Liver stiffness measurements (LSMs) offer a noninvasive method for monitoring liver disease development. This study evaluated the prognostic value of different LSM trajectories in chronic hepatitis B (CHB) and compensated advanced chronic liver disease (cACLD) patients.
Materials and MethodsWe retrospectively analyzed 1272 CHB and cACLD patients with at least two LSMs, applied group-based trajectory modeling (GBTM) to identify distinct LSM trajectories, and used a Cox model to analyze their associations with liver-related events (LREs) and mortality risk.
ResultsPatients were categorized into five groups with distinct LSM trajectories: 67 (8.5 %), 13 (11 %), 36 (23.5 %), 34 (27.6 %) and 23 (25.0 %) developed LREs in Groups 1–5. The low stable trajectory (Group 3), the medium gradual decrease trajectory (Group 4) and high quickly decrease followed by increase trajectory (Group 5) had higher LREs risks than the low gradual decrease trajectory (Group 1) (adjusted HRs 2.26, 2.39, 2.67; 95 % CIs 1.50–3.40, 1.57–3.66, 1.61–4.43, respectively). Similar elevated risks were observed for hepatic decompensation, hepatocellular carcinoma (HCC), liver-related and all-cause mortality, except that there was no significant difference in the risk of HCC between Groups 4 and 1 (aHR 0.66, 0.36–1.23). When comparing Group 1 with the medium quickly decrease trajectory (Group 2), no significant differences were noted in the prognosis (P > 0.05). Notably, age over 40, high LSM, low PLT, and high total bilirubin were linked to high-risk trajectories (Groups 3–5).
ConclusionsMonitoring LSM trajectories improves prognostic prediction in CHB and cACLD compared with single measurements and may guide personalized treatment strategies.
The global health landscape is significantly impacted by hepatitis B virus (HBV), a disease that affects an estimated 257 million individuals globally [1]. This prevalence persists even amidst the accessibility of efficacious vaccines and therapeutic options. Chronic HBV infections are notably linked to the development of cirrhosis, hepatocellular carcinoma (HCC), and liver-related mortality [2].
The evaluation of liver fibrosis plays a crucial role in estimating the prognosis of patients with chronic liver disease. Most complications associated with chronic liver diseases arise in patients with advanced fibrosis or cirrhosis due to the development of portal hypertension. Prognosis varies significantly between patients with compensated cirrhosis (with or without esophageal varices) and those with decompensated cirrhosis [3].
Consistent surveillance of liver fibrosis in CHB patients is essential, encompassing periods before, during, and after antiviral treatment, to track disease progression effectively [4]. The reversal of liver fibrosis is a pivotal long-term objective in the treatment of CHB, as it mitigates the likelihood of liver degeneration and HCC emergence [5]. A liver biopsy has been used to stage liver fibrosis. However, owing to its invasiveness, risk of complications, and difficulty in repeatedly monitoring changes in fibrosis over time, it has limited use in assessing the prognosis of chronic liver diseases [6].
Currently, noninvasive methods, including serum biomarkers and imaging devices, have been developed to assess liver fibrosis and prognosis in patients with chronic liver disease [6,7]. Transient elastography (TE) is a noninvasive method for the diagnosis of liver fibrosis that involves measuring liver stiffness. TE has good accuracy in predicting the presence of cirrhosis [8], and its application has been particularly extensive in monitoring the progression of fibrosis in hepatitis C and alcoholic liver disease patients [9,10] and has been proposed as a useful tool to assess the risk of decompensation in patients with chronic liver disease [11–13]. Owing to its low cost and noninvasiveness, TE can be repeated during the follow-up of patients with liver disease. There are few studies on the prognostic effect of repeated LSM in patients with chronic hepatitis B [14], and most recent longitudinal studies of cACLD with other etiologies consider only the baseline and last LSM levels and do not fully use the results of each LSM [15–17].
Our previous work revealed that changes in liver stiffness indeed reflect patient prognosis [18]. However, during the follow-up process, we observed that the changes in liver stiffness did not exhibit a monotonic trend. Therefore, we included all the LSM values through group-based trajectory modeling (GBTM), fitted different trajectories with similar LSMs, and initiated this study to investigate how a particular trend in changes represents what kind of prognosis. The primary objective was to assess the correlation between these trajectories and the likelihood of LREs, including HCC, liver decompensation, and death. By distinguishing distinct subgroups with unique LSM trajectories, we aimed to reveal disease progression heterogeneity and inform the development of personalized treatment approaches for patients with CHB and cACLD.
2Materials and Methods2.1Study design and populationIn this retrospective analysis, we included all individuals at the Third Affiliated Hospital of Sun Yat-sen University diagnosed with CHB and cACLD who had undergone at least two consecutive TE assessments for clinical purposes from July 2013 to December 2019. Eligibility for inclusion was based on the following: (1) hepatitis B surface antigen (HBsAg) is consistently detectable, and/or HBV DNA is detected for more than six months; (2) at least two LSMs are detected by TE; and (3) the patient is receiving antiviral treatment with either entecavir (ETV) or tenofovir disoproxil fumarate (TDF) as the initial nucleos(t)ide analogue (NA). The initiation of antiviral treatment conformed to the criteria for NA treatment defined by the American Association for the Study of Liver Diseases (AASLD) guidelines [19].
The exclusion criteria for this research were as follows: (1) individuals with an initial LSM value less than 10 kPa; (2) those with an unreliable LSM reading or a failed TE examination due to the lack of valid measurements; (3) individuals with only two LSM results during the entire follow-up period, with the interval between them being less than three months; (4) individuals under 18 years of age; (5) patients with concurrent infections of hepatitis C, D, or HIV; subjects with alcoholic liver disease, nonalcoholic fatty liver disease (NAFLD) characterized by hepatic steatosis on imaging or histology, in conjunction with risk factors such as diabetes, obesity, or hyperlipidemia, and without significant alcohol consumption, autoimmune liver disorders, or other chronic liver disease causes; (6) patients with decompensated cirrhosis, HCC, or a history of liver transplantation at the start of the study or who only obtained two LSM results during the entire follow-up period with an outcome event occurring between the two LSM intervals; (7) those who had been on antiviral therapy for a duration of less than six months at the time of onset of the study or used interferon agents. A detailed description of the screening process is presented in Figure. S1.
2.2Liver stiffness measurementIn line with standard operating procedures, a skilled practitioner, proficient operator who possessed extensive experience in more than 500 procedures, performed LSM using TE (FibroScan®, Echosens, Paris, France) [20]. The procedure required patients to lie supine and extend their right arm fully, allowing access to the right hepatic lobe through the intercostal spaces. Before the examination, patients were advised to abstain from food for at least three hours.
The appropriate probe, either medium (M) or large (XL), was selected based on the individual's body habitus, utilizing the device's built-in probe selection feature. The TE software evaluated each measurement's success, and a session's validity hinged on three criteria: (1) a minimum of ten successful shots were recorded; (2) the success rate exceeded 60 %, calculated as the quotient of successful shots over the total shots taken; and (3) the interquartile range (IQR) was kept to less than 30 % of the median LSM value (IQR/M ≤ 30 %). These measurements are expressed in kilopascals (kPa).
2.3DefinitionsThe cACLD is defined as an LSM greater than 10 kPa [3].
The FIB-4 index was derived using the following formula: [age (years) × AST (U/L)]/{platelets (109/L) × [ALT (U/L)1/2]}. The FIB-4 index categorizes individuals into three risk tiers: less than 1.30 indicates low risk, 1.30 to 2.67 indicates intermediate risk, and above 2.67 indicates high risk [21].
2.4Patient evaluations and follow-upFor patient evaluation and subsequent follow-up, the initial assessment relied on the first available record suitable for LSM calculation [22]. The follow-up duration commenced with the first test and ended with either the patient's death or the conclusion of the study on October 1, 2023. The primary outcomes were liver decompensation, HCC emergence, or death. All participants had been on antiviral therapy for a minimum of six months at the time of onset of the study and continued throughout the follow-up period. The guidelines were followed during follow-up, laboratory evaluation, ultrasound, esophagogastroscopy, and management of esophageal varices and HCC [19]. LREs were categorized into three groups: liver decompensation (manifested by ascites, bleeding varices, or encephalopathy), HCC development, and liver-related fatalities. Mortality records were cataloged and differentiated based on whether they were liver-related (including liver transplants) or due to other causes [16].
2.5Statistical analysisContinuous variables are expressed as the means ± standard deviations (SDs) or medians (interquartile ranges [IQRs]) and were evaluated using the ANOVA if the variables were normally distributed or via the Kruskal‒Wallis test if the assumption of normality was not met. Categorical variables are expressed as proportions and were evaluated using the chi-square test.
The GBTM approach was implemented utilizing the R programming environment, version 4.3.2, with the 'lcmm' package to generate distinct trajectories of the LSM. The GBTM, a specialized finite mixture modeling approach, can differentiate subpopulations that are underlying and not direct subpopulations with the highest likelihood of membership in a particular trajectory group based on their progression over time [23]. Employing a maximum likelihood estimation method permits all available data to be utilized in model estimation, assuming the randomness of the missing data.
When the GBTM is applied, it is essential to ascertain the suitable quantity and form of the latent trajectories. An upper limit of seven potential trajectory classes was predefined, and models ranging from one to seven classes were sequentially evaluated. We concurrently assessed various growth parameters (linear, quadratic, or cubic) of each trajectory to obtain the optimal polynomial function forms describing the dynamic LSM change. The Bayesian information criterion (BIC) and Akaike information criterion (AIC) were utilized to compare and select the best-fit model, with preferences given to those with the lowest absolute values. Accurate classification was indicated by an average posterior probability (APP) of 70 % or higher for assigning individuals to a specific trajectory group. Additionally, models were chosen where each trajectory group comprised more than 5 % of the sample to ensure the reliability of further analysis (Table S1).
Survival curves for varying LSM groups were constructed via the Kaplan‒Meier method and were statistically compared using the log-rank test. The relationships between different LSM groups and LREs, HCC, liver decompensation, or mortality were explored using Cox proportional hazards models in both univariate and multivariate contexts. Findings are presented as hazard ratios (HRs) with 95 % confidence intervals (CIs), and forest plots were generated for visual representation. GBTM facilitated the examination of LSM trend changes across multiple time points, allowing for the categorization and analysis of baseline variables based on these trends. To test the robustness of our findings, we performed several sensitivity analyses. First, we further adjusted for baseline LSM levels to explore whether the effect of trajectories on LREs and death was independent of baseline conditions. Second, to mitigate reverse causality, we conducted a lagged analysis excluding participants who experienced the event within the first two years. Furthermore, we excluded participants with only two LSMs from a separate analysis. Finally, we applied a Fine‒Gray competing risk model, treating non-liver-related deaths as competing risk events.
To determine which baseline variables are predictive of higher risk within LSM groups, we conducted both univariate and multivariate logistic regression analyses, employing a forward selection process for variable inclusion. All the statistical computations were executed using R, version 4.3.2, and a P value of less than 0.05 in a two-tailed test was interpreted as statistically significant.
2.6Ethical statementThe study was granted ethical approval by the Ethics Committee of The Third Affiliated Hospital of Sun Yat-sen University, with reference number [2019]02–530–01. Informed consent was obtained in writing from all participants who agreed to be part of the study.
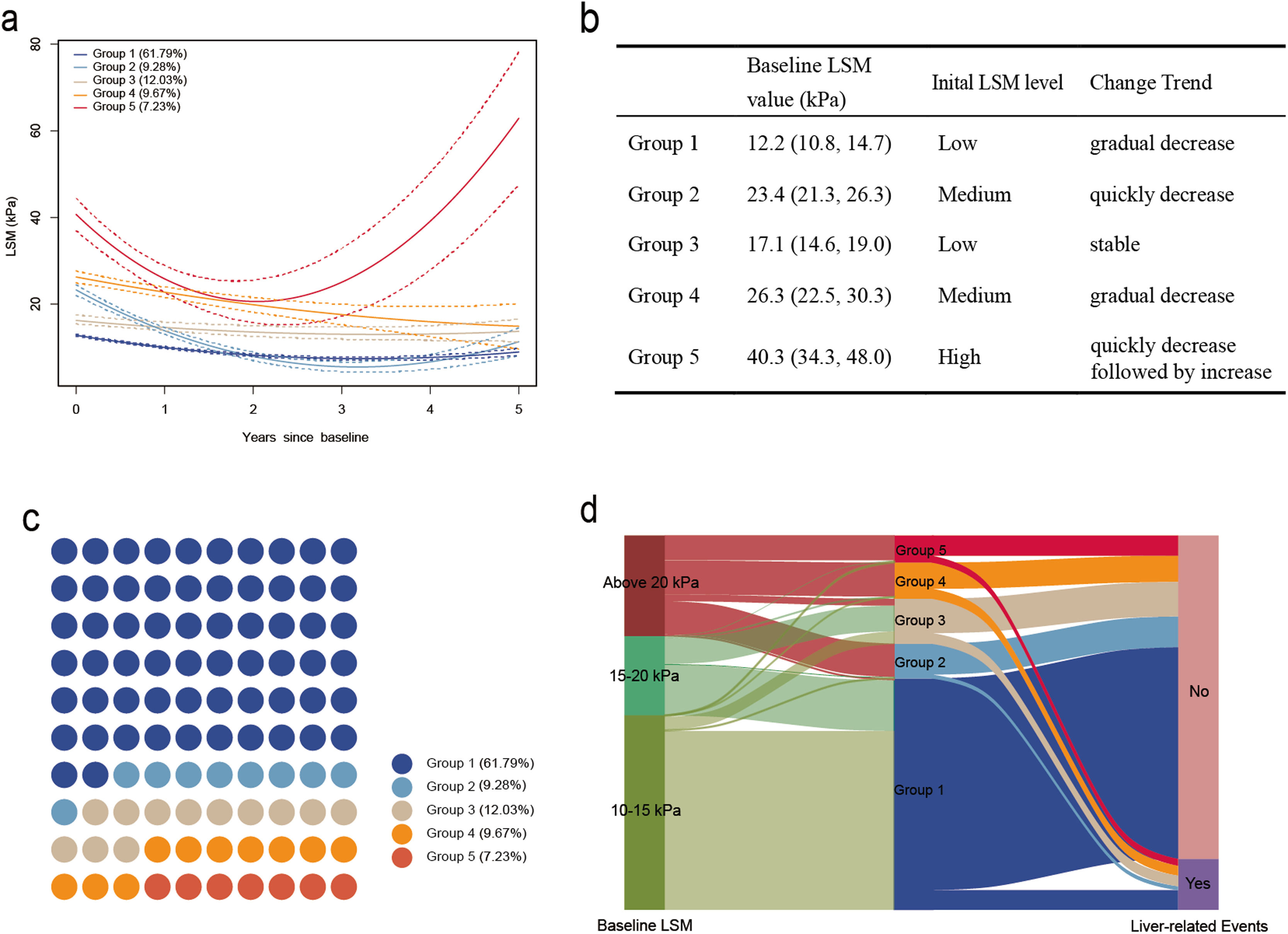
3Results3.1Study populationThrough the GBTM model, the LSM from repeated measurements was divided into trajectories, resulting in five distinct patterns of development, namely, the low gradual decrease trajectory group (Group 1, N=786, 61.79 %). This group is characterized by an initial lower level of LSM at 12.2 kPa (interquartile range, IQR: 10.8 to 14.7 kPa), with a gradual decline observed during the follow-up period. The medium quickly decrease trajectory group (Group 2, N=118, 9.28 %) is distinguished by an initially moderate level of LSM at 23.4 kPa (21.3–26.3), and it experienced a rapid decrease to a lower level during the follow-up period. The low stable trajectory group (Group 3, N=153, 12.03 %) is characterized by a relatively low level of LSM, with values of 17.1 kPa (14.6–19.0), and it remained stable throughout the follow-up period. The medium gradual decrease trajectory group (Group 4, N=123, 9.67 %) was characterized by a moderate baseline level of LSM at 26.3 kPa (22.5–30.3), and it exhibited a declining trend during the follow-up period. The high quick decrease followed by the increase trajectory group (Group 5, N=92, 7.23 %), this group is distinguished by having a relatively high baseline LSM at 40.3 kPa (34.3–48.0), and it demonstrated a pattern of initially rapid decline followed by a subsequent sharp increase during the follow-up period (Fig. 1a, 1b and 1c).
Trajectories of liver stiffness measurements (LSMs) (a). Group 1, Low gradual decrease trajectory group; Group 2, Medium quickly decrease trajectory group; Group 3, Low stable trajectory group; Group 4, Medium gradual decrease trajectory group; Group 5, High quickly decrease followed by increase trajectory group. Characteristics of different LSM trajectories (b). Population distribution of the five groups with distinct LSM trajectories (c). A total of 1272 patients were included in the study and categorized into Group 1 (786 [61.79 %]), Group 2 (118 [9.28 %]), Group 3 (153 [12.03 %]), Group 4 (123 [9.67 %]), and Group 5 (92 [7.23 %]) based on the different LSM trajectories during follow-up. Sankey diagram: Association of baseline LSM, LSM trajectories, and liver-related events in patients with chronic hepatitis B (CHB) and compensated advanced chronic liver disease (cACLD) (d). Liver-related events occurred in 8.52 % (67/786) of patients in Group 1, 11.02 % (13/118) of patients in Group 2, 23.53 % (36/153) of patients in Group 3, 27.64 % (34/123) of patients in Group 4, and 25.00 % (23/92) of patients in Group 5.
The distributions of the baseline demographics and clinical characteristics of the LSM trajectory patients are shown in Table 1. Except for sex, body mass index (BMI), HBV DNA, total bilirubin (TBIL), Alpha-fetoprotein (AFP) and Interval time between two LSM, which do not show differences among trajectory change groups (P>0.05), statistical differences exist among different trajectory change groups for all other variables (P<0.05). Compared with the other groups, Group 4 was older and had a lower platelet (PLT) count. Group 2 had higher alanine aminotransferase (ALT) and gamma-glutamyl transpeptidase (GGT) levels. Group 5 had higher levels of aspartate aminotransferase (AST) and lower levels of albumin (ALB) (Figure. S2).
Participant baseline characteristics stratified by LSM trajectories after six months of antiviral therapy.
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | P-value | |
|---|---|---|---|---|---|---|
| N | 786 | 118 | 153 | 123 | 92 | |
| Mean age (years) | 42 (35, 50) | 43 (36, 50) | 46 (40, 54) | 50 (42, 55) | 46 (37, 54) | <0.001 |
| Male sex (n, %) | 630 (80.2 %) | 100 (84.8 %) | 123 (80.4 %) | 93 (75.6 %) | 70 (76.1 %) | 0.404 |
| BMI (kg/m2) | 23.6 (21.4, 25.3) | 23.6 (20.3, 26.8) | 24.3 (20.1, 28.1) | 25.5 (21.2, 28.7) | 23.3(20.2, 26.2) | 0.702 |
| HBV DNA* (log10IU/ml) | 1.6 (1.1, 2.5) | 1.8 (1.3, 2.3) | 1.5 (1.2, 2.2) | 1.5 (1.1, 2.1) | 1.6 (1.1, 2.4) | 0.233 |
| PLT × 109/L | 145 (132, 190) | 145(111, 180) | 126 (79, 145) | 111 (76, 145) | 136 (81, 145) | <0.001 |
| ALT (U/L) | 41.5 (28.2, 71.0) | 52.0 (32.2, 158.5) | 39.0 (30.0, 58.0) | 43.0 (31.5, 64.0) | 44.0 (30.0, 81.0) | 0.015 |
| AST (U/L) | 39.0 (30.0, 63.0) | 53.5 (35.0, 116.0) | 40.0 (32.0, 57.0) | 50.0 (36.0, 69.0) | 58.5 (43.7, 109.2) | <0.001 |
| GGT (U/L) | 47.0 (30.0, 78.0) | 88.5 (55.2, 143.2) | 54.0 (34.0, 84.0) | 79.0 (51.5, 120.0) | 84.5 (61.0, 134.5) | <0.001 |
| TBIL (umol/L) | 15.4 (11.6, 21.4) | 15.6 (12.8, 24.6) | 17.1 (12.2, 22.6) | 16.1 (12.0, 23.5) | 17.2 (12.7, 24.2) | 0.080 |
| ALB (g/L) | 44.9 (42.6, 46.9) | 42.3 (39.9, 44.5) | 44.5 (40.8, 46.9) | 41.5 (38.1, 44.8) | 39.3 (35.0, 42.8) | <0.001 |
| AFP (ng/mL) | 7.2 (3.2, 11.4) | 7.2 (3.1, 13.8) | 7.0 (3.5, 16.0) | 6.9 (3.0, 16.2) | 7.3 (3.3, 18.1) | 0.128 |
| Baseline LSM value (kPa) | 12.2 (10.8, 14.7) | 23.4 (21.3, 26.3) | 17.1 (14.6, 19.0) | 26.3 (22.5, 30.3) | 40.3 (34.3, 48.0) | <0.001 |
| Baseline FIB-4 | <0.001 | |||||
| <1.30 | 599 (76.2 %) | 63 (53.4 %) | 102 (66.7 %) | 66 (53.7 %) | 40 (43.5 %) | |
| 1.30–2.67 | 119 (15.1 %) | 25 (21.2 %) | 30 (19.6 %) | 32 (26.0 %) | 31 (33.7 %) | |
| >2.67 | 68 (8.7 %) | 30 (25.4 %) | 21 (13.7 %) | 25 (20.3 %) | 21 (22.8 %) | |
| Interval time between two LSM (years) | 1.8 (1.0, 2.9) | 1.9 (1.0, 3.2) | 2.0 (1.0, 2.9) | 1.8 (1.0, 2.9) | 1.7 (0.8, 2.7) | 0.619 |
Group 1, Low gradual decrease trajectory group; Group 2, Medium quickly decrease trajectory group; Group 3, Low stable trajectory group; Group 4, Medium gradual decrease trajectory group; Group 5, High quickly decrease followed by increase trajectory group.
AFP, alpha-fetoprotein; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; FIB-4, fibrosis score based on four factors; GGT, gamma-glutamyl transpeptidase; HBV DNA, hepatitis B virus DNA; LSM, liver stiffness measurement; PLT, platelet; TBIL, total bilirubin.
We evaluated a total of 5417 individual LSMs from 1272 patients. Each patient underwent a median of 4 (IQR, 3–5) LSMs. In the present study of CHB patients and cACLD patients, the mean follow-up time was 6.15 years, with a total of 7818.31 person-years of follow-up. A total of 173 patients (13.6 %) experienced liver-related events, including 60 (4.7 %) liver-related deaths, 65 (5.1 %) cases of cirrhotic decompensation, and 137 (10.8 %) cases of HCC. In the group of 60 patients, the particular causes of mortality related to the liver were as follows: hepatic decompensation was the cause for 27 patients, HCC for 20 patients, a combination of hepatic decompensation and HCC for 11 patients, and two individuals who underwent liver transplants. LREs were observed in 8.52 % (67/786) of patients in Group 1, 11.02 % (13/118) of patients in Group 2, 23.53 % (36/153) of patients in Group 3, 27.64 % (34/123) of patients in Group 4, and 25.00 % (23/92) of patients in Group 5 (Fig. 1d). Moreover, we identified 63 instances of viral breakthrough, with the majority (39 cases) occurring in the high-risk group (Groups 3–5). During the study's follow-up period, 78 participants (6.1 %) were documented to have died.
Kaplan–Meier curve analysis confirmed the significant differences in the incidence of LREs and mortality between different groups (P<0.001) (Fig. 2). In the context of LREs and all-cause mortality, no significant differences were observed between Group 1 and Group 2 (P > 0.05). In terms of LREs, liver-related mortality, and all-cause mortality, Groups 3, 4, and 5 presented significantly higher cumulative incidence rates than Groups 1 and 2 did (P < 0.05). For HCC, Groups 3, 4, and 5 presented significantly higher rates than Group 1 did, whereas no significant difference was found between Groups 4 and 2. In terms of cirrhotic decompensation, Groups 3, 4, and 5 had significantly higher rates than Group 1 did; however, no significant difference was detected between Groups 3 and 2 (Table S2).
Kaplan‒Meier survival analysis curves for liver-related events (LREs) and deaths in the cohort of CHB patients with cACLD according to different LSM change trajectories.
Group 1, Low gradual decrease trajectory group; Group 2, Medium quickly decrease trajectory group; Group 3, Low stable trajectory group; Group 4, Medium gradual decrease trajectory group; Group 5, High quickly decrease followed by increase trajectory group.
Liver-related events (a), HCC (b), liver decompensation (c), liver-related death (d), and overall death (e). P value was determined by the log-rank test. Crude rates of liver-related primary outcomes at the end of follow-up according to different LSM change trajectory groups in the entire cohort of CHB patients with cACLD (f).
The 2, 5-year risk of the LREs with Group 1 at 1.4 %, and 6.1 %; Group 2 at 2.6 % and 9.2 %; Group 3 at 0.7 % and 14.3 %; Group 4 at 4.9 % and 18.7 %; and Group 5 at 3.3 % and 20.5 %. However, the risk of developing LREs was < 1 % in all groups within 1 year (Table 2). Considering the risk of LREs, we categorized CHB patients and cACLD patients into low-risk (Groups 1 and 2) and high-risk (Groups 3, 4, and 5) groups. Kaplan–Meier analysis revealed significant differences in LRE incidence and death between the low- and high-risk groups (P<0.001, Figure. S3).
Cumulative incidence of LREs occurrence and mortality.
Group 1, Low gradual decrease trajectory group; Group 2, Medium quickly decrease trajectory group; Group 3, Low stable trajectory group; Group 4, Medium gradual decrease trajectory group; Group 5, High quickly decrease followed by increase trajectory group.
LSM, liver stiffness measurement; LREs, liver-related events.
The relationships between different LSM trajectories and LREs, HCC, decompensation, liver-related mortality, and overall mortality are shown in Fig. 3. When Group 1 was used as the reference, in the crude model, Group 3, Group 4 and Group 5 were positively correlated with LREs; the HRs and 95 % CIs were 2.80 (1.87–4.20), 3.22 (2.13–4.87), and 3.05 (1.90–4.91), respectively. After adjusting for multiple factors, Group 3, Group 4, and Group 5 still had 2.26 times (95 % CI 1.50–3.40), 2.39 (1.57–3.66) and 2.67 (1.61–4.43) risk for LREs, respectively. Moreover, similar results were observed for decompensation, liver-related mortality and overall mortality, with aHR and 95 % CIs values of 3.31 (1.55–7.09), 4.31 (2.11–8.82) and 3.47 (1.87–6.45) in Group 3 and 3.62 (1.82–7.23), 2.58 (1.72–4.44) and 2.57 (1.81–4.06) in Group 4 and 3.03 (1.21–7.58), 3.39 (1.41–8.17) and 2.96 (1.35–6.50) in Group 5. In terms of the risk of HCC development, our findings indicate a significantly increased risk among Groups 3, and 5 in the crude model. In the adjusted model, the associations for Groups 3 and 5 remained significant, with aHR and 95 % CI values of 2.28 (1.46–3.58) and 2.22 (1.28–3.86), respectively. However, in Group 4, the risk did not significantly increase (P > 0.05). Furthermore, our results indicated that there was no statistically significant difference between Group 2 and Group 1 regarding LREs, HCC, cirrhosis decompensation, liver-related mortality, or overall mortality (P > 0.05).
Associations between the trajectories of the LSM and liver-related events (LREs) and death. Model 1: unadjusted model; Model 2: adjusted for age and sex. Model 3: adjusted for age, sex, ALB, ALT, AST, GGT, PLT, TBIL, and interval time between two LSMs. HR, hazard ratio; 95 % CI, 95 % confidence interval; LSM, liver stiffness measurement.
Group1, Low gradual decrease trajectory group; Group2, Medium quickly decrease trajectory group; Group3, Low stable trajectory group; Group4, Medium gradual decrease trajectory group; Group5, High quickly decrease followed by increase trajectory group.
Further adjustment for LSM levels at baseline did not substantially change the associations (Table S3). Sensitivity analysis by excluding outcomes within the initial 2 years of follow-up (Table S4), excluding participants with only two LSMs (Table S5), and treating deaths as competing risk events (Table S6) generated findings similar to those of the primary analysis.
3.4Baseline characteristics predictive of high-risk LSM changes groupsFrom a clinical standpoint, our study indicated that a comprehensive and dynamic evaluation of baseline LSM and LSM changes could aid in categorizing the likelihood of LREs and mortality in patients with CHB and cACLD. We stratified the patients into low-risk groups (Group 1 and Group 2) and high-risk groups (Group 3, Group 4, and Group 5) by integrating baseline LSM and the trajectory of LSM changes and further predicted the high-risk groups based on the patients' baseline characteristics. In the univariate analysis, age ≥ 40 years, high LSM, PLT < 150 × 109/L, AST ≥ 40 IU/L, GGT ≥ 40 IU/L, TBIL ≥ 17.1 µmol/L, low ALB and FIB-4 index ≥ 1.3 were identified as factors associated with high-risk LSM change groups. However, in multivariable analyses, only age ≥ 40 years (aOR=2.72, 95 % CI: 1.87–3.97), high LSM (aOR 1.24, 95 % CI: 1.20–1.28), PLT < 150 × 109/L (aOR 2.54, 95 % CI: 1.78–3.64) and TBIL ≥ 17.1 µmol/L (aOR 1.52, 95 % CI: 1.11–2.10) were also relevant risk factors for the high-risk group (Fig. 4).
Baseline characteristics predictive of high-risk LSM change groups.
ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FIB-4, fibrosis score based on four factors; GGT, gamma-glutamyl transpeptidase; LSM, liver stiffness measurement; OR, odds ratio; 95 % CI, 95 % confidence interval; TBIL, total bilirubin.
The present study provides a comprehensive analysis of LSM trajectories in patients with CHB and cACLD, offering novel insights into the prognostic implications of longitudinally monitoring LSM, as it refines risk prediction. A large cohort of 1272 patients was studied, with 5417 reliable LSMs, followed by patients for a median of more than 6.15 years; this represents the largest cohort study on LSM dynamics in patients with CHB with cACLD thus far.
It is well known that cirrhosis is a dynamic evolving condition, and it is reasonable to think that repeated LSM might better predict liver disease progression [15]. We incorporated all the measured LSM results into the model via the GBTM approach, ultimately identifying five distinct trajectories of LSM change with prognostic significance. Our findings indicate that there was no significant difference in the risk of LREs or overall mortality between Group 1 and Group 2 (P < 0.05), despite the substantial difference in their baseline LSM levels. Furthermore, we observed that Group 3, despite having a lower baseline LSM, presented a greater risk for LREs, HCC development, liver-related mortality, and overall mortality than did Group 2 (P < 0.05), as this finding indicates that the patient's liver disease remains active or insufficiently controlled. Risk stratification of patients using a single baseline LSM could misjudge a patient's actual risk and miss opportunities for individualized risk assessment [24]. Our comparisons further highlight the limitations of relying solely on the baseline LSM to predict outcomes for patients afflicted with liver conditions. Semmler et al. previously underscored this point in their research, demonstrating that the LSM dynamics relative to the baseline LSM are highly significant [25]. This notion is also supported by a study encompassing a diverse range of etiologies [16,26].
According to the pathophysiological principles, it is intuitive to treat any patient with an LSM of 20 kPa, or in whom the LSM remains steady or even increases, as a patient at risk, as this indicates that the patient's liver disease remains active or insufficiently controlled [25]. In our study, Group 5 demonstrated the highest risk for LREs, HCC, liver-related mortality, and overall mortality, with aHR values of 2.86, 2.80, 5.43, and 4.25, respectively. It had the highest baseline LSM at 40.3 (34.3 to 48.0) kPa, demonstrating an initial rapid decline in the LSM, followed by a precipitous rise and maintenance at over 20 kPa during the follow-up. However, Group 3, Group 4, and Group 5 showed no significant differences in LREs or mortality risk (P > 0.05). This could be due to the nonlinear relationship between the LSM and LREs, in which the risk associated with the LSM does not increase proportionally with every unit increase in the LSM. Instead, there may be a threshold beyond which the risk remains relatively stable despite further increases in the LSM. Furthermore, in our study, Group 5 showed a rapid initial decline in LSM, which could suggest an early positive response to treatment. This initial decrease might have had a beneficial effect on the liver, potentially reducing the risk of LREs and the mortality rate during the early stages of the follow-up period.
Compared with Group 2, where the LSM rapidly decreased to a low level, a continuous slow decline in the LSM (Group 4) does not necessarily indicate low risk, even if the LSM is less than 20 kPa after 2 years (with a decrease of more than 20 %). This further highlights the importance of including all LSMs in risk stratification. Compared with Group 1, Group 4 was found to have significantly elevated risks of LREs, liver decompensation, and both liver- and all-cause mortality. However, no significant difference in the risk of HCC was observed between Group 4 and Group 1 (P>0.05), with a P value of 0.341; consistent results were also obtained via sensitivity analyses. The lack of significant difference in the risk of HCC may be attributed to the sustained decrease in LSM, indicating that liver disease in patients is essentially under control. Despite the higher baseline LSM, compared with patients whose LSM remains stable (Group 3) or increases (Group 5), the continuous decrease in LSM can still reduce the risk of HCC.
The Baveno VII criteria for a clinically significant decrease in LSM are capable of identifying patients at low risk. The rule attributing a low risk to patients whose LSM decreases to <10 kPa might be accurate. In our study, the low-risk group (Groups 1 and 2) had a decrease in LSM to <10 kPa in 1 and 2 years, respectively, and had a lower risk of LREs, HCC, decompensation and mortality than did the high-risk group (Groups 3, 4 and 5) (P < 0.05). However, the widespread interpretation of a decrease of 20 % as “clinically relevant” might not be fully justified; in our study, patients in Group 4 still needed to be classified as high risk even if their LSM was less than 20 kPa (with a decrease of more than 20 %) two years later, Xu et al. [14]. reported that a decrease in LSM by 25 % is the optimal cutoff for predicting liver fibrosis regression (LFR) in CHB patients, Petta et al. reported that a 20 % change in LSM can stratify the risk in patients with NAFLD and cACLD [16], and Semmler et al. reported that several binary cutoffs for LSM (10 %, 20 %, and 30 % decreases) seem equally accurate in stratifying decompensation risk in cACLD patients [25]. Moreover, the absolute risk of the patient must still be considered: Although the risk of an individual patient changing from 10 kPa to 8 kPa (20 %) decreases, the overall risk of another patient decreasing from 50 kPa to 40 kPa (20 %) decreases similarly but is undoubtedly greater. Additionally, the actual changes in the LSM of the patient must still be considered. For example, patients with persistently decreasing LSM levels may exhibit significant clinical differences from those whose levels initially increase and subsequently decrease, warranting further investigation. Furthermore, fluctuations in the LSM could be attributed to factors other than the progression of liver disease. These may include inconsistencies arising from the operator or the patient's condition. Nascimbeni et al. demonstrated that approximately 49.7 % of patients exhibited a variation exceeding 20 % in their short-term paired LSM readings, despite no evidence of liver function deterioration or decompensation [27].
Although current clinical practice guidelines recommend yearly LSM, robust evidence supporting this recommendation is lacking, particularly regarding the predictive value of repeated LSM for clinical events [28,29]. This gap may stem from the limited number of studies on this topic, small sample sizes, and the absence of a standardized approach to assessing LSM dynamics [30,31]. Consequently, interpreting individual LSM changes in clinical practice becomes challenging. By including all LSM measurements in risk stratification, rather than relying solely on inflexible “high- vs. low-risk criteria” at a single baseline time point, we aim to increase the precision and generalizability of our study results and subsequent recommendations. In this cohort of 1272 adults with CHB and cACLD, patients in Groups 1, 2, 3, 4, and 5 had a 1.4 %, 2.6 %, 0.7 %, 4.9 %, and 3.3 % risk of developing LREs in the following 2 years, respectively. However, the risk of developing LREs was <1 % in all groups within 1 year, suggesting that surveillance elastography should be considered within 1 to 2 years. Moreover, patients identified as high risk by routine LSM surveillance, such as those in Groups 3–5, should be more closely monitored for HCC. In addition, the use of portal hypertension-lowering drugs should be intensified, and gastroscopy should be performed more frequently to assess the status of esophageal and gastric varices. These measures are essential for improving patient outcomes.
In our investigation, a large share of individuals with CHB and cACLD demonstrated a reduction in LSMs, aligning with observations from CHB, alcohol-related liver disease (ALD), and NAFLD populations [12,26,32]. This observation might be because our patient sample was exclusively sourced from tertiary hospitals and had received continued long-term treatment and regular follow-up [14]. Holtz Thorhauge et al. [26] and Gidener et al. [17] did not identify any clear pattern of predictors for longitudinal LSM changes in their studies. This may be due to the influence of different baseline LSM levels, where existing biochemical markers struggle to predict the trend of changes in LSM across various baseline levels. Taking this into account, we have made predictions for the trajectory of the high-risk group and recognized that age ≥ 40 years, LSM, PLT < 150 × 109/L and TBIL ≥ 17.1 µmol/L are risk factors for trajectories linked to a high-risk cohort, indicating their prognostic significance. It is important for future research to delve deeper into the correlation between these biomarkers and changes in LSM trajectories.
Our study provides a basis for future studies on noninvasive monitoring in CHB patients and cACLD patients, but our observational design has several limitations. First, the majority showed a decrease in the LSM, whereas only a small fraction exhibited an increase in the LSM. To ensure that the trajectories identified are sufficiently representative, the GBTM obtained only one LSM growth trajectory, without discerning increasing LSM trajectories at lower or moderate levels. Second, patients with identical starting and ending LSMs but different intermediate trends may exhibit significant clinical differences. Future studies will similarly require a larger sample size for comparative analysis or may necessitate alternative analytical approaches to discern these differences. Third, the study's retrospective design introduces a risk of selection bias. Finally, owing to the limited number of patients with paired liver biopsies and varying time intervals, evaluating the relationship between liver stiffness progression or remission and pathological results is challenging. Conducting paired liver biopsies at baseline and follow-up across multiple centers will enhance our findings [33].
5ConclusionsWe conducted a refined classification of patients with CHB complicated by cACLD based on baseline LSM and its dynamics, utilizing the GBTM. This stratification allowed us to investigate the risks of LREs, HCC, and liver decompensation, as well as liver and overall mortality, across different cohorts and can optimize the stratification of patient risk, guide the timing of surveillance, and further refine clinical management.
FundingThis work was supported by grants from the Natural Science Foundation of Guangdong Province for Distinguished Young Scholar (2022B1515020024), the National Natural Science Foundation of China (82070574, 82203395) and the National Key R&D Program of China (2023YFC2507500).
Data availability statementThe data that support the findings of this study are available on request from the corresponding author.
CRediT authorship contribution statementHao Jiang: Formal analysis, Investigation, Methodology, Software, Writing – original draft. Hongsheng Yu: Formal analysis, Investigation, Methodology, Software, Writing – original draft. Can Hu: Formal analysis, Investigation, Methodology, Software, Writing – original draft. Yinan Huang: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. Bilan Yang: Investigation, Methodology. Xiaoli Xi: Investigation, Methodology. Yiming Lei: Investigation, Methodology. Bin Wu: Conceptualization, Data curation, Supervision, Writing – review & editing. Yidong Yang: Conceptualization, Data curation, Supervision, Writing – review & editing.
None.
We would like to thank all the participants in this study.









![Trajectories of liver stiffness measurements (LSMs) (a). Group 1, Low gradual decrease trajectory group; Group 2, Medium quickly decrease trajectory group; Group 3, Low stable trajectory group; Group 4, Medium gradual decrease trajectory group; Group 5, High quickly decrease followed by increase trajectory group. Characteristics of different LSM trajectories (b). Population distribution of the five groups with distinct LSM trajectories (c). A total of 1272 patients were included in the study and categorized into Group 1 (786 [61.79 %]), Group 2 (118 [9.28 %]), Group 3 (153 [12.03 %]), Group 4 (123 [9.67 %]), and Group 5 (92 [7.23 %]) based on the different LSM trajectories during follow-up. Sankey diagram: Association of baseline LSM, LSM trajectories, and liver-related events in patients with chronic hepatitis B (CHB) and compensated advanced chronic liver disease (cACLD) (d). Liver-related events occurred in 8.52 % (67/786) of patients in Group 1, 11.02 % (13/118) of patients in Group 2, 23.53 % (36/153) of patients in Group 3, 27.64 % (34/123) of patients in Group 4, and 25.00 % (23/92) of patients in Group 5. Trajectories of liver stiffness measurements (LSMs) (a). Group 1, Low gradual decrease trajectory group; Group 2, Medium quickly decrease trajectory group; Group 3, Low stable trajectory group; Group 4, Medium gradual decrease trajectory group; Group 5, High quickly decrease followed by increase trajectory group. Characteristics of different LSM trajectories (b). Population distribution of the five groups with distinct LSM trajectories (c). A total of 1272 patients were included in the study and categorized into Group 1 (786 [61.79 %]), Group 2 (118 [9.28 %]), Group 3 (153 [12.03 %]), Group 4 (123 [9.67 %]), and Group 5 (92 [7.23 %]) based on the different LSM trajectories during follow-up. Sankey diagram: Association of baseline LSM, LSM trajectories, and liver-related events in patients with chronic hepatitis B (CHB) and compensated advanced chronic liver disease (cACLD) (d). Liver-related events occurred in 8.52 % (67/786) of patients in Group 1, 11.02 % (13/118) of patients in Group 2, 23.53 % (36/153) of patients in Group 3, 27.64 % (34/123) of patients in Group 4, and 25.00 % (23/92) of patients in Group 5.](https://static.elsevier.es/multimedia/16652681/0000003000000001/v1_202506020641/S1665268125000122/v1_202506020641/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)








