Peritoneal sarcomatosis is a rare disease, with multiple histological origins and poor overall prognosis. The option of radical cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) is controversial. The results of a surgical team experienced in these procedures are analyzed and discussed based on the available evidence.
MethodsStudy on a prospective database of patients with peritoneal sarcomatosis who underwent CRS and HIPEC, from 2016 to 2022, in a national reference center for sarcomas and peritoneal oncological surgery, who met the established inclusion/exclusion criteria.
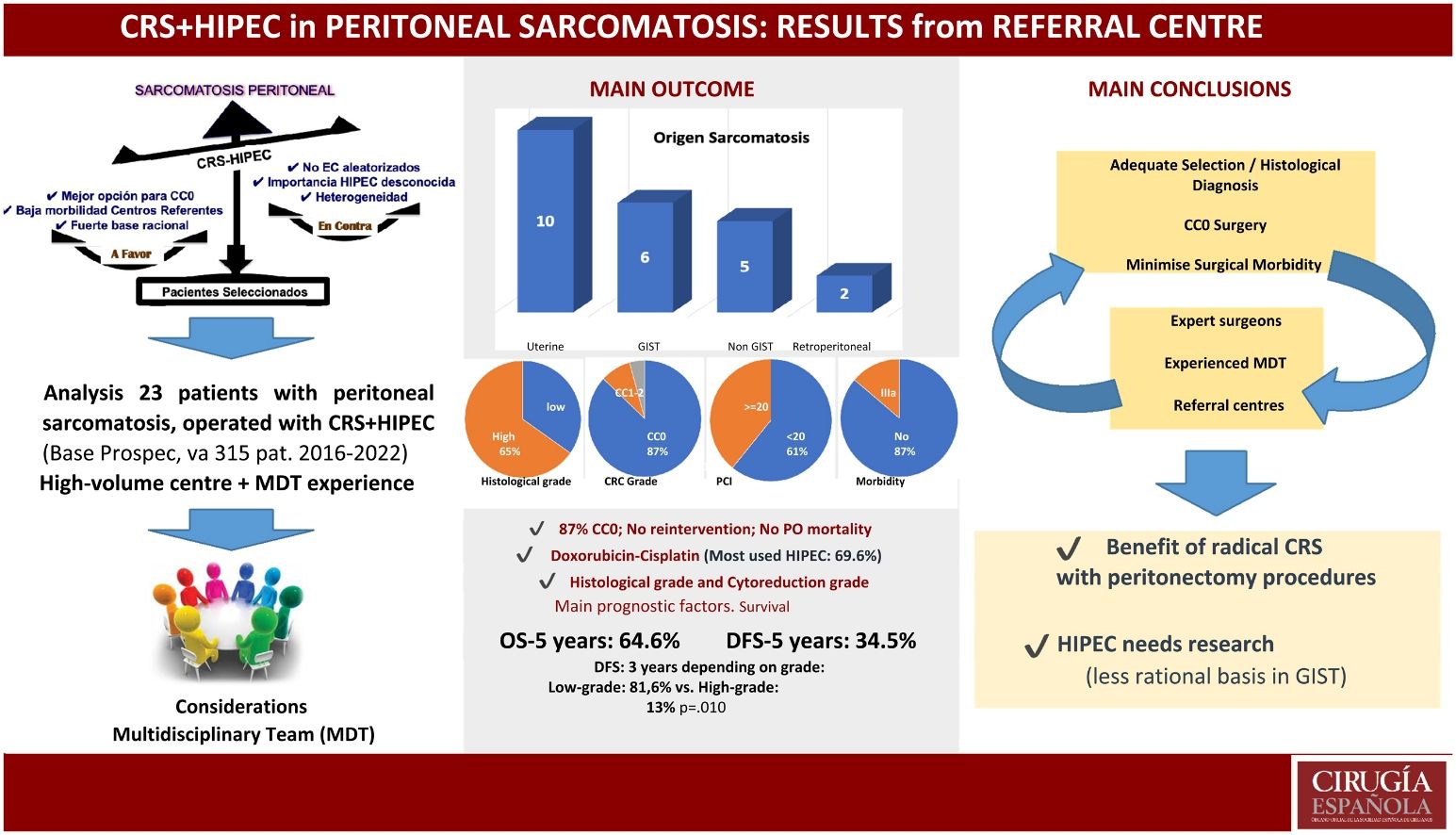
Results23 patients were included in the study, with a median age of 53 years (6−68). Recurrent/persistent clinical presentation predominated (78.3%). Visceral origin (including GIST and non-GIST peritoneal) accounted for 47.8% of patients, compared to 43.5% uterine and 8.7% retroperitoneal. The median PCI was 17 (3−36), with CC0 cytoreduction of 87%. Postoperative morbidity (Dindo Clavien III–IV) of 13%, with no postoperative mortality in the series. Overall survival and disease-free survival at 5 years were 64% and 34%, respectively. Histological grade was the most influential prognostic factor for survival.
ConclusionsThe results of the series, with low morbidity, support the benefit of radical peritoneal oncological surgery in patients with peritoneal sarcomatosis after adequate selection, as long as it is performed in high-volume centers, experienced surgeons and expert multidisciplinary teams. However, the role of HIPEC remains to be demonstrated and pending future studies.
La sarcomatosis peritoneal es una enfermedad rara, con múltiples orígenes histológicos y pronóstico global infausto. La opción de la cirugía citorreductora radical (CRS) con quimioterapia intraperitoneal hipertérmica (HIPEC) es controvertida. Se analizan y discuten los resultados de un equipo quirúrgico experimentado en estos procedimientos en base a la evidencia disponible.
MétodosEstudio sobre una base de datos prospectiva de pacientes con sarcomatosis peritoneal intervenidos mediante CRS y HIPEC, desde 2016 a 2022, en un centro referente nacional en sarcomas y cirugía oncológica peritoneal, que cumplieron los criterios de inclusión/exclusión establecidos.
ResultadosSe incluyeron en el estudio a 23 pacientes, con mediana de edad de 53 años (6−68). Predominó la presentación clínica recurrente/persistente (78,3%). El origen visceral (incluyendo GIST y peritoneal no GIST) supuso el 47,8% de los pacientes, frente al 43,5% uterino y 8,7% retroperitoneal. La mediana de PCI fue 17 (3−36), con citorreducción CC0 del 87%. Morbilidad postoperatoria (Dindo Clavien III–IV) del 13%, sin mortalidad postoperatoria en la serie. La supervivencia global y supervivencia libre de enfermedad, a 5 años, fueron del 64,6% y 34,5%, respectivamente. El grado histológico fue el factor pronóstico de supervivencia más influyente.
ConclusionesLos resultados de la serie, con baja morbilidad, apoyan el beneficio de cirugía oncológica peritoneal radical en pacientes con sarcomatosis peritoneal tras una adecuada selección de los mismos, siempre que se realice en centros de alto volumen, cirujanos experimentados y equipos multidisciplinares expertos. Sin embargo, el papel de HIPEC está por demostrar y pendiente de futuros estudios.
Soft tissue sarcomas (STSs) are malignant mesenchymal tumours, with a wide variety of histological subtypes, representing only 1% of solid cancers and 30% of these are located in the abdominopelvic or retroperitoneal cavity. In this location, we distinguish visceral gastrointestinal stromal sarcomas (GISTs), visceral or non-GIST peritoneal sarcomas, gynaecological sarcomas, and retroperitoneal sarcomas. Most of these are associated with high rates of locoregional recurrence after surgery and are characterised by the possibility of peritoneal dissemination during their evolution, frequently as a consequence of intraoperative handling of the primary tumour and its possible rupture during surgical resection.1
In such circumstances, peritoneal sarcomatosis is considered a rare disease, with multiple histological origins and a poor overall prognosis. On the other hand, unlike other epithelial tumours that develop peritoneal malignancy such as the ovary, appendix, colon or peritoneum itself, among others, the option of radical cytoreductive surgery (CRS) following peritonectomy procedures with hyperthermic intraperitoneal chemotherapy (HIPEC) is controversial.2 However, there is a strong rationale supporting aggressive locoregional treatment in these patients, based on two key circumstances. On the one hand are the clinical and epidemiological similarities that we find between STSs that can trigger peritoneal sarcomatosis and peritoneal carcinomatosis itself, which we frequently treat radically. On the other hand, we have the therapeutic quality conditions required of these procedures, which may coincide in the both clinical situations.
The aim of this work was to undertake a study on what an updated radical surgical approach can mean in patients with peritoneal sarcomatosis, operated on with CRS + HIPEC at a national referral centre for sarcomas and peritoneal oncological surgery, with a large volume of - and great experience in - these procedures that limit morbidity and reduce variability in results. Also included are considerations in this regard, based on current scientific evidence.
MethodsPatients and study designObservational analytical study based on a prospective database of 315 patients with peritoneal malignancy operated on, during the period from July 2016 to December 2022, with CRS + HIPEC at the Peritoneal and Retroperitoneal Oncological Surgery Unit (COPER) within the Virgen del Rocío University Hospital in Seville, CSUR (Centres, Services, Reference Units) under the Spanish National Health System for Mesenchymal Tumours and Sarcomas, and a referral centre for peritoneal oncological surgery. A consecutive series of 23 patients diagnosed with peritoneal sarcomatosis were included in the study, operated on by the same surgical team, with extensive experience in these procedures.
All cases were previously discussed in an expert multidisciplinary committee. Likewise, all patients signed a written informed consent form and all of them were treated in line with a protocol approved by the Institutional Ethics Committee. Follow-up of the study was continued until December 2023.
Inclusion and exclusion criteriaAll patients met the following criteria: i) histological confirmation by pathologists of the institution, of peritoneal sarcomatosis due to soft tissue sarcoma of any histological subtype, both synchronous with the primary tumour and metachronous after previous surgery. ii) surgical treatment performed using CRS + HIPEC procedures, and iii) an ECOG (Eastern Cooperative Oncology Group) performance status of grade 2 or lower. The exclusion criteria were as follows: i) presence of extra-abdominal metastatic disease that had not been completely treated previously or with incomplete intraoperative resection, ii) patients with tumour progression despite neoadjuvant treatment, iii) significant organ dysfunction (cardiovascular, respiratory, renal or hepatic), iv) another coexisting malignancy without curative treatment, v) patients with local recurrence exclusively at the same site of primary resection, and vi) patients operated on using CRS without application of HIPEC.
Surgical procedure (CRS + HIPEC)CRS + HIPEC is based on the technique described by Sugarbaker,3 widely defined and used by our surgical team.4 The goal of surgery was the complete excision of all macroscopically visible neoplastic disease (Fig. 1). Once surgical cytoreduction had been completed and before any digestive anastomosis was performed, HIPEC was given using an FDA-approved hyperthermia pump (Belmont Hyperthermia Pump™) with a high flow rate (1 l/min). The infusion of chemotherapy into the abdomen, diluted in 1.5% dextrose solution, was performed through two catheters, and extraction was through four aspiration drains placed in the abdominal cavity (subdiaphragmatic and pelvic). Four temperature probes were also used to control this during the procedure performed with an open abdomen, following the Colosseum4,5 technique. As regards cytostatics, the most commonly used regimen was cisplatin (50 mg/m2/2 l solution) with doxorubicin (15 mg/m2/2 l solution), versus doxorubicin isolate at the same dose in cases of cisplatin intolerance, or paclitaxel (60 mg/m2/2 l solution) in some gynaecological cases, also coinciding with possible platinum toxicity. Duration was 60 min at 41–43 °C in all cases (Table 1).
Images of different stages of radical cytoreductive surgery (CRS) with peritonectomy procedures in a patient with peritoneal sarcomatosis, included in the series studied: A) Exposure of the abdominopelvic cavity with peritoneal sarcomatosis; B) En bloc cytoreductive surgery with centripetal peritonectomy in the pelvic area; C) Surgical specimen removed after radical cytoreduction of the pelvic area (includes bilateral parietocolic peritoneum and pouch of Douglas, uterus, fallopian tubes, ovaries, recto-sigma, descending colon, transverse colon and previous ileocolic anastomosis); D) Pelvis with complete cytoreduction (CC0).
Patient demographics and clinics.
| Characteristics of patients in the series (n:23) | Number | % |
|---|---|---|
| Gender | ||
| - Male | 7 | 30.4 |
| - Female | 16 | 69.6 |
| Median age in years (range) | 53 (6−68) | |
| Presentation | ||
| - Primary | 5 | 21.7 |
| - Recurrent/persistent | 18 | 78.3 |
| Tumour Histology | ||
| - Visceral | 11 | 47.8 |
| - Gastrointestinal (GIST) | 6 | 26.1 |
| - Peritoneal non GIST | 5 | 21.7 |
| - Epithelioid inflammatory myofibroblastic tumour | 1 | 4.3 |
| - Desmoplastic Round Cell Tumour | 1 | 4.3 |
| - Sarcoma with NUTM 1 rearrangement | 1 | 4.3 |
| - Low-grade calcifying fibrous tumour | 1 | 4.3 |
| - Follicular dendritic cell sarcoma | 1 | 4.3 |
| - Uterine | 10 | 43.5 |
| - Leiomyosarcoma | 3 | 13.0 |
| - Endometrial stromal sarcoma | 5 | 21.7 |
| - Undifferentiated | 2 | 8.7 |
| - Retroperitoneal | 2 | 8.7 |
| - Well-differentiated liposarcoma | 1 | 4.3 |
| - Dedifferentiated liposarcoma (Grade II) | 1 | 4.3 |
| Histological Grade | ||
| - Low | 8 | 34.8 |
| - High | 15 | 65.2 |
| Median PCI (range) | 17 (3−36) | |
| - PCI < 10 | 7 | 30.4 |
| - PCI: 10−20 | 7 | 30.4 |
| - PCI > 20 | 9 | 39.1 |
| Extraperitoneal metastatic disease | ||
| - Liver | 1 | 4.3 |
| - Lung | 2 | 8.7 |
| Pretreatment | ||
| - None | 1 | 4.3 |
| - Isolated surgery | 1 | 4.3 |
| - Chemotherapy/Hormonal Therapy (QT/HT) isolated | 4 | 17.4 |
| - Isolated radiotherapy (RT) | 0 | 0 |
| - Combination (Surgery + QT/HT ± RT) | 17 | 73.9 |
| PSS score | ||
| - PSS:0−1 | 10 | 43.5 |
| - PSS:2−3 | 13 | 56.5 |
| Degree of Cytoreduction (CC) | ||
| - CC0 | 20 | 87.0 |
| - CC1 | 2 | 8.7 |
| - CC2 | 1 | 4.3 |
| Peritonectomy Procedures | ||
| - Pelvic/local (< 3 procedures) | 6 | 26.1 |
| - Extensive (3−4 procedures) | 11 | 47.8 |
| - Total (>4 procedures) | 6 | 26.1 |
| HIPEC Type | ||
| - Doxorubicin | 4 | 17.4 |
| - Doxorubicin-Cisplatin | 16 | 69.6 |
| - Paclitaxel | 3 | 13.0 |
| Median Surgical Time in Hours (Range) | 7 (5−10) | |
| Severe morbidity (Dindo-Clavien III-IV) | 3 | 13 |
| Postoperative mortality (30 days) | 0 | 0 |
| Median hospital stay in days (range) | 14 (9−42) | |
| Current status | ||
| - Alive without disease | 8 | 34.8 |
| - Alive with illness | 9 | 34.8 |
| - Died with disease | 5 | 26.1 |
| - Died from other cause | 1 | 4.3 |
The study variables, as well as the characteristics of the patients included in the series, are shown in Table 1.
The original tumour histology of the sarcomas was classified into 4 groups: visceral or peritoneal GIST type (gastrointestinal stromal tumour); visceral or peritoneal non-GIST; gynaecological (uterine), and retroperitoneal. The grade of the tumour was evaluated according to the FNCLCC (Federation Nationale des Centres de Lutte Contre le Cancer) classification. For the purposes of this study, grade 1 and 2 sarcomas are described as low-grade, and grade 3 sarcomas as high-grade. The PSS (Prior Surgical Score) variable was used to determine the history of previous surgery (PSS-0 in patients with no history of surgery for cancer; PSS-1 in patients who had previously undergone surgery in an abdominopelvic region or simple biopsy; PSS-2 in those patients who had previously undergone surgery in two to five regions; and as PSS-3 those who had undergone surgery in more than five abdominopelvic regions). The Peritoneal Cancer Index (PCI) was determined prior to any surgical excision and the extent of cytoreduction, expressed as a function of the number of procedures performed and the degree of cytoreduction obtained after the end of the intervention (CCR score), were previously defined by Sugarbaker.4
Postoperative morbidity at hospital discharge, always including the first 30 days postoperatively, was determined according to the Dindo-Clavien classification,6 with grade III and IV morbidity considered as serious.
Statistical analysisData were expressed as frequencies and medians. The endpoints of the study were OS (overall survival) and DFS (disease-free survival). They were estimated from the CRS + HIPEC data using Kaplan-Meier analysis. OS was dated from the day of CRS + HIPEC surgery until the time of death; DFS was dated from the day of CRS + HIPEC surgery up to the time of local or distant disease progression. Multivariate analysis was performed using the Cox multiple regression method. Confidence intervals were calculated at 95%. A P < .05 was considered statistically significant. Statistical analysis was performed using IBM SPSS version 20.0 statistical software for windows (SPSS Inc., Chicago, IL).
ResultsDescriptive analysisThe descriptive data of the patients with peritoneal sarcomatosis included in the study are shown in Table 1. Among these, the predominance of a recurrent or persistent nature was observed after previous suboptimal surgery (78.3%), compared to patients with peritoneal disease synchronous with primary sarcoma (21.7%). Among the previous suboptimal surgeries, the main distinctive feature was a 6-year-old child patient with multiple surgical interventions and chemotherapy schedules, including total abdominal RT, for a desmoplastic small round cell tumour. The predominant histological origin of the tumours was uterine (10/23, 43.5%), half of which were endometrial stromal tumours (3 low-grade and 2 high-grade). The reported serious postoperative morbidity of 13% consisted of 3 patients with Dindo-Clavien grade IIIa. There are currently 16 patients (69.6%) living, with 7 patients (30.4%) having died, including one patient who died during the second month after hospital discharge, due to SARS-CoV-2 disease and septic shock (Table 1).
Survival analysisWith a median follow-up of 23 months, 5-year OS was 64.6% and DFS was 34.5%. Of all the variables studied, the histological grade was the most influential in survival, although this did not reach statistical significance for OS (52% high-grade 3-year survival vs. 100% low-grade, P = .062). However, this did reach statistical significance in DFS (13% high-grade 3-year survival vs. 81.6% low-grade, P = .010) (Fig. 2).
Another variable that obtained better OS results, without statistical significance, was the primary character of peritoneal sarcomatosis compared to recurrent or persistent sarcomatosis (100% survival at 5 years versus 56.3%, respectively, P = .118). On the other hand, the multivariate study confirmed the prognostic importance of histological grade in terms of DFS, as well as the degree of cytoreduction (Table 2).
Univariate and multivariate analysis of clinical-pathological variables.
| Variables | Overall survival (OS) | Disease-Free Survival (DFS) | ||
|---|---|---|---|---|
| Univariant (P) | Univariant (P) | HR (CI 95%) | Multivariant (P) | |
| Gender (male vs. female) | .870 | .172 | ||
| Presentation (primary vs. recurrent/persistent) | .118 | .897 | ||
| Tumour Histological Origin (GIST vs. visceral vs. uterine vs. retroperitoneal) | .275 | .817 | ||
| Histological Grade (low vs. high) | .062 | .010 | .126 (.160−.993) | .049 |
| PCI (Peritoneal Cancer Index) (<10 vs. 10−20 vs. >20) | .727 | .918 | ||
| Extraperitoneal metastatic disease (no vs. yes (liver/lung) | .861 | .527 | ||
| Pretreatment (no vs. surgery vs. QT/HT vs. combined) | .455 | .585 | ||
| PSS score (0−1 vs. 2−3) | .817 | .930 | ||
| Degree of Cytoreduction (CC) (CC0 vs. CC1/CC2) | .812 | <.001 | .305 (.135−.686) | .004 |
| Peritonectomy Procedures (<3 vs. 3−4 vs. >4) | .935 | .283 | ||
| HIPEC Type (doxorubicin vs. doxo-cisplatin vs. paclitaxel) | .937 | .176 | ||
In the univariate analysis with respect to histological origin, OS at 3 years was 100% for peritoneal gistosis and sarcomatosis of retroperitoneal origin, compared to 56% for uterine sarcomatosis (P = .328). As for DFS at 3 years, this was 50% for low-grade retroperitoneal sarcomas, 33.3% for peritoneal gistosis and 33.8% for uterine sarcomatosis (P = .817). Specific analysis of gynaecological sarcomas showed the best survival results in cases of low-grade endometrial stromal sarcomatosis, with 100% OS and 66.7% DFS, at 5 years (Fig. 3).
Kaplan-Meier survival curves: A) Overall survival (OS) according to histological origin of tumour; B) Disease-free survival (DFS) according to histological origin of tumour; C) OS in the uterine sarcomatosis subgroup, according to histological subtype; D) DFS of the uterine sarcomatosis subgroup, by histological subtype.
LMS: leiomyosarcoma; LG-ESS: low-grade endometrial stromal sarcoma; HG-ESS: high-grade endometrial stromal sarcoma; (UUS: undifferentiated uterine sarcoma.
The initial analysis of this series shows that peritoneal sarcomatosis, despite its numerous histological variants, accounted for only 7.3% of the total percentage of patients with peritoneal malignancy who received CRS + HIPEC at a referral centre with a Unit specifically devoted to the treatment of these pathologies. This fact, observed in other published series,7,8 confirms its rarity and justifies the small number of patients analysed in the scientific literature, as well as the difficulty of developing large and well-designed prospective studies that enable correct validation of the results9–19 (Table 3).
Literature review on radical cytoreductive surgery (CRS) + hyperthermic intraperitoneal chemotherapy (HIPEC) in peritoneal sarcomatosis with a series of 20 or more patients (Source: PubMed Database).
| Author | Year | Study Design | Patients (n) | Primary Tumour histology | HIPEC (cytostatic) | PCI | Cytoreduction (%) | Morbidity Grades 3−4 (%) | Mortality (%) | DFS at 5 years (%) | OS at 5 years (%) | Median OS (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Berthet et al.10 | 1999 | Retrospective | 43 | 22 LMS, 9 LPS, 4 FS, 4 DSRCT, 1 MPNST, 1 SFT | HIPEC with cisplatin (3 HIPEC, 13 HIPEC + EPIC, 14 EPIC, 13 No | 9 with <13 | CC0-1: 63 | 19 | 7 | ND | CC0-CC1: 39 | 20 |
| Single-centre | 34 with >13 | |||||||||||
| Rossi et al.11 | 2004 | Multicentre Prospective | 60 | 14 GIST, 12 uterine (8 ULMS, 4 EES), 34 RPS (20 LPS, 6 UPS, 4 MPNST, 2 FS, 2 DSRCT) | doxo + cisplatin | Medium 7.7 (2−21) | CC0: 68 | 23 | 0 | ND | 38 | 34 |
| CC0-1: 100 | ||||||||||||
| Lim et al.12 | 2007 | Prospective | 28 | 17 LMS/GIST, 5 DSRCT, 2 LPS, 4 others | 19 HIPEC: cisplatin | ND | CC0-1: 95 (CDDP) vs. 100 (CDDP + MITOX) | 16 (CDDP) in front of 44 (CDDP+ MITOX) | 0 | ND | 16.9 (CDDP) as opposed to 5,5 (CDDP + MITOX) | |
| Phase 1 | 9 HIPEC: cisplatin + mitoxantrone | |||||||||||
| Comparative | ||||||||||||
| Non-randomised | ||||||||||||
| Barter et al.13 | 2010 | Retrospective | 37 | 13 LPS, 11 ULMS, 8 GIST pre-Imatinib, 5 other | doxo + mitomicin C or cisplatin | Medium 14.7 (2−34) | CC0: 76 | 21.6 | 2.7 | 17.8 | 24.3 | 26,2 (34 in LPS) |
| Single-centre | CC0-1: 84 | |||||||||||
| Hayes-Jordan et al.14 | 2015 | Retrospective | 34 | 21 DSRCT, 7 RMS, 2 LPS, 4 other sarcomas, 12 other tumours | Cisplatin | median 16 | CC0: 95 | 28 | 0 | ND | ND (DSRCT 30) | 31,4 |
| Single-centre | CC0-1: 100 | |||||||||||
| Sardi et al.15 | 2017 | Retrospective | 36 | 29 ULMS, 3 ESS, 3 AS, 1 Other | 22 doxo + cisplatin, 10 melphalan, rest cisplatin/mitom C | Median 16 (2−39) | CC0-1: 94 | 21 | 2.8 | 32 | 32 | 37 |
| multicentric | ||||||||||||
| Hayes-Jordan et al.16 | 2018 | Prospective | 20 | 14 DSRCT, 2 RMS, 2 UPS, 1 T. Granulosa, 1 Ewing Sarcoma | Cisplatin | median 15 | CC0-1: 100 | 40 | 0 | ND | ND (83 at 3 years) | 58.4 for DSRCT |
| Phase 2 | ||||||||||||
| Non-randomized | ||||||||||||
| Karamveri et al.17 | 2019 | Retrospective | 20 | 5 DDLPS, 5 RMS, 4 LMS, 2 WDLPS, 4 others | doxo + cisplatin | Mean 6 (2−24) | CC0: 86 | 20.7 | 0 | ND | 43 | 55 |
| Single-centre | ||||||||||||
| Spiliotis et al.18 | 2021 | Retrospective | 21 | 7 LPS, 6 LMS, 4 RMS, 4 FS | 11 mitomicin C | median 10 (3−20) | CC0: 52,4 | 14.3 | 4.7 | ND | ND | 20,5 |
| multicentric | 7 doxo, 3 cisplatin | CC0-1: 90 | ||||||||||
| Almasri et al.19 | 2024 | Retrospective | 29 | 12 LPS, 7 LMS, 3 FS, 2 UPS, 5 others | Ifosfamide iv +HIPEC (24 doxo + cisplatin, 5 doxo + mitom C) | median 6 (3−12) | CC0: 51,7 | 31 | 0 | ND (35 at 2 years) | ND (73 at 2 years) | NR |
| Single-centre | CC0-1: 68,9 | |||||||||||
| Serie actual | 2024 | Retrospective | 23 | 10 uterine (5 EES, 3 ULMS, 2 NEW), 6 GIST, 5 visceral non-GIST, 2 LPS | 16 doxo-cisplatin, 4 doxo, 3 paclitaxel | median 17 (3−36) | CC0: 87 | 13 | 0 | 34 | 64 | NR |
| Single-centre | CC0-1: 96 |
NR: not reached; ND: not determined; LMS: leiomyosarcoma; LPS: liposarcoma: DDLPS: dedifferentiated liposarcoma; WDLPS: Well-differentiated liposarcoma; FS: fibrosarcoma; DSRCT: desmoplastic small round cell tumour; MPNST: malignant tumour of the peripheral nerve sheath; SFT: solitary fibrous tumour; GIST: gastrointestinal stromal tumour; RPS: retroperitoneal sarcoma; UPS: undifferentiated pleomorphic sarcoma; ULMS: uterine leiomyosarcoma; UUS: undifferentiated uterine sarcoma; EES: endometrial stromal sarcoma; RMS: rhabdomyosarcoma; AS: angiosarcoma; doxo: doxorubicin; mitom C: mitomycin C; MITOX: mitoxantrone; CDDP: cisplatin.
In these circumstances, through the design of this study, focussed on a single institution with great experience in the management of this pathology, we have sought to refine as much as possible the morbidity inherent in these procedures that were performed in patients with a considerable tumour burden and a high percentage of CC0, in order to verify the contribution that radical surgery could make to this locally advanced disease.
The first conclusion that could be drawn in this regard would be that serious morbidity of 13%, without associated surgical reinterventions or postoperative mortality, in a series of 23 patients and in line with other recent publications,17 considerably improved other previous results that linked these procedures to excessive morbidity, thus limiting their performance.12 On the other hand, the overall results of series such as ours, with 5-year survival rates of more than 60% and recurrence-free survival of 34%, enable surgery to continue to be considered as a therapeutic cornerstone for patients adequately selected by an expert multidisciplinary team, even in situations of peritoneal metastasis.
Furthermore, in line with the vast majority of published studies, histological grade has been shown to be the key survival factor in the prognosis of patients with peritoneal sarcomatosis.11 The excellent results obtained in low-grade sarcomatosis are a stimulus to attempt to achieve complete, radical cytoreduction, the other prognostic factor associated with these procedures, and which reinforces the recommendation of referral to specialised centres.
Albeit with the limitation of the number of patients included, the subgroup analysis shows some interesting details. The results for retroperitoneal sarcomas are thus positively surprising, although probably not quite so much if we take into account that they only include two patients and are low-grade liposarcomas, in which complete cytoreduction is a key factor.20 On the other hand, the excellent results obtained with peritoneal gistosis, unlike other series from the pre-imatinib13 era, are probably a consequence of having performed the intervention after the first 6–12 months of treatment and response with tyrosine kinase inhibitors (TKI), prolonging their therapy after surgery, as shown by Bryan et al.21 and as advised by current guidelines.22,23 avoiding intervention in times of resistance to treatment.24 On this point, the only patient who has died, up till the present date, in the peritoneal gistosis subgroup had a long history of the disease and was undergoing second line treatment with TKI (tyrosine kinase inhibitors), to which he finally became resistant. Thus, excluding radical surgery in pathologies that have a good initial sensitivity to chemotherapy or targeted therapies, or performing this in situations of progression, in our understanding, could be a great strategic error. With regard to uterine sarcomas, leiomyosarcomas, as indicated by the studies by Sardi’s group15,25 and low-grade endometrial stromal sarcomas in which complete surgery is the most effective therapeutic weapon, these patients are postulated to be the most benefitted from these radical cytoreductive procedures.26 Finally, the subgroup of visceral/non-GIST visceral/peritoneal sarcomatosis, which includes different histological types of sarcomas, such as small round cell desmoplastic or inflammatory myofibroblastic tumour, with a high affinity for peritoneal involvement and more frequent at young ages, could also benefit from aggressive cytoreductive surgery with peritonectomy procedures. as reflected in the scientific literature.14,16,27,28
With regard to HIPEC, the most commonly used combination was doxorubicin + cisplatin, in line with the recent meta-analysis by Wong and most of the studies published.9 However, the benefit of HIPEC is as yet unknown and we cannot attribute a clear leading role to it. The washing of disseminated cells by the surgeons themselves, during such long surgeries, to prevent their implantation, as well as the cytolytic action on the residual microscopic disease in those cases with demonstrated chemosensitivity, could be its benefits. However, in the absence of prospective randomised studies, we cannot propose this as a standard of treatment. This is even less so in patients with peritoneal gistosis responding to TKIs (tyrosine kinase inhibitors, for whom the administration of routine cytostatics may lack a rational basis.
Finally, a large percentage of patients in our series with oncological treatment were observed prior to the radical surgical procedure. It is essential that this decision and the type of treatment given be argued out by an expert multidisciplinary team, as recommended by the STS consensus guidelines. In some cases, the lack of control response to this previous treatment could even be a key factor in determining the non-indication of these procedures.
In summary, the results of the series show selected patients with peritoneal sarcomatosis who could receive radical surgical treatment with high rates of complete cytoreduction and low associated morbidity – always provided this is performed in high-volume centres by experienced surgeons and expert multidisciplinary teams. The histopathological heterogeneity and small number of patients included in the series published make it difficult to discriminate the histological subtypes that obtain the most benefit. However, low histological grade seems to be a particularly favourable prognostic factor for these surgical procedures, where we do not know the true role of HIPEC, which is pending future studies.
FundingThis work has not received any funding.
Conflict of interestThe authors declare no conflict of interest.