An efficient microwave-assisted hydrothermal method was developed for the preparation of nanocrystalline 3 and 8mol% yttria-doped zirconia (named as Z3Y and Z8Y, respectively) from commercially-available ZrOCl2·8H2O, YCl3·6H2O and KOH. The synthesis was conducted at low temperature (180°C for sample Z3Y and 200°C for sample Z8Y) and short reaction time (30min) by simultaneous precipitation of both chlorides with KOH and dehydration of hydroxides. Analytical control of Zr and Y (standardization of stock solutions, precipitation degree) throughout the entire synthetic process was carried out by ICP-OES. Quantitative precipitation (greater than 99.999%) of both elements was obtained. The as-synthesized powders were calcined at 500°C (sample Z3Y) and 800°C (sample Z8Y), and all the resulting products were characterized by XRD, FE-SEM, HR-TEM and SAED. Both the as-synthesized and calcined nanoparticles were highly crystalline. The crystalline phases obtained were tetragonal phase (with a fraction of about 20% monoclinic) for sample Z3Y, and pure cubic phase for sample Z8Y. No impurities from other phases were detected. The average crystallite sizes for the as-synthesized samples Z3Y and Z8Y were 6.2±1.0 and 3.5±0.7nm, respectively, while for the calcined ones the values were 8.0±1.2 and 11.3±1.3nm, respectively.
Se ha desarrollado un eficiente método hidrotermal asistido por microondas para la preparación de circona nanocristalina dopada con 3 y 8 moles % de itria (denominadas Z3Y y Z8Y, respectivamente) a partir de ZrOCl2·8H2O, YCl3·6H2O y KOH comercialmente disponibles. La síntesis se ha realizado a baja temperatura (180°C para la muestra Z3Y y 200°C para la muestra Z8Y) y corto tiempo de reacción (30min) mediante precipitación simultánea de ambos cloruros con KOH y deshidratación de hidróxidos. El control analítico de Zr e Y (estandarización de soluciones madre, grado de precipitación) a lo largo de todo el proceso sintético se realizó mediante ICP-OES. Se obtuvo una precipitación cuantitativa (superior al 99,999%) de ambos elementos. Los polvos sintetizados fueron calcinados a 500°C (muestra Z3Y) y 800°C (muestra Z8Y), y todos los productos resultantes fueron caracterizados por XRD, FE-SEM, HR-TEM y SAED. Tanto las nanopartículas sintetizadas como las calcinadas fueron altamente cristalinas. Las fases cristalinas obtenidas fueron la fase tetragonal (con una pequeña fracción de aproximadamente el 20% de monoclínica) para la muestra Z3Y, y la fase cúbica pura para la muestra Z8Y. No se detectaron impurezas de otras fases. El tamaño medio de los cristales de las muestras sintetizadas Z3Y y Z8Y fue de 6,2±1,0 y 3,5±0,7nm, respectivamente, mientras que el de las muestras calcinadas fue de 8,0±1,2 y 11,3±1,3nm, respectivamente.
Zirconia (ZrO2) is considered one of the most important ceramic materials due to its superior mechanical, thermal, chemical, electrical, optical, and catalytic properties, including high strength and fracture toughness, high hardness, high thermal expansion coefficient, excellent thermal and chemical stability, low thermal and high ionic conductivity, high refractive index, good biocompatibility, etc. These properties explain the wide range of industrial and medical applications of ZrO2, such as structural and high temperature ceramics, thermal barrier coatings, solid electrolyte for solid-oxide fuel cell, catalysts and catalytic supports, electrochemical and electronic devices, gas sensors, optical coatings, medical and surgical instruments and implants, etc. [1–8]. It is also important to note that, compared to bulk materials, nanostructured ceramics present a wide functional diversity and exhibit enhanced or different properties [9]. The properties of nanocrystalline ZrO2 differ from those of conventional micrometer-sized ZrO2 due to the unusual properties occurring at the nanoscale and the surface phenomena [10,11].
ZrO2 has three polymorphs at atmospheric pressure depending on the temperature: monoclinic (up to 1170°C), tetragonal (1170–2370°C) and cubic (2370–2680°C). Above 2680°C, ZrO2 transforms to liquid phase [12]. The transformations from monoclinic to tetragonal phase and from tetragonal to cubic phase are reversible, so the high temperature polymorphs do not retain on cooling back to room temperature. Of these, the martensitic transformation from tetragonal to monoclinic phase, which occurs at a lower temperature (around 950°C) and is accompanied by a volume increase of about 3–5%, is of great importance because it is the basis of the “transformation toughening mechanism” exhibited by zirconia-based materials [13].
Pure zirconia with only monoclinic phase is used quite rarely due to the volume increase associated with the martensitic transformation from tetragonal to monoclinic phase. However, high temperature phases (tetragonal and cubic), which are more valuable for technological applications than the monoclinic phase, can be partially or completely stabilized at room temperature by doping with appropriate amounts of compounds such as CaO, MgO, Sc2O3, Y2O3 or rare-earth metal oxides. Among them, Y2O3 is the most widely used additive to stabilize the ZrO2. Doping with concentrations of approximately 3mol% Y2O3 results in the formation of tetragonal phase, whereas doping with higher concentrations of approximately 8mol% Y2O3 results in the formation of cubic phase.
The physico-chemical characteristics required for nanocrystalline yttria-doped zirconia include precise chemical composition (dopants, impurities), defined crystalline structure (monoclinic, tetragonal, cubic), controlled phase transformations, uniform morphology, low crystallite size, narrow particle size distribution, high crystallinity, etc. Its properties and, consequently, its applications depend on these characteristics, which in turn depend on the synthesis procedure and reaction conditions.
A large number of traditional methods to synthesize yttria-doped zirconia have been reported in the literature, including co-precipitation [14], hydrothermal [15], solvothermal [16], sol–gel [17], sol–gel combustion [18], Pechini [19], pyrosol [20], etc. Many of these methods require complex and tedious procedures, expensive precursors, multi-step reactions, stabilizing or chelating agents, etc., and thus are difficult to control accurately. Typical drawbacks are inhomogeneity, varied particle size distribution, long reaction times, poor crystallinity particles requiring an additional crystallization step at high temperatures, etc.
The use of microwave-assisted hydrothermal synthesis is a very promising route to produce inorganic nanostructured powders [21–26]. The advantages over conventional methods and the characteristics of the resulting nanopowders are detailed in a previous publication [27]. To the best of our knowledge, there are only a few reports on the microwave-assisted hydrothermal synthesis of yttria-doped zirconia nanoparticles [28–31] in the available literature.
Guo et al. [28] synthesized nanocrystals of 3mol% yttria-doped zirconia from ZrOCl2.8H2O and YCl3·6H2O as precursors, NH4OH as precipitation agent, triethanolamine (TEOA) as mineraliser, and polyethylene glycol (PEG) as dispersant, and studied the effects of ZrOCl2.8H2O concentration, reaction temperature and time, mineralizer concentration and dispersant type. The results showed that the concentration of ZrOCl2·8H2O had little effect on the powder properties, whereas microwave time and temperature, TEOA concentration and PEG molecular weight greatly influenced the resulting nano-sized zirconia powders. The optimal parameters for preparing yttria-doped zirconia included the use of a temperature of 200–240°C, a time of 30–50min, a TEOA concentration of 0.3–0.5M and a PEG1000/PEG2000/PEG4000 concentration of 1.5wt%. The synthesised doped zirconia nanocrystals, tetrahedral and spherically shaped and with a size of approximately 20–30nm, had a tetragonal phase without any trace of monoclinic or cubic phases.
Pakharukova et al. [29] synthesized nanocrystalline 3.4mol% yttria-doped zirconia powders by co-precipitation of hydroxides from nitric acid solutions with aqueous ammonia at pH 9 followed by the microwave drying and calcination in the temperature range from 300 to 1000°C, but they did not provide any details on the synthesis conditions and procedure. At 300°C, the obtained particles, with sizes around 10nm, exhibited very poor crystallization. As expected, crystallinity and particle size increased with calcination temperature, leading to the formation of tetragonal phase (with a small amount of monoclinic phase in the samples treated at 800 and 1000°C). The particles in all samples had almost spherical shape.
Combemale et al. [30] synthesized 8mol% yttria-doped zirconia nanocrystals from ZrCl4, YCl3·6H2O, CH3CH2ONa (sodium ethoxide, a strong base) as mineralizer and ethanol as solvent, in an inert atmosphere (argon gas) within the reactor, at low temperature (160°C) and short heating time (2min). The powders had an average size of 5–10nm, while snowflake-shaped aggregates with size close to 150nm were observed. Thermal treatment at 300, 600, 800 and 1200°C was made to induce growing of crystals in order to reveal cubic phase. The as-prepared doped zirconia, as well as the powders treated at 300°C, exhibited very poor crystallinity. Only from 600°C the appearance of cubic monophase was observed. Moreover, the stability of cubic phase was maintained up to 1200°C.
Khollam et al. [31] prepared 10mol% yttria-doped zirconia powders from ZrO(NO3)2·xH2O and YCl3 as starting precursors and KOH as mineralizer. The microwave parameters (temperature, pressure and reaction time) were varied systematically in order to find the lowest temperature and time for the formation of cubic single-phase zirconia. The results indicated that cubic zirconia was obtained at a temperature as low as 125°C and a reaction time equal to 5min, although the powders were poorly crystalline. Because of this, to achieve better crystallinity, the powders were prepared under microwave conditions of 200°C and reaction time of 30min. The powders consisted of agglomerated particles with diameters ranging from 0.2 to 3μm and varying shapes, while the crystallite size, which was determined using the Scherrer equation, was found to be 14.4nm. To check the stability of the cubic phase with temperature, the synthesized powders were heat-treated at different temperatures ranging from 500 to 1000°C. The cubic phase remained stable up to 800°C, but at 900°C it completely transformed into the monoclinic phase.
The goal of the present study was to establish a simple and rapid route for the synthesis of nanoparticles of 3 and 8mol% yttria-doped zirconia, and their characterization, with high crystallinity and low particle size through a microwave-assisted hydrothermal method.
ExperimentalSamples preparationMicrowave ovenA commercial Milestone ETHOS 1 (Sorisole, Italy) microwave oven specifically designed for synthetic applications operating at 2450MHz and capable of programming and adjusting the most important reaction parameters (power, temperature, pressure and time) was used. A detailed description of the apparatus is shown in previous publications [32,33].
ChemicalsAll chemical reagents in present work were of analytical grade and used as received without further purification. All aqueous solutions were prepared with deionized water with a resistivity of >18MΩcm, produced by a Milli-Q Plus pure water generating system from Millipore (Bedford, MA, USA). ZrOCl2·8H2O (Sigma-Aldrich, St. Louis, MO, USA), YCl3·6H2O (Sigma-Aldrich, St. Louis, MO, USA) and KOH (Panreac, Barcelona, Spain) were used as starting materials.
Stock solutions ≈1M of ZrOCl2·8H2O and YCl3·6H2O were prepared, and they were standardized by inductively coupled plasma optical emission spectrometry (ICP-OES) by using a Thermo Jarrell Ash spectrometer, Model Iris Advantage Duo (Waltham, MA, USA). For the standardization of both stock solutions, as well as for the determination of Zr and Y in the supernatant liquids, the calibration was performed with standard solutions of appropriate concentration prepared by serial aqueous dilution from standard stock solutions of 1.000±0.002gl−1 of Zr and Y (Merck, Darmstadt, Germany). As analytical lines, the wavelengths at 339.197nm of Zr and at 371.029nm of Y were used.
ProcedureAll the reactions were carried out in a 100ml sealed vessel made of high-purity TFM, which is surrounded by a safety shield. Temperature and pressure during synthesis were monitored and controlled with the aid of a shielded thermocouple inserted directly into the vessel and with a pressure transducer sensor connected to the vessel. Built-in magnetic stirring (Teflon-coated stirring bar) was used. The evolution of time, temperature, pressure and power were continuously recorded during each experiment. The synthesis procedure was performed by precipitation and dehydration of hydroxides.
Synthesis of 3mol% yttria-doped zirconiaTwenty-five millimoles of ZrOCl2·8H2O (from standardized stock solution), 1.55mmol of YCl3·6H2O (from standardized stock solution) and 17ml of H2O were put into the 100ml vessel of the microwave oven, keeping a constant stirring. Under continuous stirring, 5.36ml of 10N KOH were added dropwise, and then 1N KOH (about 1ml), also dropwise, until a pH of 10±0.2 was reached. Immediate formation of a white large colloidal solid was observed. The vessel was sealed and placed in the microwave oven, where it was heated, with a heating ramp of 10min (rate of about 0.26°Cs−1), to a temperature of 180°C, with a dwell time of 20min. This sample was labeled as Z3Y.
Synthesis of 8mol% yttria-doped zirconiaTwenty-five millimoles ZrOCl2·8H2O (from standardized stock solution), 4.35mmol of YCl3·6H2O (from standardized stock solution) and 13.5ml of H2O were put into the 100ml vessel of the microwave oven, keeping a constant stirring. Under continuous stirring, 6.20ml of 10N KOH were added dropwise, and then 1N KOH (about 1ml), also dropwise, until a pH of 10±0.2 was reached. Immediate formation of a white large colloidal solid was observed. The vessel was sealed and placed in the microwave oven, where it was heated, with a heating ramp of 10min (rate of about 0.26°Cs−1), to different temperatures (180, 200 and 220°C), with a dwell time of 20min. This sample was labeled as Z8Y.
In all cases, after cooling down at room temperature, a white suspension was obtained. The separation of the solid from the liquid was performed by centrifugation using a multi-purpose bench top centrifuge at a gyration speed of 2400rpm (Núve NF800, Ankara, Turkey). The obtained particles, of white color, were collected, carefully washed several times with deionized water until chloride ions were no longer detectable in the washing water (AgNO3 test) and centrifuged every time, and finally dried overnight in air at 110°C. The dried material was crushed using an agate mortar and sieved through a 100μm sieve. The colorless supernatant liquid and the washing water were transferred to a 1000ml volumetric flask and made up to volume with deionized water, and it was analyzed by ICP-OES to determine the Zr and Y content as explained in the previous section. A portion of the powders was calcined at 500°C (sample Z3Y) and at 800°C (sample Z8Y) in air for 1h at a heating rate of 10°Cmin−1.
Characterization techniquesThe crystalline phases of the as-synthesized and calcined powders were determined by X-ray diffraction (XRD) on a D8 Advance (Bruker, Karlsruhe, Germany) diffractometer using Cu Kα radiation. The measurements were performed within 2θ angles ranging from 20 to 80° at 25°C, and the step size and time of reading were 0.02° and 2s, respectively. The following XRD patterns collected at the ICDD© databank (Joint Committee for Powder Diffraction Standards, JCPDS-The International Centre for Diffraction Data©, Newtown Square, PA, USA) were used as references for the analysis of our XRD patterns: PDF card 00-037-1484 of monoclinic zirconia, PDF card 00-048-0224 of tetragonal zirconia and PDF card 00-030-1468 of cubic zirconia.
The morphology, size and microstructure of the powders was studied by electron microscopy: field emission scanning electron microscopy (FE-SEM) on a S-4800 Type I (Hitachi, Tokyo, Japan) microscope; and high-resolution transmission electron microscopy (HR-TEM) on a JEM-2100F (JEOL, Tokyo, Japan) microscope working at 200keV, complemented by selected area electron diffraction (SAED). The image processing was performed using the DigitalMicrograph® software. The particle size distribution, mean size diameter (D0) and standard deviation (σ) were determined using the ImageJ software after measuring at least 100 particles in random fields of view on the HR-TEM micrographs.
Results and discussionPowder synthesisThe synthesis of 3 and 8mol% yttria-doped zirconia has been carried out by the route of precipitation and dehydration of hydroxides, which takes place in two steps. For the synthesis of Z3Y sample, in the first step, the precipitation of zirconium and yttrium hydroxides under strong alkaline pH conditions by the addition of KOH occurs according to the Eqs. (1) and (2), respectively. The total reaction occurring when both Zr- and Y-precursors are present in the solution is shown in Eq. (3). In the second step, the dehydration of the hydroxides by microwave heating leads to the formation of the corresponding oxides, according to the Eq. (4). The overall chemical reaction is shown in Eq. (5).
Similarly, the chemical reactions corresponding to the synthesis of the Z8Y sample are shown in Eqs. (6) and (7), and the overall chemical reaction is shown in Eq. (8). The preparation of this composition was performed at temperatures of 180, 200 and 220°C because, as will be seen later, at 180°C a poorly crystalline compound was obtained.
ZrOCl2·8H2O and YCl3·6H2O are very hygroscopic compounds. In addition, the melting point of YCl3·6H2O is 100°C and decomposes at this temperature with elimination of water of crystallization. Consequently, these compounds cannot be dried to eliminate moisture, so it is very difficult to quantitatively weigh an accurate quantity of both products. Because of this, stock solutions of both ZrOCl2·8H2O and YCl3·6H2O were prepared with a concentration approximately 1M, and they were standardized by ICP-OES, which ensures the use of stoichiometric amounts of Zr and Y. Thus, taking into account that the volume range in the vessel is 8–50ml, ≈25ml of ZrOCl2·8H2O stock solution are added to the vessel for all syntheses, and ≈1.55 or ≈4.35ml of YCl3·6H2O stock solution for synthesis of 3 or 8mol% yttria-doped zirconia, respectively, in addition to ≈7ml of KOH. Also, ≈17 and ≈13.5ml of H2O were incorporated for synthesis of Z3Y and Z8Y samples, respectively. Under these conditions, the volume of all reagents in all syntheses was practically the same (about 50ml). This is a faster and more precise method than the one used by other authors [31], who determined the ZrO2 content of the starting precursor ZrO(NO3)2·xH2O by taking the weight of the residual zirconia obtained by heating said reagent at 1000°C for 5h.
As stated in a previous publication [27], it is very important to perform a complete washing of the particles obtained directly from the microwave reactor in order to completely eliminate the KCl formed according to global reactions (5) and (8). The presence of this compound causes two problems: first, it presents a strong diffraction peak at 2θ≈28° (PDF card 00-041-1476 of sylvite, KCl), which overlaps with the reflection (−111) at 2θ=28.2° of the monoclinic phase of zirconia; and second, it contaminates the synthesized zirconia powders. In this regard, silver nitrate is a common and simple test for chloride ions in aqueous solutions and is extremely sensitive.
The global time of the microwave-assisted hydrothermal synthetic procedure is lower than 1h (10min for heating from room temperature to 180–220°C, 20min of dwell time and about 20–30min for cooling to room temperature). Although the heating rate from room temperature to the reaction temperature can be much higher (several degrees per second or even tens of degrees per second) than those used in this work (0.26, 0.29 and 0.32°Cs−1 for the syntheses performed at 180, 200 and 220°C, respectively), it is not advisable, since it avoids possible hot spots phenomena or superheating effect (which is related to the boiling point of a liquid under microwave irradiation and represents a basic problem of microwave heating). Gentle heating, as carried out in this work, is preferable. Fig. 1 illustrates the real-time evolution of temperature and pressure inside the reaction vessel during the microwave synthesis of Z8Y sample at temperatures of 180, 200 and 220°C. It is noticeable the great temperature and pressure stability throughout the synthesis. This can be explained by the mechanism of microwave dielectric heating (involving dipolar polarization and ionic conduction processes), which results in very efficient internal heating, increasing the temperature of the whole volume simultaneously and uniformly (the so-called volumetric heating). As can be observed, the pressure reached during heating was around 9, 18 and 24bar for the syntheses at 180, 200 and 220°C, respectively. Obviously, accurate control of reaction temperature and pressure within the vessel allows for more reproducible microwave synthesis conditions.
Analytical control of the microwave synthesisIn order to study the degree of precipitation of the elements involved in the microwave synthesis, the content of Zr and Y in the supernatant liquid after the completion of microwave-assisted synthesis was determined by ICP-OES.
The analytical determination by ICP-OES of Zr in the supernatant liquids for all microwave syntheses (i.e., for samples Z3Y and Z8Y prepared at 180, 200 and 220°C) showed that the concentration of this element was below its quantification limit (QL, about 0.007mg Zrl−1 for our ICP-OES equipment). This means that the content of Zr in the supernatant liquids was lower than 0.0007mg, corresponding to 0.000008mmol ZrOCl2·8H2O, which verified the quantitative precipitation (higher than 99.9999%) of this element for all samples. This result is the same as that obtained for the microwave-assisted hydrothermal synthesis of undoped zirconia [27].
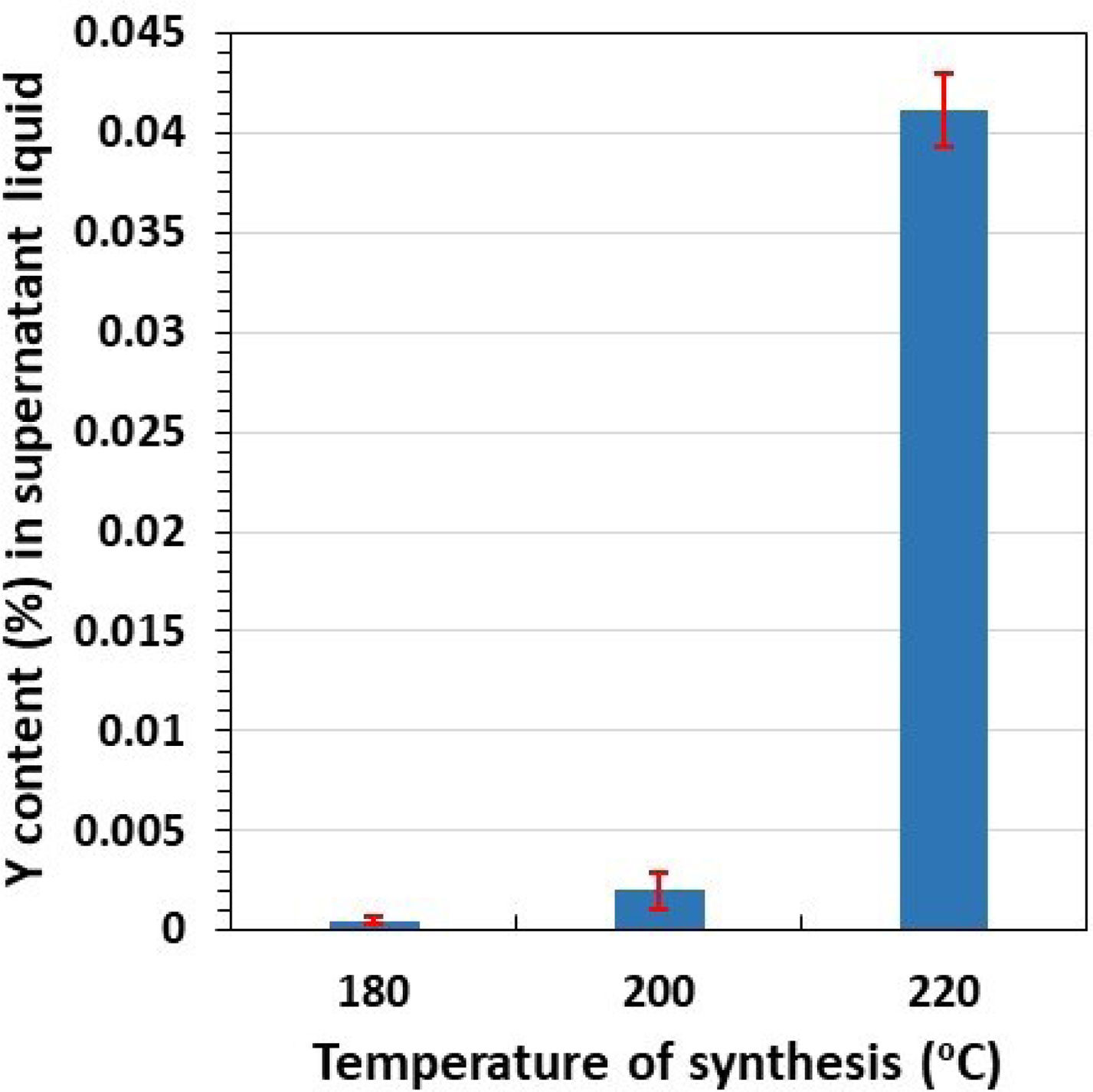
The behavior of Y is quite similar for Z3Y sample. Its determination by ICP-OES in the supernatant liquid showed that the concentration of this element was below its QL (about 0.008mg Yl−1 for our ICP-OES equipment) for microwave synthesis of this sample. In this case, the content of Y in the supernatant liquids was lower than 0.0008mg, corresponding to 0.000009mmol YCl3·6H2O, which confirmed the quantitative precipitation (higher than 99.999%) of this element. However, for the samples with the highest Y content (Z8Y prepared at 180, 200 and 220°C), the concentration of this element in supernatant liquid was higher than the QL, and could therefore be quantified. The analytical results are plotted in Fig. 2, which shows the Y content (in weight percentage with respect to the starting Y) in supernatant liquids as a function of synthesis temperature. As can be seen, the higher the reaction temperature, the higher the level of Y in solution. Nevertheless, the non-precipitated Y is very small for the reaction temperatures of 180 and 200°C (0.0005±0.0002 and 0.002±0.001%, respectively, which represents a quantitative precipitation of about 99.999%). Increasing the synthesis temperature to 220°C leads to a concentration of Y in supernatant liquid of 0.041±0.002%, which means that the Y precipitation level is 99.96%. Although this percentage of precipitation is high, it affects the stoichiometry of the synthesized 8mol% yttria-doped zirconia, and therefore should be taken into account.
Phase developmentThe crystalline phases of all the powders were determined by XRD. The XRD patterns of sample Z3Y as-synthesized and calcined at 500°C are depicted in Fig. 3. The XRD patterns of sample Z8YK prepared at temperatures of 180, 200 and 220°C as-synthesized and calcined at 800°C in air are illustrated in Fig. 4.
The XRD pattern of the as-synthesized sample Z3Y (Fig. 3a) shows a well-crystallized powder with all the typical reflections of the tetragonal phase, although some low intensity peaks reveal the presence of a fraction of about 20% of monoclinic phase. Furthermore, heat treatment at 500°C has no significant effect on either the crystallinity or the relative phase ratio, which remain almost constant (Fig. 3b).
The XRD pattern of the as-synthesized sample Z8Y prepared at 180°C (Fig. 4a, bottom spectra) shows the most important reflections of the cubic phase, but reveals low crystallinity. Because of this, the microwave synthesis was also performed at temperatures of 200 and 220°C. The XRD patterns of both the as-synthesized powder are shown in Fig. 4b and c (bottom spectra), respectively, where all the typical reflections of the cubic phase are well developed, revealing a higher degree of crystallinity. As expected, the higher the synthesis temperature, the higher the crystallinity of the powders. The calcination at 500°C of the three samples produces powders with relatively low crystallinity, mainly for the sample synthesized at 180°C. However, the thermal treatment at 800°C results in a large increase of the crystallinity which is similar for all three cases, as evidenced by the well-resolved XRD pattern (Fig. 4, top spectra). As clearly shown in Fig. 4, the only phase obtained is the cubic one.
The XRD patterns of all samples displayed in Figs. 3 and 4 do not show any additional phase within the detection limit of XRD, indicating a high purity of the obtained powders. On the other hand, the significant broadening of the peaks can be attributed to the nanocrystalline nature of the prepared powders, as will be shown below.
Considering that the reactions at 180 and 200°C take place with quantitative precipitation of both Zr and Y, as demonstrated in the previous section, and that the powders synthesized at 200°C present a higher crystallinity than those synthesized at 180°C, as well as that the crystallinity of the three samples (prepared at 180, 200 and 220°C) calcined at 800°C is similar, the temperature of 200°C was selected to perform the microwave synthesis of 8% mol yttria-doped zirconia.
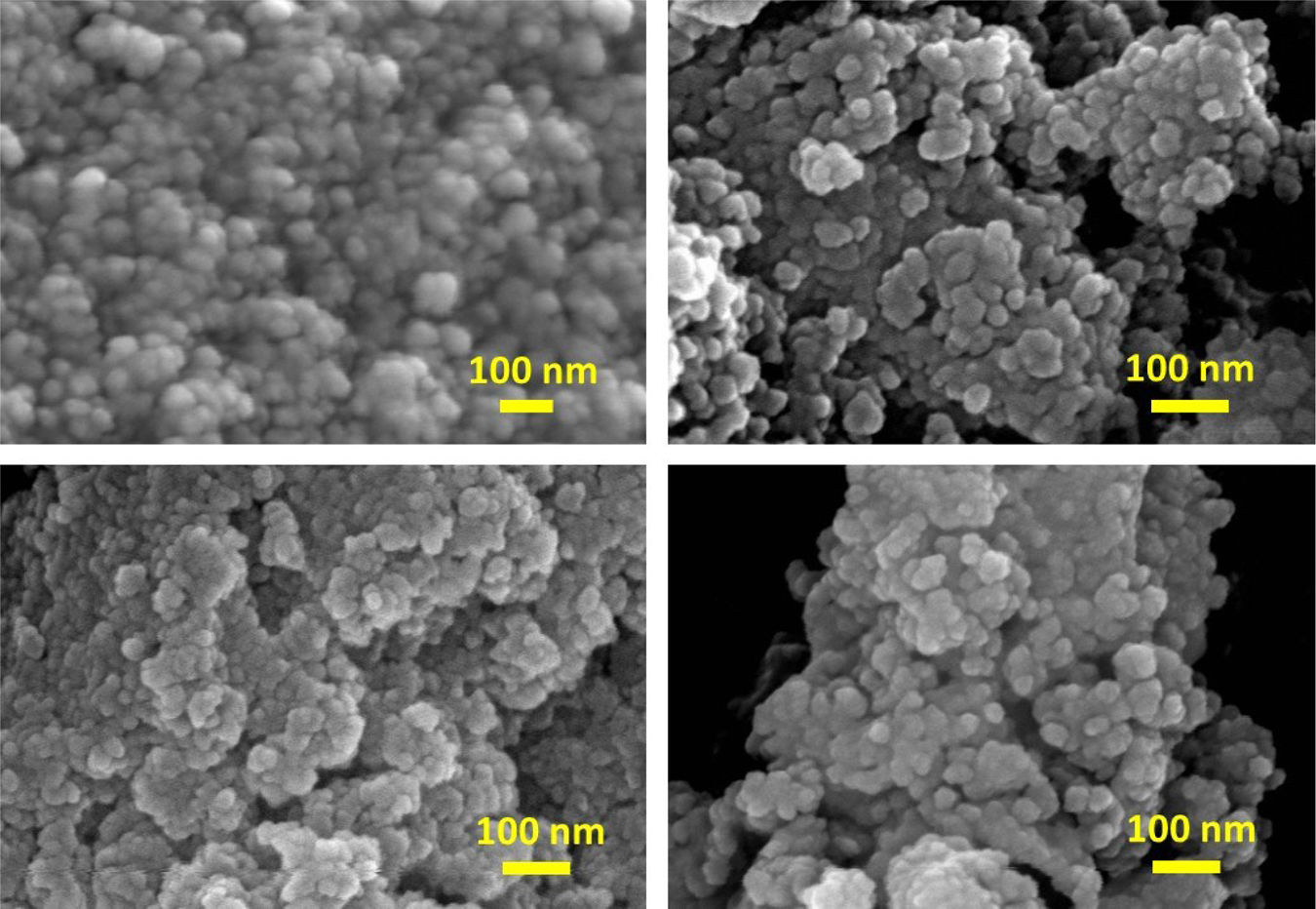
Microstructure and morphologyThe surface morphology and approximate particle size were evaluated for all samples by FE-SEM observations. Fig. 5 shows the FE-SEM images of the samples Z3Y, as-synthesized and calcined at 500°C, and Z8Y prepared at 200°C, as-synthesized and calcined at 800°C. In all cases, the images reveal nanometer-sized particles with an apparently spherical shape. These particles are agglomerated due to the effect of inter-particle forces, forming clusters of approximately 50 and 25nm for the as-synthesized Z3Y and Z8Y samples, respectively, and approximately 25nm for the corresponding calcined samples. Considering that the particles have a size range of about 5–10nm, as shown below, the small size of these clusters indicates that they are formed by agglomeration of very few particles.
HR-TEM and SAED studies were carried out in order to obtain structural information (i.e., morphology, crystallinity, crystal size and nanostructure) about the prepared powders.
Figs. 6 and 7 show representative HR-TEM images at different magnifications of the samples Z3Y, as-synthesized and calcined at 500°C, and Z8Y prepared at 200°C, as-synthesized and calcined at 800°C, respectively. From these images, it is clear that, in all cases, the particles are polycrystalline, consisting of smaller single crystallites with a typical diameter lower than about 10nm. The particles of the as-synthesized and calcined Z3Y sample (Fig. 6) show high uniformity, with spherical or nearly spherical shapes and sizes of approximately 10nm. However, there are some differences in the size and shape of the nanoparticles in Z8Y sample (Fig. 7), which could be attributed to the exclusive presence of the cubic phase. Thus, the nanoparticles in this sample are mainly spherical or nearly spherical and with rounded platelet-shaped morphology, although irregularly shaped particles can also be observed. Furthermore, there is a significant difference in particle size, which is about 5nm or smaller in the case of as-synthesized sample and about 10nm or larger in the case of calcined sample.
Fig. 8 illustrates the particle size distribution of the samples Z3Y, as-synthesized and calcined at 500°C, and Z8Y prepared at 200°C, as-synthesized and calcined at 800°C. It includes the D0 and σ values, which have been accurately calculated by fitting the particle size distribution histogram to the normal distribution function, in the case of as-synthesized samples, and to the log-normal distribution function, in the case of calcined samples. As it can be seen, D0 values for as-synthesized samples Z3Y and Z8Y are 6.2 and 3.5nm with σ values of 1.0 and 0.7nm, respectively. As expected, the thermal treatment of the samples increases the D0 values, slightly for Z3Y sample and considerably (due to the higher calcination temperature) for Z8Y sample: 8.0 and 11.3nm with σ values of 1.2 and 1.3nm, respectively. These obtained D0 values confirm that the clusters observed in Fig. 5 are formed by agglomeration of very few particles: approximately 7 or 8 on average for the as-synthesized samples and only about 2 or 3 on average for the calcined samples, which explains why the agglomerates of calcined samples are equal in size or smaller than those of as-synthesized samples. Also, the results quantify and confirm the previous observations made in Figs. 6 and 7 regarding particle size.
Particle size distribution of samples Z3Y (top) and Z8Y prepared at 200°C (bottom) as-synthesized (left side), fitted with a normal function (solid line), and calcined at 500°C, in the first case, and at 800°C, in the second case (right side), fitted with a log-normal function (solid line). Mean size diameter (D0) and standard deviation (σ) values are included.
Fig. 9 depicts the SAED patterns of the samples Z3Y, as-synthesized and calcined at 500°C, and Z8Y prepared at 200°C, as-synthesized and calcined at 800°C. It also includes the measured interplanar distances (the d-spacing, i.e., the distance between crystal planes), which have been calculated from the diameter measurement of each ring, the corresponding Miller indices, and the monoclinic, tetragonal and cubic crystalline phases. For all samples, the SAED pattern exhibits a series of clear concentric diffraction rings with intermittent spots, indicating the polycrystalline nature of the nanosized yttria-doped zirconia powders. As it can be seen, the SAED patterns of the as-synthesized and calcined Z3Y sample are similar, and all the rings can be clearly indexed to the monoclinic and tetragonal crystal systems. This indicates that the crystallinity of both samples is comparable, which is in agreement with the XRD patterns depicted in Fig. 3. The SAED pattern of the as-synthesized Z8Y sample shows the rings corresponding to the most intense reflections of the cubic phase, which confirms the presence of this monophase of ZrO2. The SAED pattern of the calcined Z8Y sample is similar to that of the corresponding as-synthesized sample, but exhibits brighter rings due to its higher crystallinity, which agrees with the XRD patterns illustrated in Fig. 4. It can be said that, regarding crystallinity and composition of the crystalline phases, all SAED results are consistent with the XRD results presented above.
SAED patterns of samples Z3Y (top) and Z8Y prepared at 200°C (bottom) as-synthesized (left side) and calcined at 500°C, in the first case, and at 800°C, in the second case (right side). The measured interplanar distances (d), the Miller indices (hkl) and the crystalline phases monoclinic (M), tetragonal (T) and cubic (C) are included.
Figs. 10 and 11 illustrate HR-TEM images at higher magnifications of the samples Z3Y, as-synthesized and calcined at 500°C, and Z8Y prepared at 200°C, as-synthesized and calcined at 800°C, respectively. They also include the measured distance between every two successive fringes (i.e., the d-spacing), the corresponding Miller indices and the monoclinic, tetragonal and cubic crystalline phases. The insets show well-ordered equidistant parallel lattice fringe patterns of individual nanocrystals, which confirms the high crystallinity of the two samples, even of the as-synthesized ones. In Fig. 10, crystallites belonging to both the monoclinic and tetragonal phases of zirconia can be noticed. Crystallites with measured d-spacing of 3.14–3.17Å and 2.84–2.85Å are consistent with the most characteristics (−111) and (111) planes, respectively, of monoclinic zirconia. Crystallites with measured d-spacing of 2.95–2.98Å can be attributed to the well-recognized (101) plane of tetragonal zirconia, and crystallites with measured d-spacing of 2.55–2.59Å can be assigned to (110) plane of this crystalline phase. In Fig. 11, crystallites belonging exclusively to the cubic phase of zirconia can be observed. Crystallites with measured d-spacing of 2.92–2.99Å and 1.79Å are consistent with the most characteristics (111) and (220) planes, respectively, of cubic zirconia, and crystallites with measured d-spacing of 2.52–2.59Å can be attributed to the (200) plane of this crystalline phase. These results agree with those obtained by XRD and SAED reported previously.
HR-TEM images at higher magnifications showing lattice fringe patterns (insets) of individual nanocrystals of Z3Y sample as-synthesized (top) and calcined at 500°C (bottom). The measured interplanar distances, the Miller indices (hkl) and the crystalline phases monoclinic (M) and tetragonal (T) are included.
HR-TEM images at higher magnifications showing lattice fringe patterns (insets) of individual nanocrystals of Z8Y sample prepared at 200°C as-synthesized (top) and calcined at 800°C (bottom). The measured interplanar distances, the Miller indices (hkl) and the crystalline phase cubic (C) are included.
Microwave-assisted hydrothermal synthesis has been shown to provide a simple and energy-efficient route for the preparation of nanometer-sized powders of 3 and 8mol% yttria-doped zirconia. In both cases, the synthetic procedure is very gentle (180–200°C) and rapid (30min) and is carried out in an aqueous medium, without the need for organic solvents, templates, stabilizing or chelating agents, etc., so it can be considered as environmentally friendly. At reaction temperatures above 200°C, the crystallinity of the synthesized powders increases, but the non-precipitated Y also increases, and therefore the stoichiometry of the synthesized powders decreases.
The intrinsic mechanism of microwave heating (volumetric heating) leads to compounds with a high homogeneity. Precise control of reaction temperature and pressure within the vessel allows for more reproducible microwave synthesis conditions. Standardization of Zr and Y stock solutions using an appropriate analytical technique, such as ICP-OES, ensures the use of stoichiometric quantities of both elements in the reactions involved in the synthesis, leading to the quantitative precipitation of Zr and Y and, consequently, to the formation of compounds with controlled stoichiometry.
The synthesis of 3 and 8mol% yttria-doped zirconia leads to the formation of tetragonal zirconia (with a fraction of about 20% monoclinic), in the first case, and pure cubic zirconia, in the second one. No impurities from other phases were detected. The as-synthesized nanoparticles obtained present a high degree of crystallinity and a very small average diameter (about 6 and 3nm for the 3 and 8mol% yttria-doped samples, respectively).
Declaration of competing interestThe author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This research has been carried out with financial support from the Spanish Ministry of Science, Innovation and Universities (MCIU), the State Research Agency (AEI) and the European Regional Development Fund (FEDER) through the Project PID2021-124521OB-I00.