Abstracts Asociación Mexicana de Hepatología (AMH) 2024
More infoThe Connective Tissue Growth Factor (CTGF) is a multifunctional protein recognized as an important mediator in fibrogenic pathways in liver diseases. We aimed to establish the correlation between serum CTGF levels using Enzyme-Linked ImmunoSorbent Assay (ELISA) and the degree of hepatic fibrosis measured by transient elastography in patients with cholestasis diagnosed with Primary Biliary Cholangitis (PBC).
Materials and PatientsProspective, analytical, experimental study. Three groups were recruited: the first group comprised patients with cholestasis, the second group comprised patients with cirrhosis due to Hepatitis C Virus (HCV), and the third group comprised healthy subjects. Anthropometric and biochemical data were collected. A blood sample was collected to quantify serum levels of CTGF using ELISA. The degree of fibrosis was determined by transient elastography. Statistical analysis: Data are presented as Mean±SD or Median (IQR 25-75). They were analyzed by one-way ANOVA with Tukey's post-hoc test or Kruskal-Wallis with Dunn's post-hoc test. The following parameters were calculated: Sensitivity (S), Specificity (E), Positive Predictive Values (PPV), Negative Predictive Values (NPV), and the area under the ROC curve (AUROC). A p-value <0.05 was considered significant.
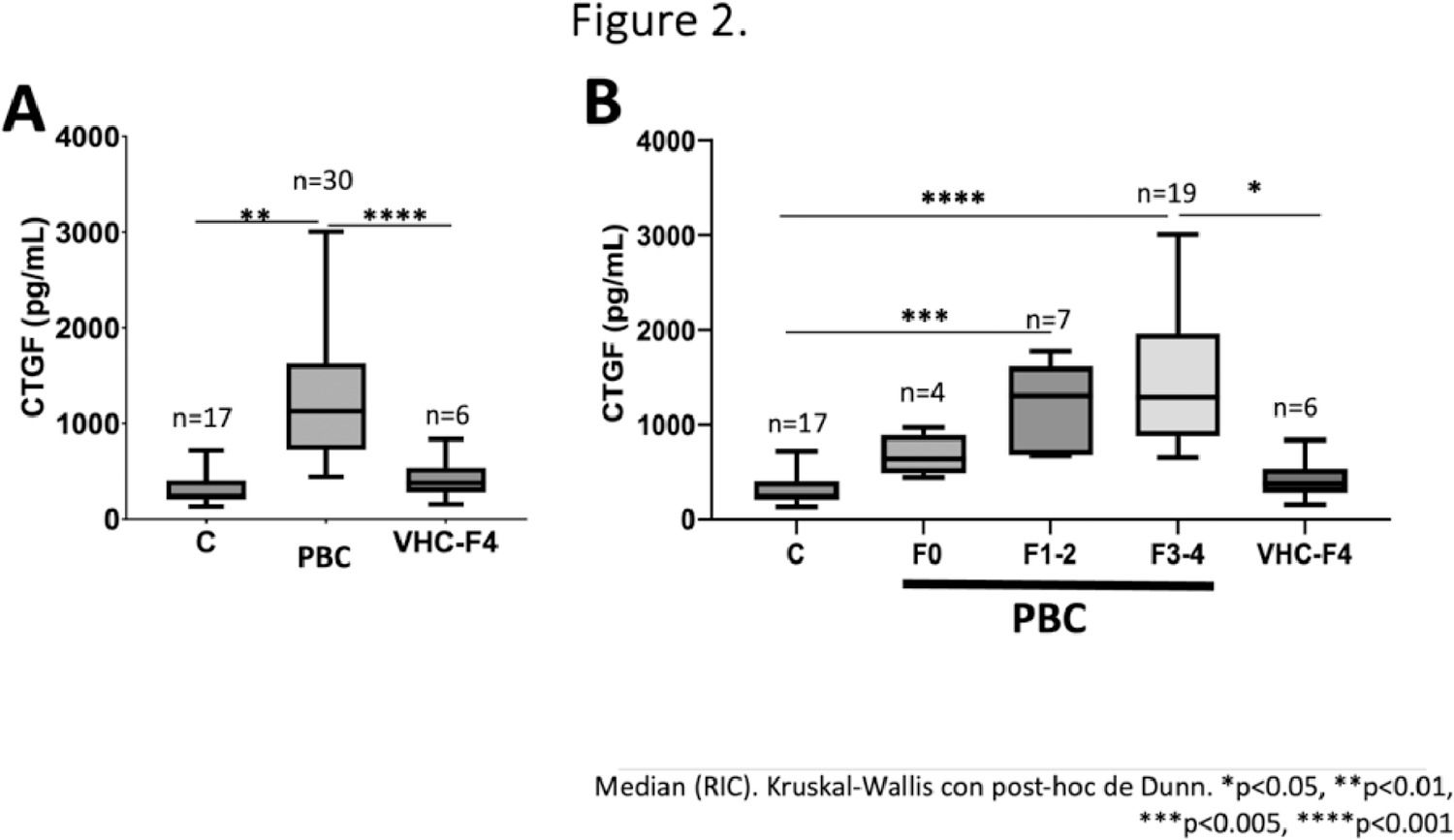
ResultsThirty patients with cholestasis diagnosed with PBC were included, along with a group of subjects with cirrhosis due to Hepatitis C Virus (VHC-F4, n=6), and a control group without liver disease (C, n=17). It was observed that there is a positive correlation between CTGF levels and the degree of fibrosis in patients with cholestasis (PBC), but not in patients with cirrhosis due to HCV. Using a cutoff point of 630 pg/mL, a sensitivity (S) of 0.93, specificity (E) of 0.91, positive predictive value (PPV) of 0.93, negative predictive value (NPV) of 0.91, and an area under the ROC curve (AUROC) of 0.97 with a Youden index of 0.85 were obtained (Figure 1). With a serum CTGF value of 520 pg/mL in patients with PBC without fibrosis or with moderate fibrosis compared to controls and HCV-F4, a sensitivity (S) of 0.75, specificity (E) of 0.87, and AUROC of 0.88 for F0, and a sensitivity (S) of 0.91, specificity (E) of 0.87, and AUROC of 0.94 for F2 were identified (Figure 1). Regarding the degree of fibrosis, CTGF was significantly higher in F4 compared to F0 in patients with PBC. In the case of the VHC-F4 group, there were no differences compared to the group without liver disease, suggesting a specificity of CTGF for fibrosis due to cholestatic disease (Figure 2).
ConclusionsThere is a direct correlation between serum levels of CTGF in patients with cholestasis and the degrees of fibrosis measured by transient elastography, as well as specific cutoff points for discrimination with and without fibrosis for PBC.