Introduction
Carrying out research in primary care (PC) involves an increase in the care activity of the professionals involved in the scientific project. It requires effort as regards motivation, planning of care activity and training needs. primary care doctors and nurses have to spend more time in the clinic to carry out research,1 since there is no time set aside for these tasks. Also, they have a large patient workload and overcrowded clinics that do not have the space to separate these activities from purely care ones.2
When we design a study we must take care in the selection of suitable research professionals, taking into account motivation and the current difficulties as regards recruitment and follow up3 of the patient cohort, the time that has to be invested as well as the human and economic costs.4
Some factors that are known to influence the acceptance of the project by researchers in PC are: the training that the professionals receive, the working material and tools, the time required to carry out the intervention, the workload pressure in the clinics, the usefulness of this intervention in a normal clinic, and the expectations generated.2,3
The objective of our study is to find out the overall appreciation of the organisation of the clinical study by the researchers.
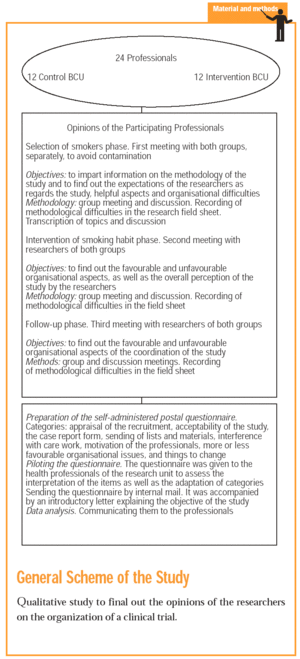
General Scheme of the Study. Qualitative study to final out the opinions of the researchers on the organization of a clinical trial.
Methods
The subjects of the study are 24 professionals who participate in a controlled, randomised by group, multi-centre and open clinical study, for the evaluation of the effectiveness of a stepped primary care smoking cessation intervention programme (ISTAPS).5 The randomisation unit in this study is the basic care unit (BCU), which is the doctor and nurse who tend to the same group of patients in the normal clinic.
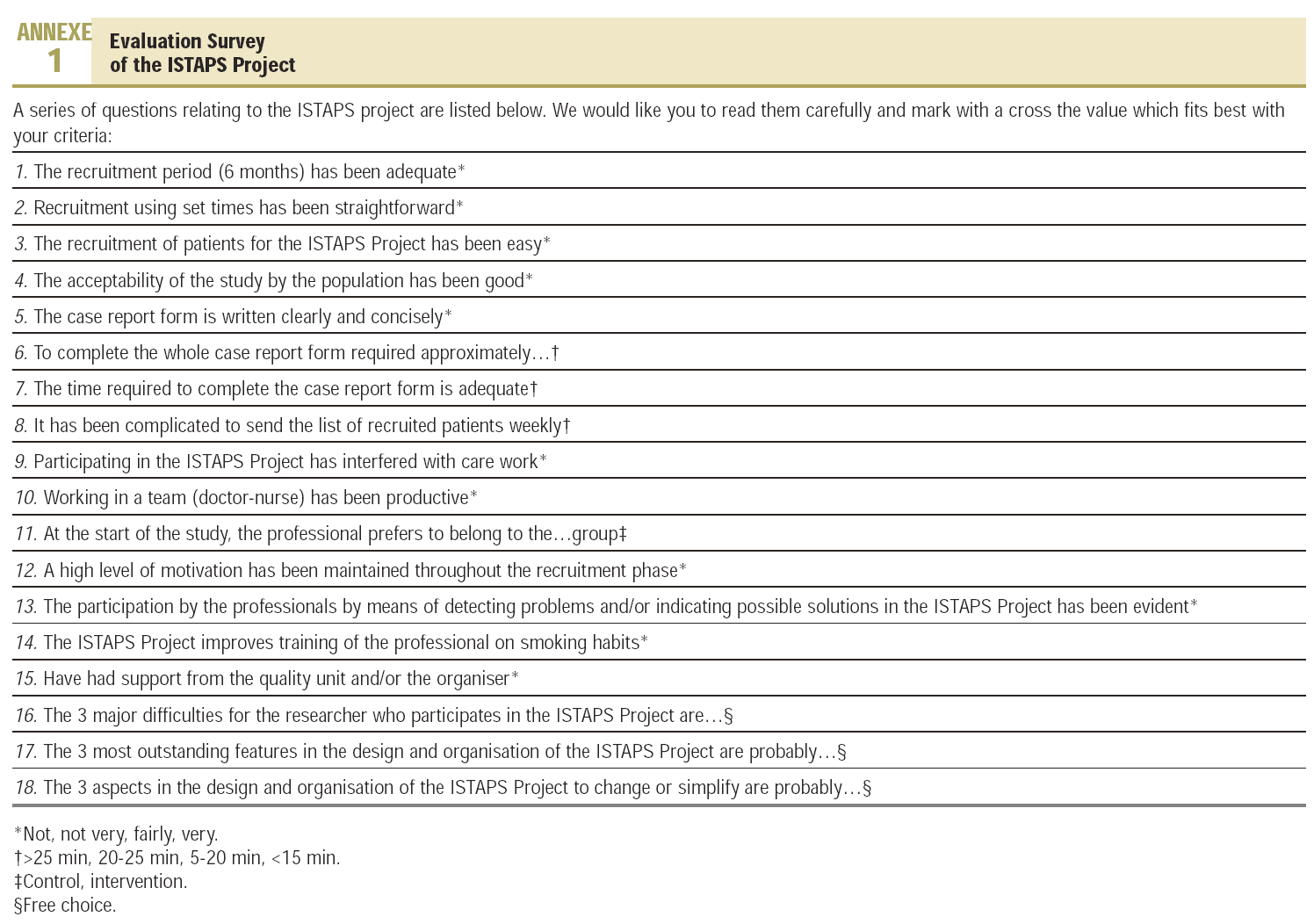
We chose a qualitative focus. In a first approach to the objective of the study, we had three formal meetings lasting 30 minutes with the control and intervention groups, respectively, in which we discussed aspects regarding the design, organisation and the possible factors that had to be changed in future studies, which we noted in the research information sheet.6 From the categories selected we prepared a structured, self-administered questionnaire with 18 items, 15 closed and 3 open (to confirm saturation), which was sent by post to the 24 participants (Annexe 1).
Results
The reliability of the questionnaire has been calculated using the split-half method. The Spearman-Brown coefficient was 0.74 (P<.010). The questionnaire was returned by post by 79.1% (19/24) of the participants. For each one of the items, the non-response rate was ≤10%. Of the 19 researchers who returned the questionnaire, 42.1% (8/19) belonged to the control group and 57.9% (11/19), to the intervention group. 47.4% (9/19) were doctors and 52.6% (10/19), nurses. The distribution by gender was: 63.2% (12/19) male and 36.8% (7/19) female. 63.2% (12/19) worked the morning shift and 36.8% (7/19), the afternoon shift. 68.4% (13/19) had previous experience in smoking problems, and 42.1% (8/19) had participated in clinical studies before.
The closed items provided information on:
1. Motivation and acceptability7: 89.5% (17/19) considered that they had been sufficiently motivated. 57.9% (11/19) would have preferred being assigned initially to the intervention group.
The acceptability of the population was very good for 31.6% (6/19) or fairly good for 57.9% (11/19).
2. Design of the study2: the case report forms (CRF) were fairly clear and concise for 63.2% (12/19). To complete the CRF took 20-25 minutes for 57.9% (11/19). Overall, the time dedicated was not quite adequate (31.6%; 6/19), or inadequate (31.6%; 6/19) for their clinics.
3. Organisational issues6: the ISTAPS study interfered a lot (36.8%; 7/19) or a little (57.9%; 11/19) with their care activities. The doctor-nurse model (BCU) was very productive (26.3%; 5/19) or fairly productive (47.4%; 9/19) for the investigation. They considered the support of the organiser to be very useful (52.6%; 10/19) or fairly useful (36.8%; 7/19).
4. Methodological aspects8: the posting of the completed CRF was not very complicated (36.8%; 7/19) or uncomplicated (42.1%; 8/19). The selection of patients at predetermined time intervals (by protocol) was not very easy (52.6%; 10/19) or not easy at all (21.1%; 4/19).
As regards the open questions in the questionnaire and the discourse analysis, the issues highlighted were:
1. Importance of participating in a national multi-centre project. They appreciated the coordinated organisation of a large project: "the coordination has generally been quite good, taking into account that it was nationwide."
2. Improvement of knowledge.1 The training received by the intervention group was motivating: "the training of the professional on smoking habits was the best." The control group also expressed interest in being trained about smoking. "When are you going to give us a training course?"
3. Research topic and assigned groups.9 The researchers considered that the research top was important: "I believe that smoking habits aroused motivation and interest." The control group expressed lacked of motivation: "I believe that the control does not do very much," "there is less intervention in the control group."
4. Support during the investigation.8,10 They emphasised the doctor-nurse work model: "I think working with the BCU model is important." If the doctor-nurse relationship is damaged, it could become an obstacle: "It's because they only think of themselves." They also appreciate the personal contact and external supervision8,10: "the good communication there is between the groups and the organisation of the study is important," "also the availability of the Organiser." The control group needs more attention11: "I feel a bit left aside as regards support and coordination."
5. Accessibility of the patient4: "the patient does not have to attend any talks or lectures. They only have to receive telephone calls at a time of their convenience."
6. Applicability in clinical practice.1,8 Clinical trial conditions are not the same as in a normal clinic due to there being less time available1,7: "it should stick to the reality of PC as regards clinic times" and a greater clinical workload: "finally, the recruitment phase coincided with the higher clinical workload months with vaccines and all."
7. Methodological and design issues. They disagreed with some of the patient selection criteria and with the wording and thoroughness of some items: "some patients could not enter due to smoking other substances," "the questions on alcohol consumption are not very clear"; they disagreed with methodological aspects of the protocol: "I think the recruitment hours should be more flexible."
Discussion
We obtained a high response rate to the questionnaire. The professionals show a high level of motivation and knowledge of smoking habits. They have previous experience in clinical studies and appreciate being participants of a multi-centre trial. They are concerned professionals, eager to receive the training offered in the clinical trial. These factors could explain the initial wish to be randomised to the intervention group, which is already evident in almost all studies published.
As regards the design, the professionals pointed out that the questionnaire, although extensive and repetitive, was of high quality. The subsequent applicability of the clinical trial, as regards design and methodology, is a factor that causes concerns and decreases motivation in our professionals.6 They devote between 20 and 25 minutes to the patient interview, a situation that is not very feasible in clinical practice. The researcher would like more straightforward forms, probably to cut down the time spent on it.9 We have to take into account that the average consulting time per patient is around 5 minutes. On the other hand, the acceptability by the population is good. The professional dedicates more time than usual to them, and this gives them satisfaction.
The organisational delays had a negative influence due to the start of the study coinciding with the vaccination campaign and holidays, when there is more pressure in the clinics.12 Even so, they were able to comply with the protocol as regards sending in the CRF. The demands of the "protocol" to spread recruitment according to specific days and times showed an unfavourable relationship between the expected benefit (to select a representative population sample) and the human cost generated (to devote clinic time to recruitment on days with a heavy workload). The professional devoted time to the research activity "when able," and this time did not necessarily coincide with the hour or day of the week specified in the trial. We must assess the importance of designing clinical trials in such a way that they are feasible in primary care clinics.
The professionals dissented with some of the selection procedures, the wording of the case report forms and methodology followed in the trial. We believe that when designing a trial we must collaborate with the professionals who carry out their care activities in PC, since it would allow us to adjust them more to the reality of their clinics.12
Another factor which appears to influence the obtaining of good results is the doctor-nurse research work group (BCU), since it helps in sharing the tasks involved. However, like any other human relationship, it is subjected to personal and work conflicts. In our opinion, the possible difficulties in the doctor-nurse relationship should be examined,10 since they could be an obstacle to obtaining the targeted selection of the trial.
What Is Known About the Subject
*There are organisational aspects that have an influence on clinical trials:
The topic investigated and the training received.
The time required to carry out the intervention, and pressure of care work in the clinics.
The clinical usefulness of the trial or intervention.
What This Study Contributes
*Positively valued organisational aspects:
Doctor-nurse work models with sharing of tasks.
External support of the organiser, particularly to the control group.
Collaboration of the professionals who will carry out the care activity in PC in the design, to adapt the intervention to the reality of the clinics.
Conclusions
The study highlights some organisational factors which we must take into account when designing a clinical trial. The favourable organisational aspects are: to participate in a national multi-centre trial, to receive training in new skills, to have quality material available, the doctor-nurse work model, the external support of the Organiser and to belong to the intervention group. The unfavourable organisational aspects are: the delays in starting the trial, questionnaires too long, methodological and design aspects which are far from the reality of PC clinics.
The most important limitation of our study lies in the fact that our professionals may not be representative, since they had an increased interest in smoking habits and experience in clinical trials. The results obtained must be verified by other groups to be able to make generalisations.
Correspondence:
Dra. A. Ramos Martín.
Flora Tristán, 12, 5.º, 6. 28021
Madrid. España.
E-mail: alrama@terra.com
Manuscript received September 12, 2005.
Manuscript accepted for publication January 23, 2006.