Olfactory neuroblastoma (ONB) is a rare entity that constitutes less than 5% of nasosinusal malignancies. Mainstream treatment consists in surgical resection+/−adjuvant radiotherapy. By exposing results observed with apparition of new therapeutic options as neoadjuvant chemotherapy, the objective is to evaluate a series and a review of the current literature.
MethodsA retrospective review was conducted including patients diagnosed and followed-up for ONB from 2008 to 2015 in our institution.
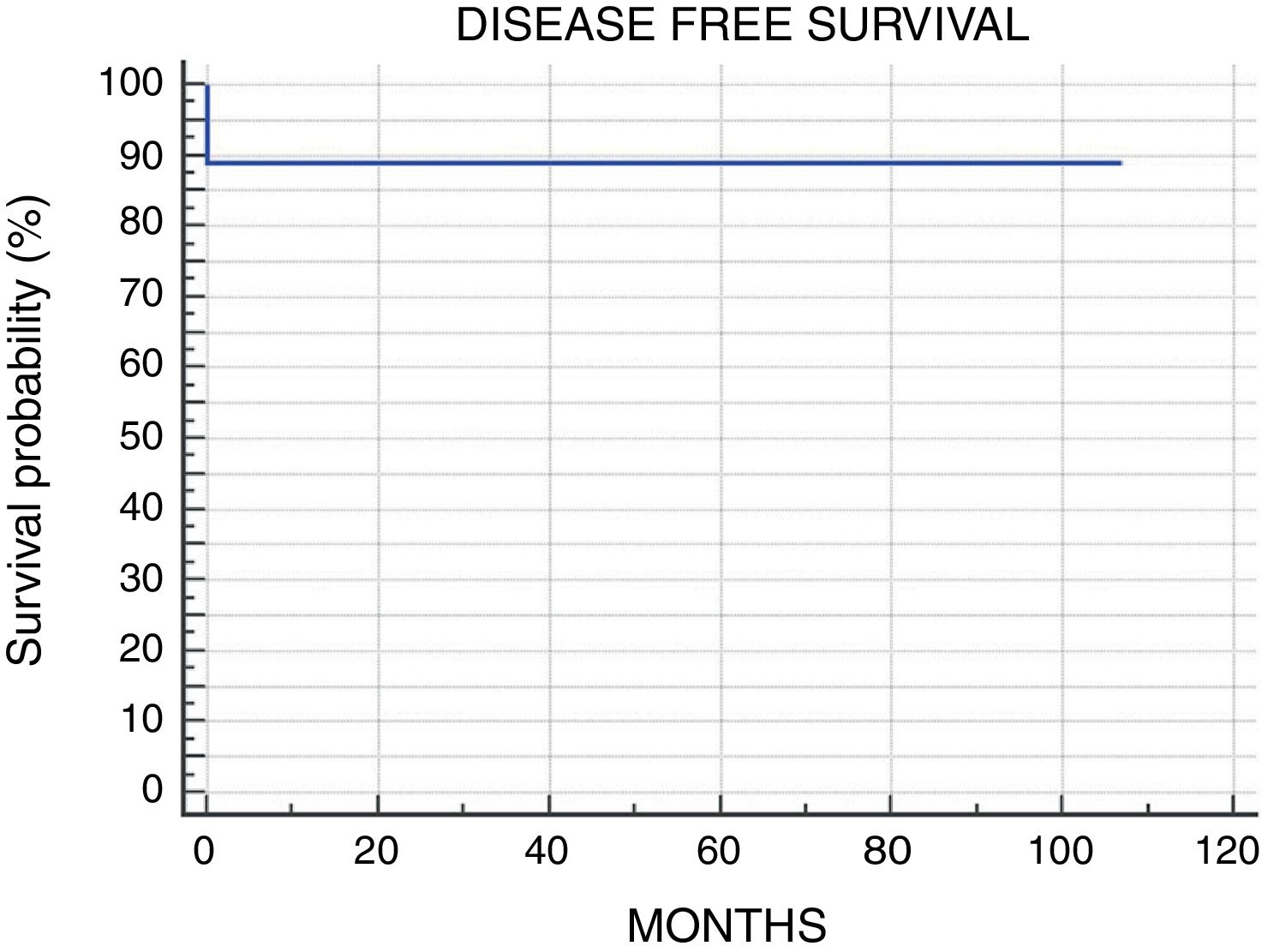
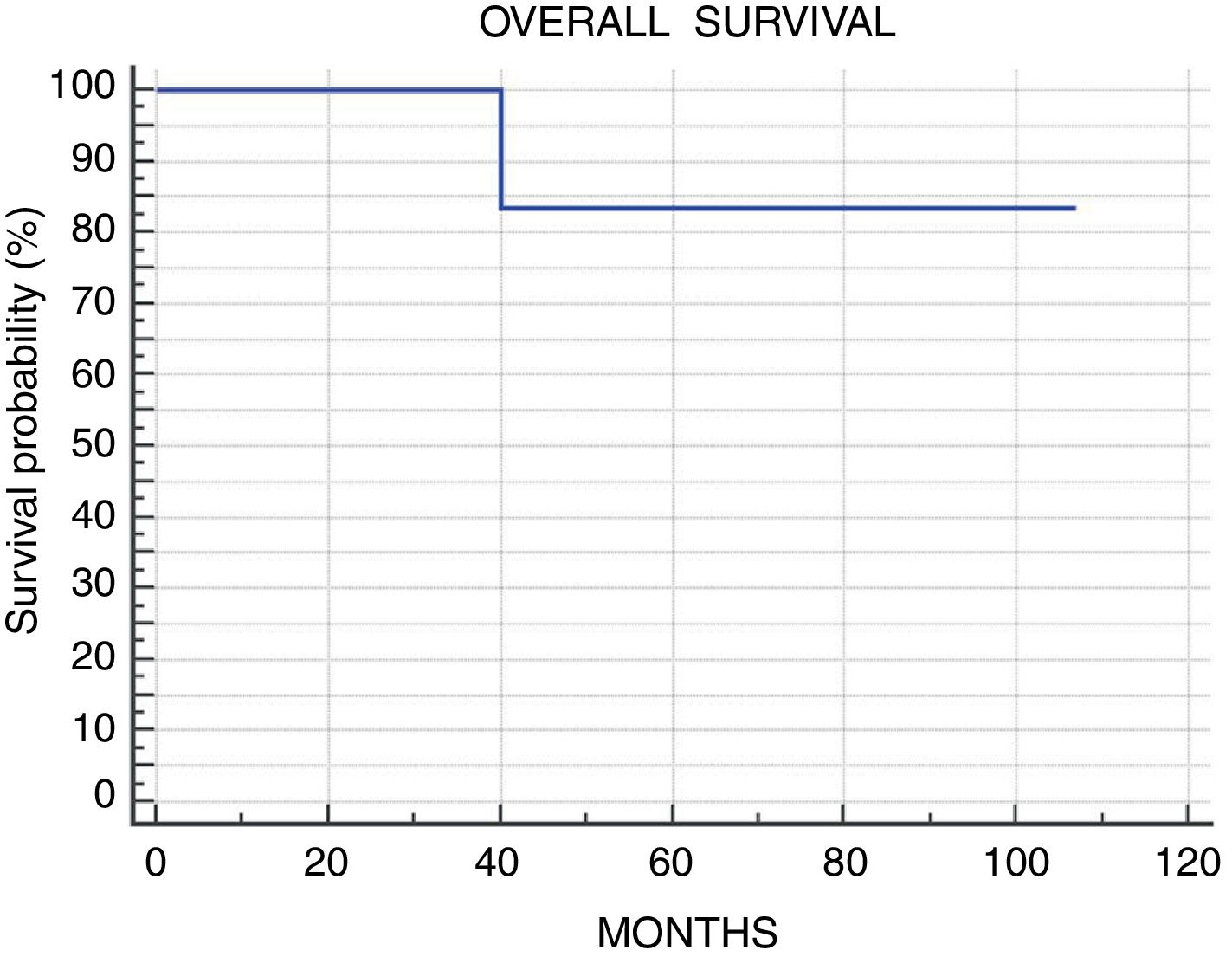
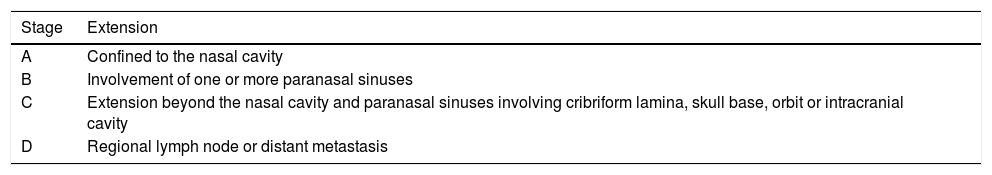
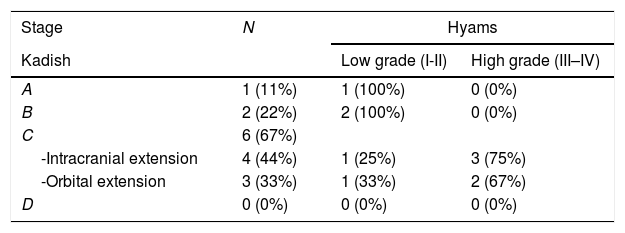
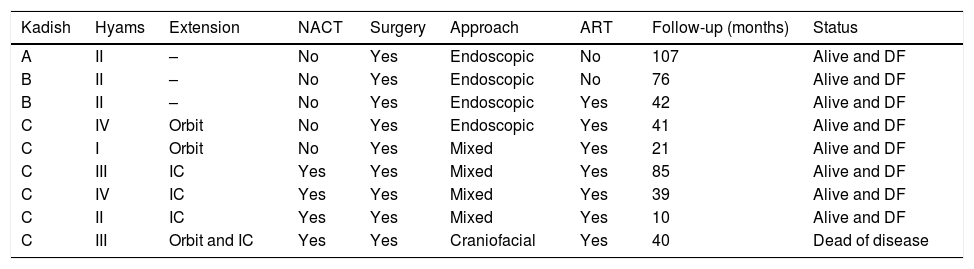
Results9 patients were included. Mean follow-up of 52.5 months (range 10–107). Kadish stage: A, 1 patient (11.1%) treated with endoscopic surgery; B, 2 patients (22.2%) treated with endoscopic surgery (one of them received adjuvant radiotherapy); C, 6 patients (66.7%), 4 patients presented intracranial extension and were treated with neoadjuvant chemotherapy followed by surgery and radiotherapy. The other 2 patients presented isolated orbital extension, treated with radical surgery (endoscopic or craniofacial resection) plus radiotherapy. The 5-year disease free and overall survival observed was 88.9%.
ConclusionNeoadjuvant chemotherapy could be an effective treatment for tumor reduction, improving surgical resection and reducing its complications.
Elneuroblastoma olfatorio es una entidad rara que se corresponde con menos del 5% de las neoplasias nasosinusales. El tratamiento principal consiste en la resección quirúrgica±radioterapia adyuvante. El objetivo es evaluar la sobrevida en una serie de casos y la literatura actual, mostrando resultados observados con la aparición de nuevas opciones terapéuticas como la quimioterapia neoadyuvante.
MétodosSe realizó un estudio retrospectivo incluyendo pacientes tratados y seguidos en nuestro centro desde 2008 a 2015.
ResultadosDentro del estudio fueron incluidos 9 pacientes. El seguimiento medio fue de 52,5 meses (rango 10-107). Estadio Kadish: A) un paciente (11,1%) fue tratado con resección endoscópica; B) 2 pacientes (22,2%) tratados con resección endoscópica (uno de ellos recibió radioterapia adyuvante); C) 6 pacientes (66,7%), de los cuales 4 presentaron extensión intracraneal y fueron tratados con quimioterapia neoadyuvante, cirugía y radioterapia adyuvante. Los otros 2 pacientes presentaron invasión intraorbitaria aislada, tratados con cirugía radical y radioterapia adyuvante. La sobrevida y periodo libre de enfermedad a 5 años fue del 88,9%.
ConclusiónLa quimioterapia neoadyuvante puede ser un tratamiento efectivo para la reducción del tamaño tumoral, mejorando la resección quirúrgica y reduciendo sus complicaciones.