The presence of cervical lymph node metastases in patients with oral cavity squamous cell carcinoma reduces survival by up to 50%.
ObjectiveThe aims of this study are to assess the accuracy of clinical N staging versus pathological N staging and its impact on survival in order to identify predictive factors associated with the presence of occult neck metastases.
MethodsOutcomes of 105 patients with oral cavity squamous cell carcinoma who underwent surgical treatment of the primary tumor and neck were retrospectively evaluated.
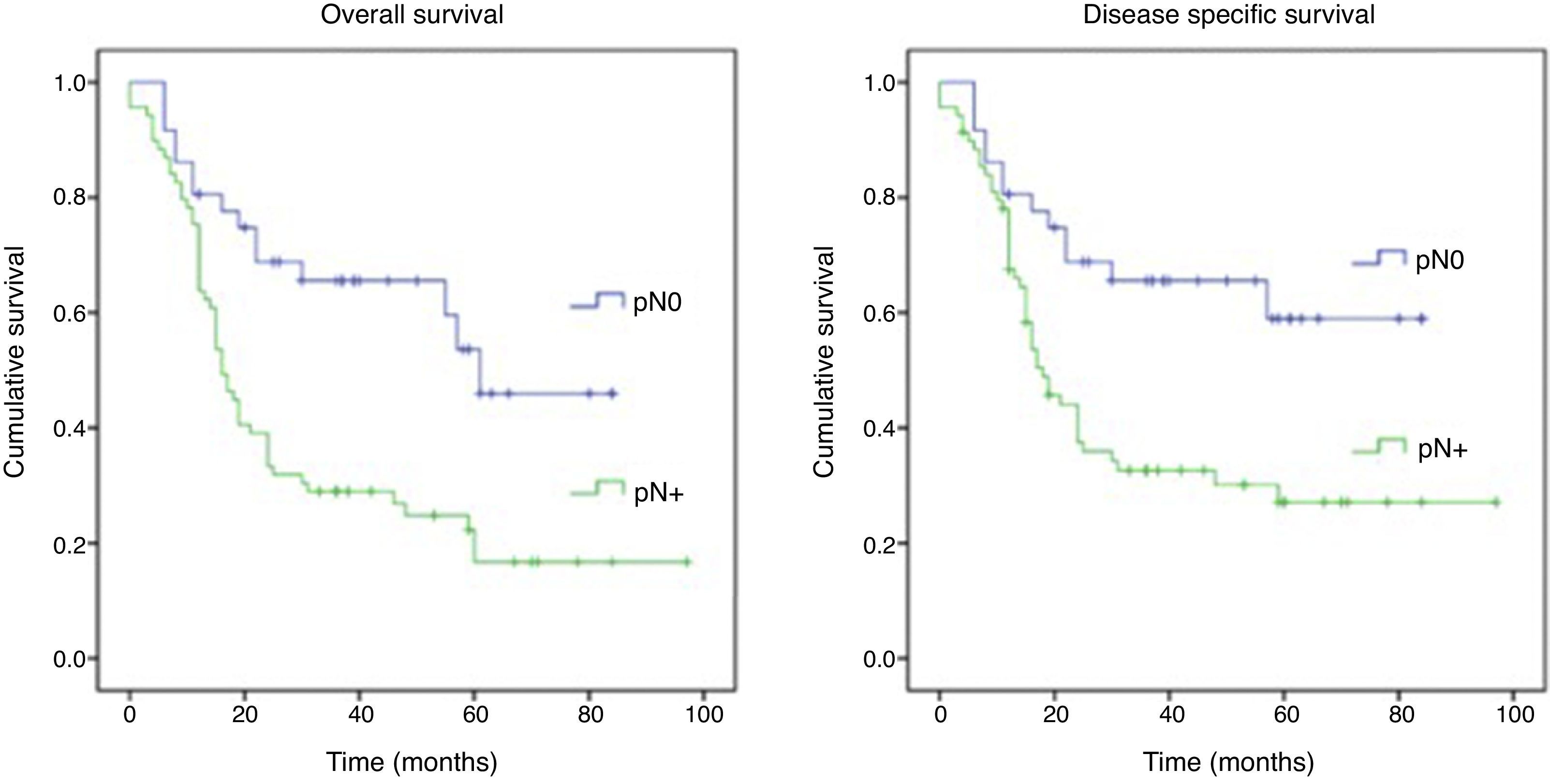
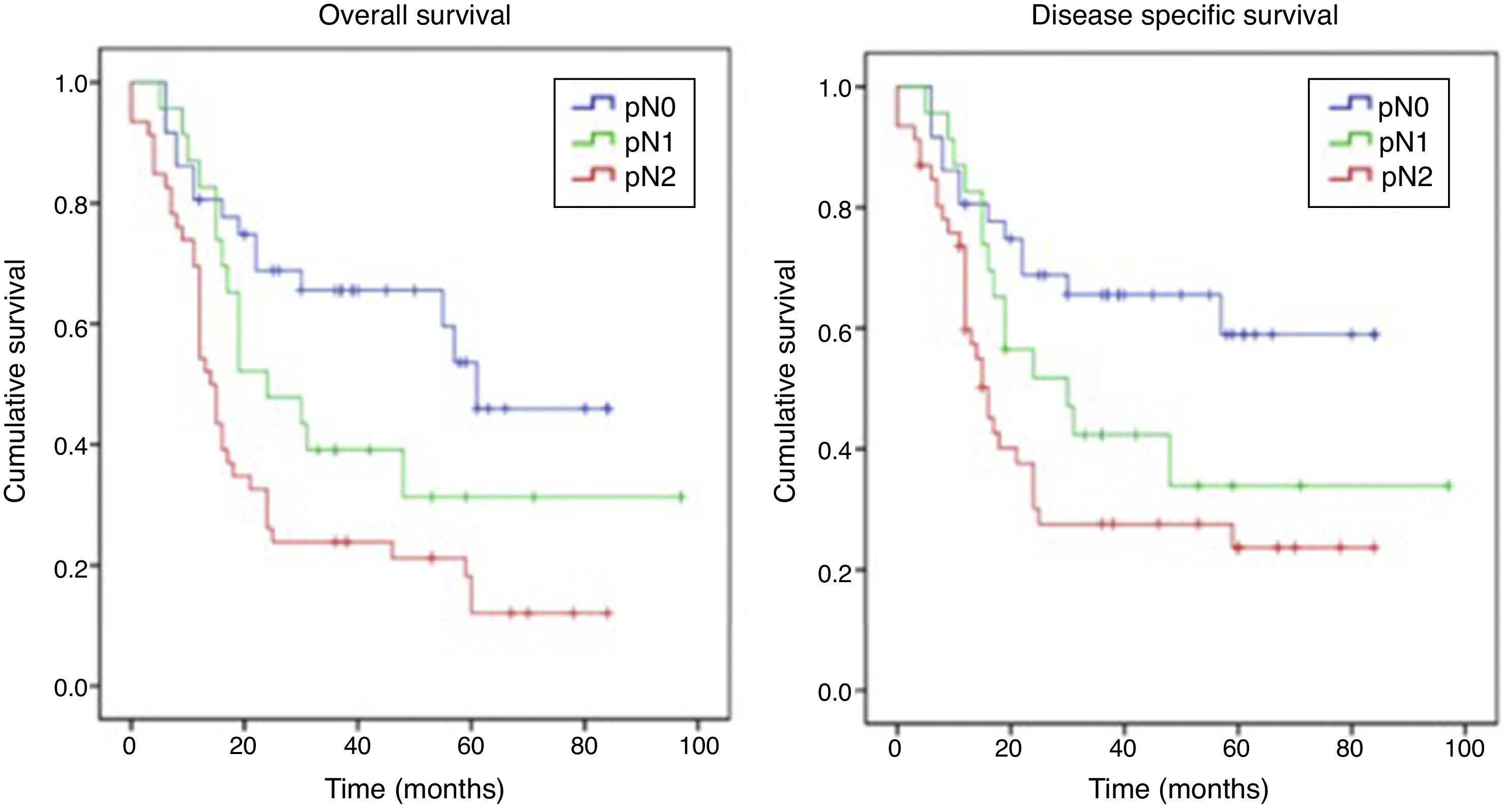
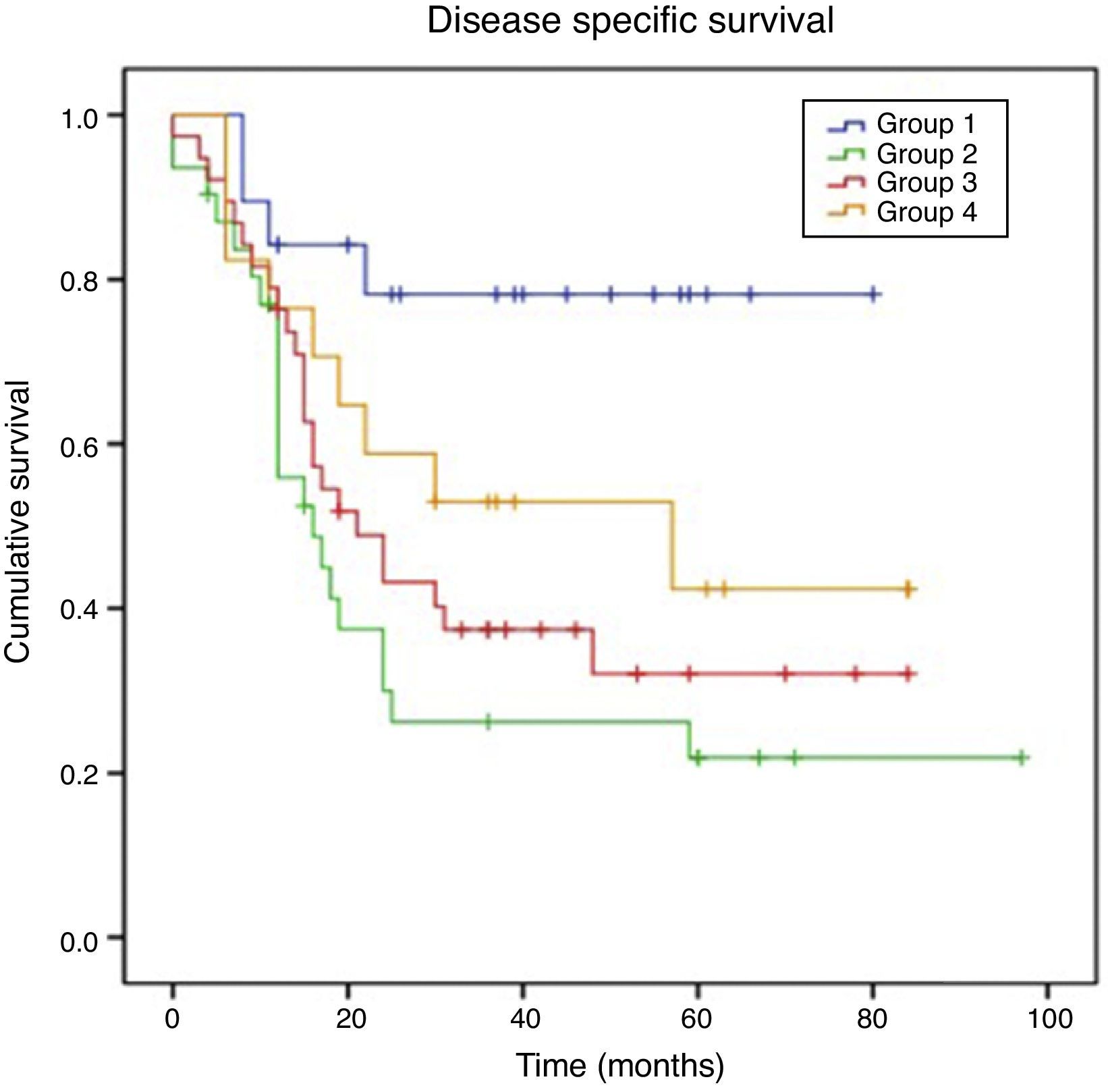
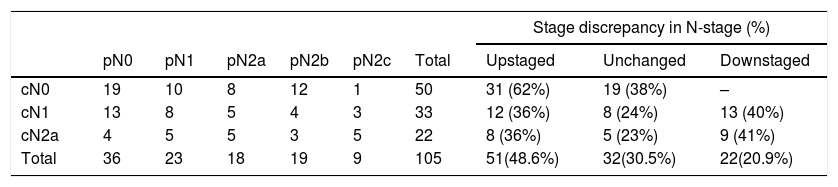
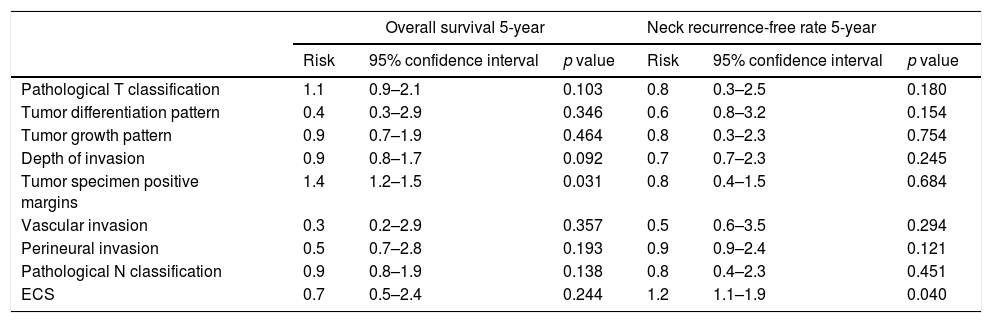
ResultsFor pN0 and pN+ patients 5-year overall survival was respectively 53% and 27%; disease specific survival was 66% for pN0 and 33% for pN+. Patients with clinical negative lymph nodes were pathologically upstaged in 62% of cases. Disease specific survival according to staging discrepancy had statistically significant impact on survival (p=0.009).
ConclusionClinical staging usually underestimates the presence of nodal disease. Neck dissection should be performed in cN0 oral cavity squamous cell carcinoma.
La presencia de metástasis ganglionares cervicales en los pacientes con carcinoma de la cavidad oral reduce la supervivencia hasta en un 50%.
ObjetivosLos objetivos de este estudio son evaluar la exactitud del estadiaje N clínico frente al estadiaje N patológico y su impacto en la supervivencia, de cara a identificar los factores predictivos asociados a la presencia de metástasis ocultas cervicales.
MétodosSe han evaluado retrospectivamente los resultados de 105 pacientes con carcinoma epidermoide de la cavidad oral tras tratamiento quirúrgico del tumor primario y vaciamiento cervical.
ResultadosEn los pacientes pN0 y pN+ la supervivencia global a los 5 años fue del 53 y del 27%, respectivamente, y la supervivencia específica libre de enfermedad fue del 66% para los pN0 y del 33% para los pN+. En los pacientes estadiados clínicamente como negativos se verificó un subestadiaje en el 62% de los casos. La supervivencia específica libre de enfermedad en función de la discrepancia del estadiaje tuvo un impacto estadísticamente significativo en la supervivencia (p=0,009).
ConclusiónEl estadiaje clínico suele subestimar la presencia de enfermedad ganglionar. La disección cervical debe realizarse en los carcinomas de cavidad oral aunque se trate de uno cN0.