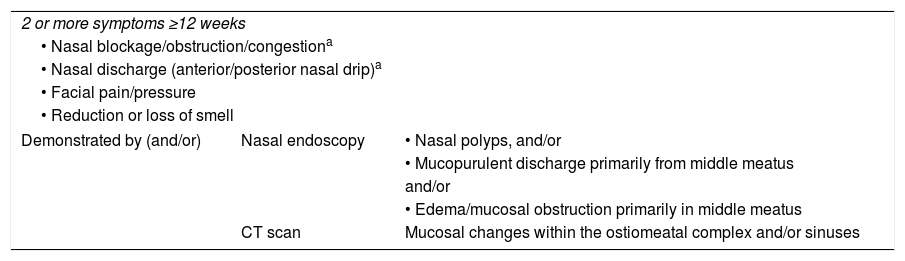
Considering that all the evidence indicates that chronic rhinosinusitis without nasal polyps (CRSsNP) and chronic rhinosinusitis with nasal polyps (CRSwNP) are distinct entities, the aim of this study was to compare the concentrations obtained in plasma and in sinonasal mucosa with oral and nasal topical ciprofloxacin, in patients with and without nasal polyps, without evaluating the effectiveness of the use of an antibiotic.
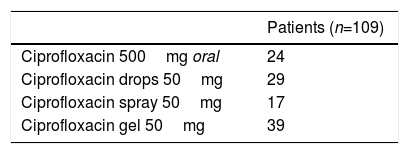
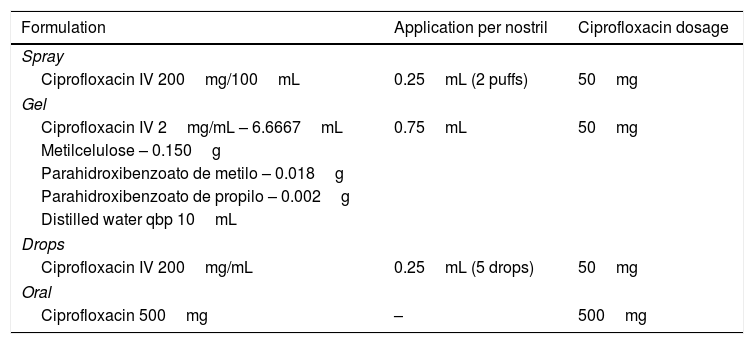
MethodsProspective clinical study with single-blind randomization. The population consisted of patients with chronic rhinosinusitis with eligible for endonasal surgery, over 18 years old. It took place between January 2010 and December 2014. A single preoperative dose of ciprofloxacin (oral or nasal topic- spray, gel or drops) was given and samples of plasma and nasal mucosa (inferior turbinate, middle turbinate, ethmoid and maxillary sinus) were collected prior to surgery. The plasma and mucosal ciprofloxacin concentrations were assayed with high performance liquid chromatography (HPLC) with fluorescence detection (FD).
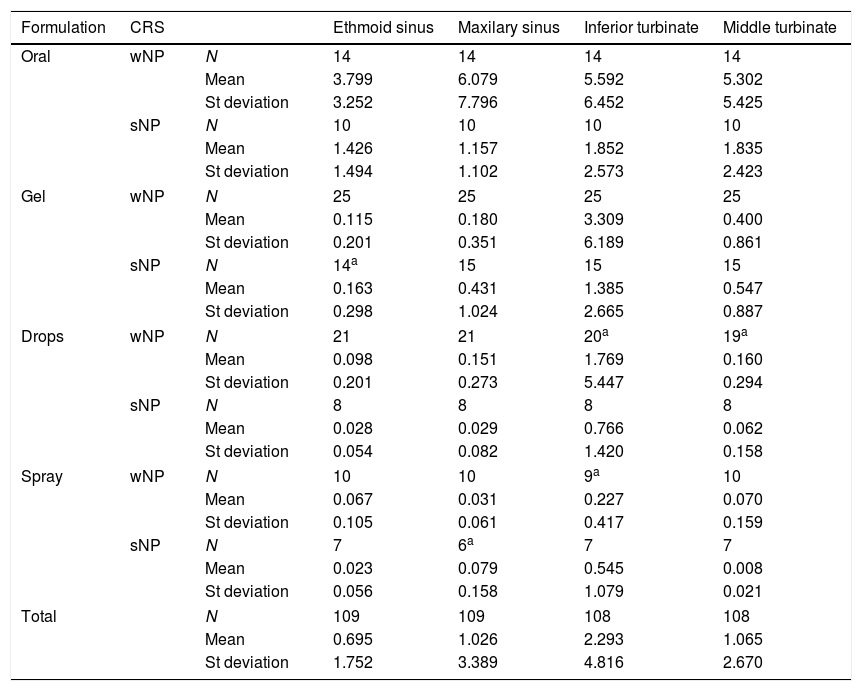
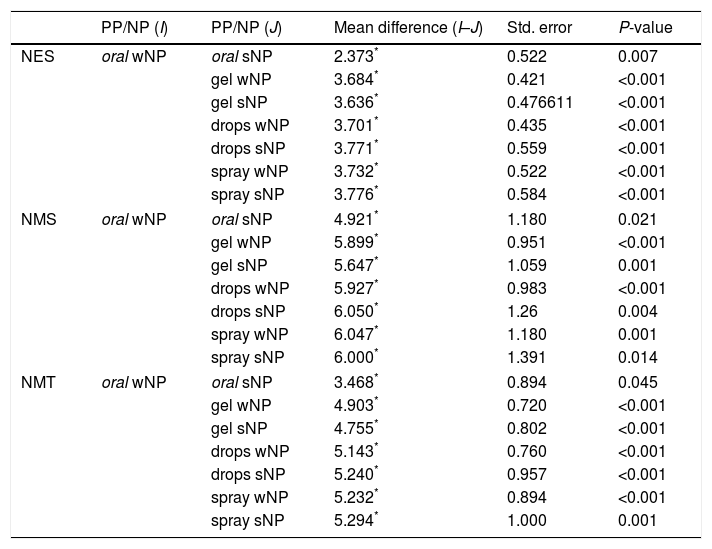
ResultsThe oral ciprofloxacin achieved better mucosal concentrations but had a significant plasmatic expression in all patients. None of the topical formulations achieved measurable ciprofloxacin plasmatic levels. Among the topical formulations, the gel had the best mucosal results, despite the existence of polyposis.
Considerando todas las evidencias de que la rinosinusitis crónica sin poliposis nasal (RSCsPN) y la rinosinusitis crónica con poliposis nasal (RSCcPN) son entidades distintas, el objetivo de este estudio fue comparar las concentraciones obtenidas en el plasma y en la mucosa nasal con ciprofloxacino oral y tópico nasal en pacientes con y sin pólipos nasales, sin evaluar la efectividad del uso del antibiótico.
MétodosEstudio clínico prospectivo con asignación aleatoria. La población se componía de pacientes con rinosinusitis crónica propuestos para cirugía endonasal, mayores de 18 años. Se desarrolló entre enero de 2010 y diciembre de 2014. Se administró una dosis única preoperatoria de ciprofloxacino (oral o tópico nasal, en aerosol, gel o gotas) y se recogieron muestras de plasma y mucosa nasal (cornetes, etmoides y seno maxilar) antes de la cirugía. La concentración de ciprofloxacino en el plasma y en la mucosa se ensayó mediante cromatografía líquida de alto rendimiento con detección de fluorescencia.
ResultadosEl ciprofloxacino oral logró las concentraciones mucosas más altas pero tuvo una expresión plasmática significativa en todos los pacientes. Ninguna de las formulaciones tópicas ha generado niveles plasmáticos de ciprofloxacino medibles. Entre las formulaciones tópicas, el gel fue el que presentó mejores resultados mucosos, a pesar de la existencia de poliposis.