isosorbide mononitrate added to misoprostol versus misoprostol alone in cervical ripening and labor induction in post-term pregnancy.
MethodsIn this double-blind controlled trial study, 150 pregnant women in post-term pregnancy who were candidates for labor induction were selected. The participants were assigned randomly to receive either vaginal isosorbide mononitrate (IMN) (40mg) or placebo. Misoprostol (25mg) was added to both groups as needed. Time to full cervical ripening, time to delivery, and the amount of misoprostol used in each group were assessed.
ResultsThe time interval from the administration of IMN to full cervical ripening was shown to be significantly lower in the IMN+ misoprostol groups versus the comparison group (p=.032). The adjusted analysis of this time interval after controlling for age, BMI, gravidity, and Bishop score on administration remained significantly less (p=.045),the mean difference being −4.85h, CI 95% −9.58 to −.12. Isosorbide treatment resulted in significantly less misoprostol used versus misoprostol alone (2.37±1.02 versus 3.08±1.29), adjusted p-value=.001, CI 95% −1.09 to −.32. We found no significant increase in maternal–fetal outcomes or side effects of the IMN+ misoprostol group compared with the misoprostol group.
ConclusionThis study found that intravaginal IMN added to misoprostol is more effective in reducing time to full cervical ripening versus misoprostol alone in post-term pregnancy. It also reduces the need for more misoprostol.
El objetivo de esta investigación es determinar si el mononitrato de isosorbida vaginal, agregado al misoprostol, acorta el tiempo hasta la maduración cervical completa en el embarazo postérmino.
MétodosEn este estudio de prueba controlado doble ciego, se seleccionaron 150 mujeres embarazadas en embarazo postérmino candidatas para la inducción del trabajo de parto. Los participantes fueron asignados al azar para recibir mononitrato de isosorbida vaginal (NMI) (40mg) o placebo. Se añadió misoprostol (25mg) a ambos grupos según fuera necesario. Se evaluaron el tiempo hasta la maduración cervical completa, el tiempo hasta el parto y la cantidad de misoprostol utilizado en cada grupo.
ResultadosEl intervalo de tiempo desde la administración de la NMI hasta la maduración cervical completa se mostró significativamente más bajo en los grupos de NMI versus el grupo de comparación (P=0,032). El análisis ajustado de este intervalo de tiempo después de controlar la edad, el IMC, la gravidez y la puntuación de Bishop en la administración se mantuvo significativamente menor (P=0,045) con la diferencia media -4,85h, IC 95% -9,58 a -0,12. El tratamiento con isosorbida dio como resultado una menor cantidad de misoprostol usado significativamente en comparación con el misoprostol solo (2,37±1,02 versus 3,08±1,29), valor de P ajustado=0,001, IC 95% -1,09 a -0,32. No se encontró un aumento significativo en los resultados materno-fetales y los efectos secundarios del grupo de NMI en comparación con el grupo de misoprostol.
ConclusiónEste estudio ha encontrado que la NMI intravaginal agregada al misoprostol es más eficaz para reducir el tiempo hasta la maduración cervical completa en comparación con el misoprostol solo en el embarazo postérmino. También reduce la necesidad de más misoprostol.
Prolonged pregnancy is at risk for an adverse outcome either maternal or fetal. Most studies in post-term pregnancy have shown a greater neonatal acidemia, presence of meconium, neonatal mortality, dystocia in delivery, and also greater cesarean delivery rate.1 The more adverse outcome will be especially when labor is electively induced in the presence of an unripened cervix.
Prostaglandins have been known for cervical ripening before labor induction and Misoprostol is the most commonly used synthetic prostaglandin. Previous investigations were noted that Misoprostol is quite effective in cervical ripening.2–4 Unfortunately, except for the risk of uterine hyperstimulation that may compromise the fetus, its side effects are also the disadvantages of this drug.3,5,6 To avoid hazards surrounding the use of Misoprostol, researchers have shown an increased interest in other safe agents with similar efficacy. Isosorbide mononitrate (IMN) is a Nitric oxide (NO) donor that its enzymatic activity for ripening of cervix has been already proved.7 Recently, a relatively small body of literature is concerned with the increased cervical ripening associated with Isosorbide,8 nevertheless, a considerable amount of these studies has been addressed the beneficial effects of that.8,9 More recently, evidence has emerged that offers contradictory findings of the combination of both for cervical ripening at term.8–12 In this study, we compared the effect of vaginal isosorbide mononitrate added to Misoprostol versus misoprostol alone in cervical ripening and labor progression in post-term pregnancy. Moreover, administrations of the first dose of Isosorbide until delivery of the neonate, the Bishop score 24h after administration the medicines, the number of Misoprostol used during labor and the adverse effects of drugs compared in both groups.
MethodThe ethical committee of Babol University of Medical Science approved this double-blind randomized clinical trial study. It was registered with the number IRCT20180922041083N1in the Iran Registry of Clinical Trials (IRCT). Participants were selected among the pregnant women who were admitted to the maternity unit of Rouhani Hospital in Babol from April 2018 to May 2019. The reason for admission in all mothers was the termination of post-term pregnancy (gestational age>41 weeks) through the induction of labor.
Inclusion criteria were healthy nulliparous women at post-term (after 42 weeks of pregnancy) candidate for labor induction, BMI (19.8–26kg/m2) in the first trimester of pregnancy, singleton and cephalic pregnancy, Bishop score less than 6, having a normal biophysical test or fetal electrocardiogram (ECG) 48h before to selection. Exclusion criteria include pregnant women with a serious disease, history of headache, intolerance to Isosorbide mononitrate, contraindications to Misoprostol (severe renal or hepatic disorder, heart disease, asthma or glaucoma), use of alcohol, preeclampsia, and eclampsia, uncontrolled diabetes, intrauterine growth restriction, polyhydramnios, oligohydramnios AFI≤5, placenta previa, placenta abruption. Using 90% power, α error 0.05, and intervention to comparison ratio 1:1, a sample size of 75 women per arm (with adjusting for the attrition rate) was calculated to detect the primary outcome of a change in at least 20% between the two groups. After obtaining written consent from the mothers, a checklist containing patients’ demographic information was completed. Effect of stimulation of labor was determined by Bishop scoring that was according to the status of cervical examination and consisting of 5 components: dilatation (0–3), lengh (0–3), consistency (0–2), and position (0–2) and station of presenting part (0–3) and total scoring was 0–13.13
RandomizationRandomization was performed using an online random block assignment website that generated permuted block-sized numbers (block-4). Both tablets were inserted in the capsules of a similar design. For concealment, they were packed in the uni-shape pocket and coded A or B. A trained obstetrics resident as a third party allocated the participants according to the blocked numbers list. The participants received either Isosorbide Mononitrate (IMN) tablet 40mg (Arya Pharma co.) as a single vaginal dose in group A or Vitamin B1 tablet 100mg (Shafa pharm Co, Iran) in group B vaginally. The blocklist was exterminated after the allocation. The participants and the outcome assessor were unaware of the allocation. The resident who was in charge of insertion, recorded the demographic and labor parameters of patients in the checklist and a statistician analyzed them.
InterventionThe resident placed the related capsules to the posterior fornix of the cervix through a vaginal applicator. Bishop score was measured 24h after administering IMN or placebo. Then in both groups, 25mcg of vaginal Misoprostol (Cytotec, Searle Pharmaceuticals, High Wycombe, Bucks, UK) was placed in the cervix. Both mother and fetal heart rate (FHR) and maternal blood pressure were monitored after the initiation of the intervention by ECG every 15min. This monitoring was repeated after the placement of each Misoprostol. Notably, amniotomy was performed at 6cm dilatation or in conditions diagnosed by the same resident. Six hours later, mothers in both groups were remeasured for the Bishop score and uterine contractions. If the Bishop score did not improve or contractions were inappropriate (less than 3 contractions in 10min last 40s), Misoprostol was continued every 3–6h for a maximum of 6 doses.14 If a mother failed to have desirable contractions after 6h of the last dose of Misoprostol, stimulation is initiated with oxytocin 10 units per 1000cc Ringer serum in 4–8 drops per minute. Every 15min, 4 drops were added until the appropriate contractions rate was achieved and continued with the same number of drops. If the appropriate contraction was not achieved, stimulation was continued to the maximum drops (64 drops per minute). If the mother did not have enough contractions yet or if 8h passed from initiation of the labor, it was considered as induction failure. The mother was a candidate for the cesarean section, but she did not drop out of the study.
Outcome assessmentAs the primary outcome, the time interval between administration of the first dose of Isosorbide until the cervix is fully ripened, and dilated (hours) was studied. The maximum dilated cervix was considered 10 cm. The secondary outcomes were considered the time interval between administration of the first dose of Isosorbide until delivery of the neonate, the effect of Isosorbide on the Bishop score 24h after administration, the number of Misoprostol used during labor in the two groups, and also the adverse effects in both groups and compare them.
Outcome analysisStatistical tests were done using SPSS 18 (SPSS Inc., Version 18, Chicago, IL, USA). To minimize attrition bias, intention to treat analysis (ITT) was applied. Kolmogorov–Smirnov and Shapiro–Wilk Test tests were used to evaluate the normality of quantitative data. Also, skewness and kurtosis indices were used to examine the normality of the data. Data were presented as mean and standard deviations (mean±SD). A 2-tailed p-value of 0.05 or less was considered statistically significant. Chi-square was compared to qualitative variables. T-test determined the mean difference of parameters between the study groups and the comparison group. Multiple linear regression analysis was applied to determine the association between IMN and the outcome parameters with controlling the potential covariates. The effect size of treatment (Cohen's d) was showed the difference between Hedge's g of the intervention and comparison groups and calculated according to Lenhard15 and Cohen's d categories.16
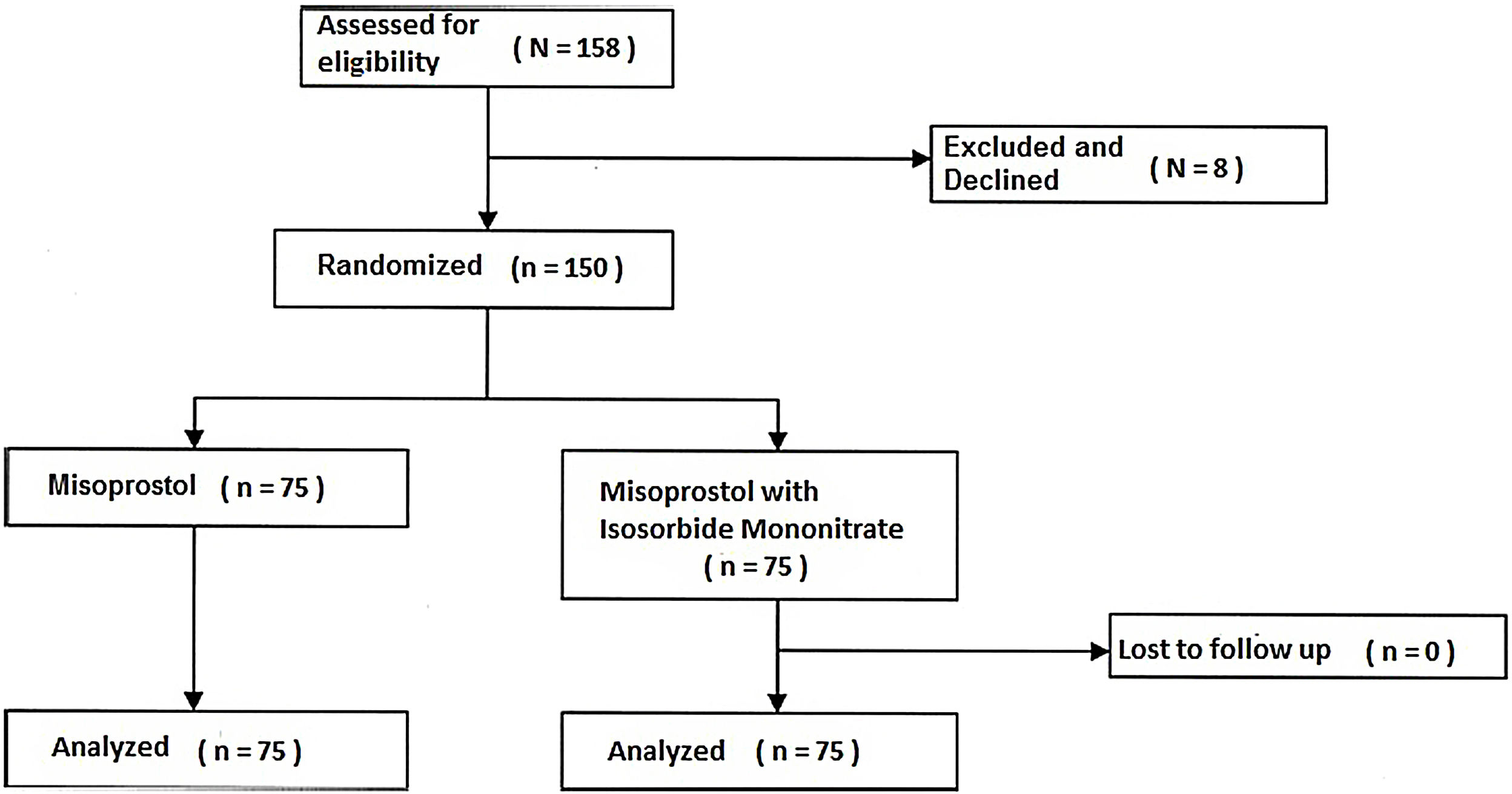
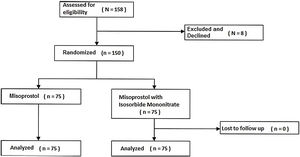
ResultsEight women were excluded or unwilling to attend. Finally, 150 women were selected and divided into two groups (75 women in the Isosorbide group and 75 women in the placebo group). Nobody declined to participate after allocation (Fig. 1).
Table 1 was demonstrated the demographic and gestational characteristics of the participant. No significant difference between the two groups was evident (p=0.41).
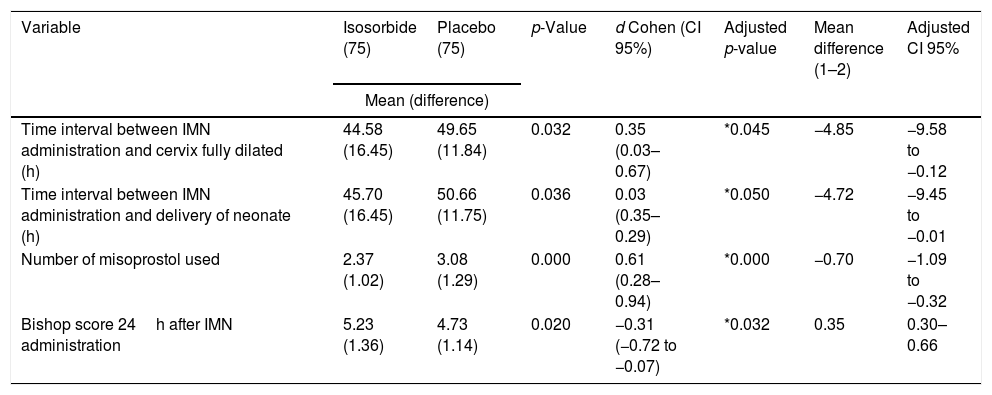
Table 2 presents the primary and secondary outcomes measurements. An independent t-test was used to examine the significant differences between the two groups. From the data in Table 2, it is apparent that the length of time left between the first dose of IMN administration and fully cervix dilated in the study group was significantly less than the comparison group (p=0.032). Size of treatment effects (d Cohen) with the IMN in the time interval between the dose of IMN and fully cervix dilated showed the small to medium strength 0.35, CI 95% 0.03–0.67 according to Cohen categories.16 General linear model univariate analysis showed that after eliminating the potential confounders affected (age, BMI, gravidity and, Bishop score at the administration), the difference of the time interval (Intervention group – comparison group) remained significantly less (p=0.045) with the mean difference −4.85h, CI 95% −9.58 to −0.12. Also after controlling the baseline criteria, the intervention group showed less time interval −4.72h (CI 95% −9.45 to −0.01) between IMN administration and delivery versus the controls. This result was significant at the p=0.05 level (Table 2).
Comparison of primary and secondary outcome measurement between two groups.
| Variable | Isosorbide (75) | Placebo (75) | p-Value | d Cohen (CI 95%) | Adjusted p-value | Mean difference (1–2) | Adjusted CI 95% |
|---|---|---|---|---|---|---|---|
| Mean (difference) | |||||||
| Time interval between IMN administration and cervix fully dilated (h) | 44.58 (16.45) | 49.65 (11.84) | 0.032 | 0.35 (0.03–0.67) | *0.045 | −4.85 | −9.58 to −0.12 |
| Time interval between IMN administration and delivery of neonate (h) | 45.70 (16.45) | 50.66 (11.75) | 0.036 | 0.03 (0.35–0.29) | *0.050 | −4.72 | −9.45 to −0.01 |
| Number of misoprostol used | 2.37 (1.02) | 3.08 (1.29) | 0.000 | 0.61 (0.28–0.94) | *0.000 | −0.70 | −1.09 to −0.32 |
| Bishop score 24h after IMN administration | 5.23 (1.36) | 4.73 (1.14) | 0.020 | −0.31 (−0.72 to −0.07) | *0.032 | 0.35 | 0.30–0.66 |
To compare the difference between the frequencies of the doses of Misoprostol received in each group, the Chi-square test was used. All patients in both groups were received the initial dose of Misoprostol. 37.3%28 of the comparison group needed the fourth dose and further of Misoprostol while 8%6 in the intervention group needed that (p=0.000). The difference between the doses of Misoprostol used was highlighted in Table 2. It is apparent from this table that, there is a significantly descending trend in the number of misoprostol used by patients was clear (p=0.000). The difference of Misoprostol used revealed a strong effect size of 0.61 (CI 95% for d Cohen (−0.28 to −0.94))16 (Table 2).
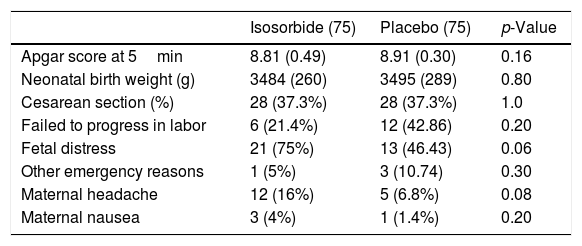
Table 3 compares maternal and fetal complications and also the side effects of medicines in both groups. The most common cause of cesarean section in the two groups was fetal hypoxia due to meconium-stained amniotic fluid passage. Other common cause includes failure to progress in labor such as prolonged labor (labor slower than normal progress or long active phase) or secondary arrest (complete cessation of progress). 28 (37.3%) women in the intervention group underwent cesarean section and 47 (62.7%) women had vaginal delivery the same as comparison group. One patient in the IMN group and three patients in the placebos that led to cesarean section had an acute situation threatening life of mother and fetus (prolapse of the umbilical cord or unknown vaginal bleeding) (Table 3). There were no differences in terms of maternal headache and maternal nausea between the two groups (p=0.08 and p=0.2 respectively). No other side effects of IMN were observed.
Between-groups comparison of maternal and fetal complications.
| Isosorbide (75) | Placebo (75) | p-Value | |
|---|---|---|---|
| Apgar score at 5min | 8.81 (0.49) | 8.91 (0.30) | 0.16 |
| Neonatal birth weight (g) | 3484 (260) | 3495 (289) | 0.80 |
| Cesarean section (%) | 28 (37.3%) | 28 (37.3%) | 1.0 |
| Failed to progress in labor | 6 (21.4%) | 12 (42.86) | 0.20 |
| Fetal distress | 21 (75%) | 13 (46.43) | 0.06 |
| Other emergency reasons | 1 (5%) | 3 (10.74) | 0.30 |
| Maternal headache | 12 (16%) | 5 (6.8%) | 0.08 |
| Maternal nausea | 3 (4%) | 1 (1.4%) | 0.20 |
Values presented as mean±SD or number (%).
Concerning the first research question, it was found that IMN could reduce the time to fully cervix dilated in women given IMN versus the controls. The difference was significant even after the eliminating of confounders, however, the size of the treatment effect revealed a small to medium strength.
This study supports evidence from Elsoky et al. who used IMN for cervical ripening at full term and concluded Isosorbide is more effective than Misoprostol in shortening cervical ripening time.8 Also, our result is in agreement with Abotorabi's finding, however, she presented a large effect size of Bishop Score on behalf of the IMN.17
To the best of our knowledge, there is relatively much controversy regarding the effectiveness of IMN and its safety for cervical softening. Haghighi et al. compared Misoprostol alone with IMN in his double-blind trial and revealed Misoprostol could shorten the time to delivery more than IMN. He also reported more adverse effects of IMN versus Misoprostol.4 This difference can be explained in part by the number of IMN suppository used in Haghighi's study that was more than we administered.4 In the current study, the intervention group was received one single dose of IMN, and needed less Misoprostol significantly, hence the adverse effects of Misoprostol were shown to be similar in both groups.
Lotfalizadeh et al. in their study administered more IMN and Misoprostol in combination and found no significant differences for cervical ripening versus Misoprostol alone in the two studied groups. Nevertheless, he recommended using IMN due to lesser side effects versus Misoprostol.6 This study also is contrary to that of Collingham et al. In their study, cervical ripening and time to vaginal delivery appeared to be less affected by the combination therapy of IMN and Misoprostol versus Misoropstol alone.11 There are likely two causes for the difference between Collingham's study and the present study. He administered oral IMN and our participants received vaginally that may not have a controlled release over 24h without a mesh. Second, his study was lack of blinded or placebo-controlled, while we tried to eliminate some bias through masking in our study. However, in our study, the staff were necessarily aware of the type of drugs and probably that may a source of uncontrolled unconscious bias that may be a source of limitation in this study.
In the present study, the fetal distress was greater in the women received IMN+Misoprostol versus the women received Misoprostol alone and the reason for this is not clear but it may have something to do with sample size. However, it did not show statistically difference and the apgar score of neonates were compared in both groups. Accordingly, although the IMN did not increase vaginal delivery in the intervention group versus comparisons, however helped them to experience shorter time interval to fully cervical ripening (the mean difference −4.85h, CI 95% (−9.58 to −0.12). Therefore, a note of caution is due here since the current study is limited to the effectiveness of IMN and it lacks a detailed census assessment of its safety profile, hence further research should be undertaken to investigate IMN safety despite its promising efficacy.
ConclusionAdding intravaginal Isosorbide to Misoprostol is more effective to shorten the time to the cervix fully dilated than Misoprostol alone. It also lessens the need for further Misoprostol.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingDeputy of research and technology of Babol University of Medical Science supported us financially.
Conflict of interestNo conflict of interest was declared.
We thank Dr. Aghajani Delavar for assistance with the methodology that greatly improved the manuscript.