To prospectively study the value of PET-CT with fluorine-18 fluorodeoxyglucose (FDG) to predict neoadjuvant chemotherapy (NAC) response of locoregional disease of stages II and III breast cancer patients.
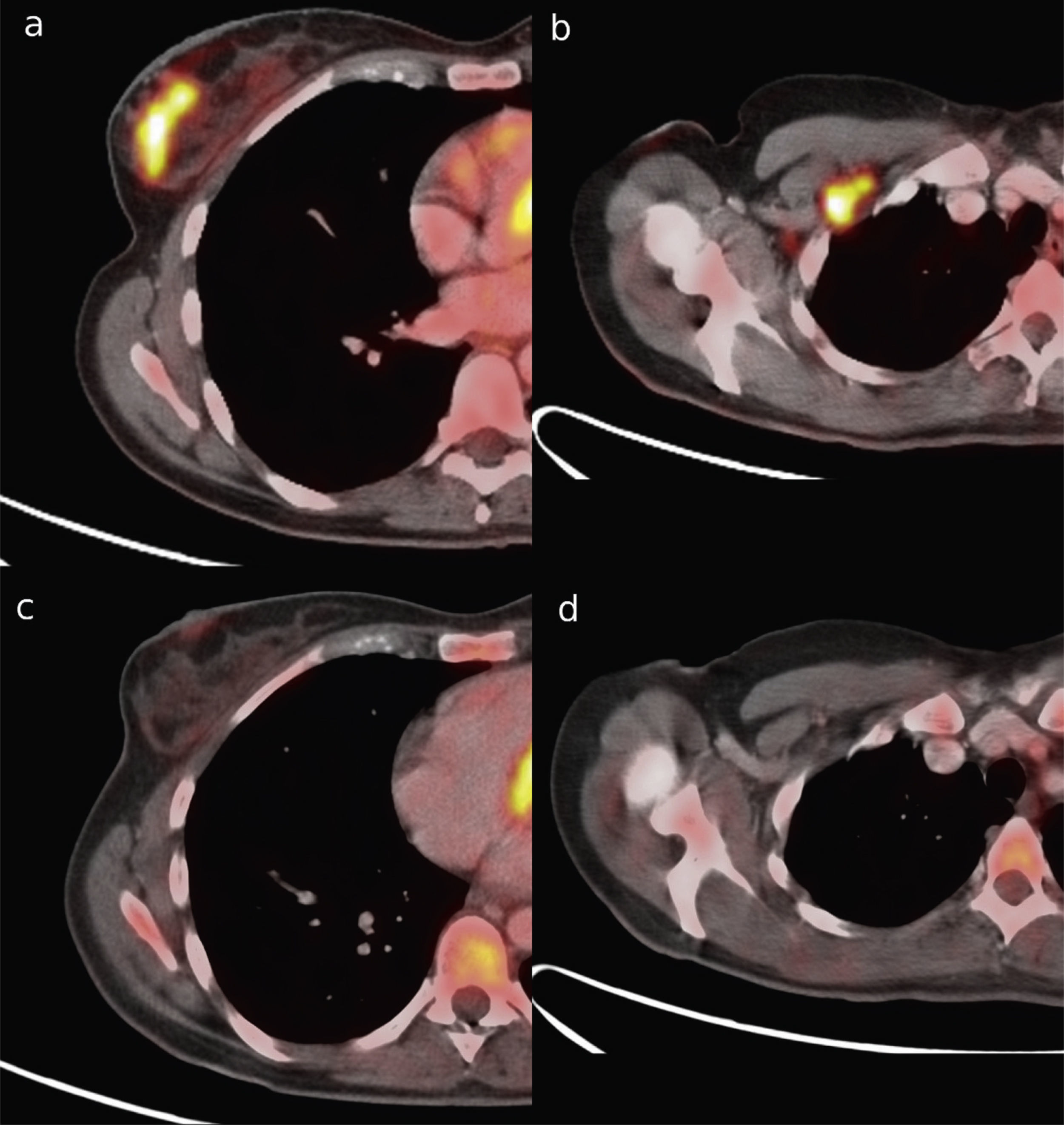
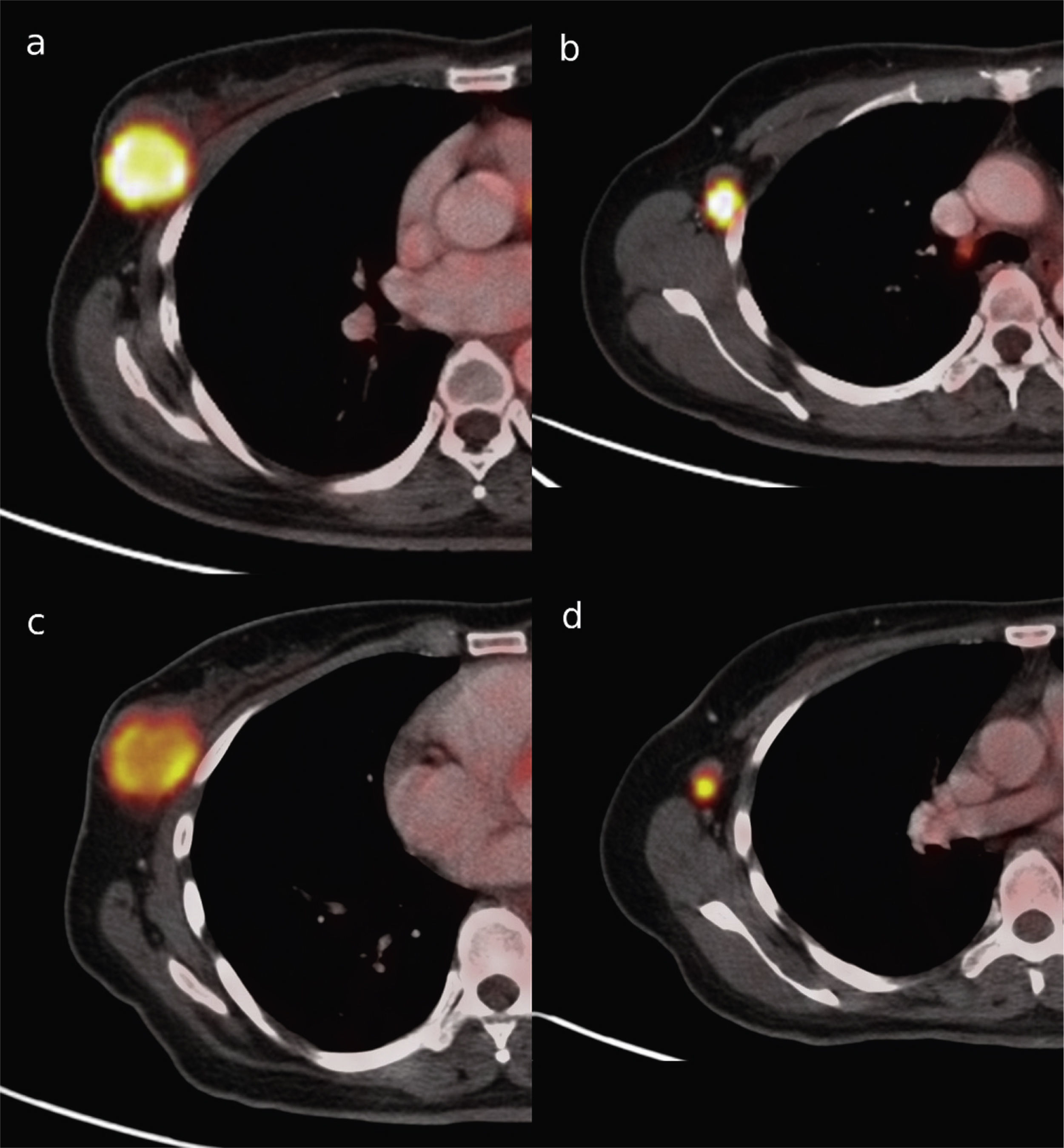
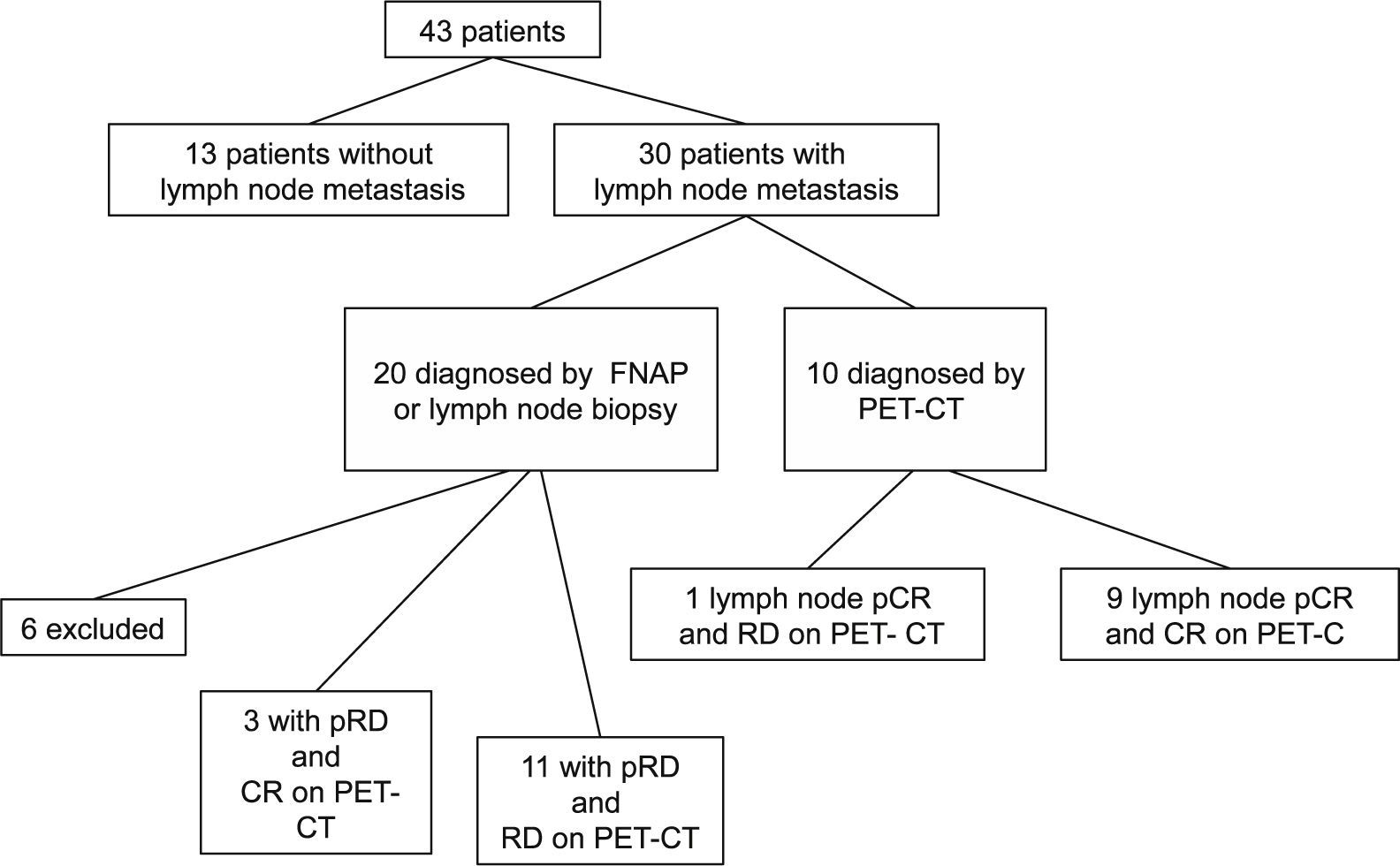
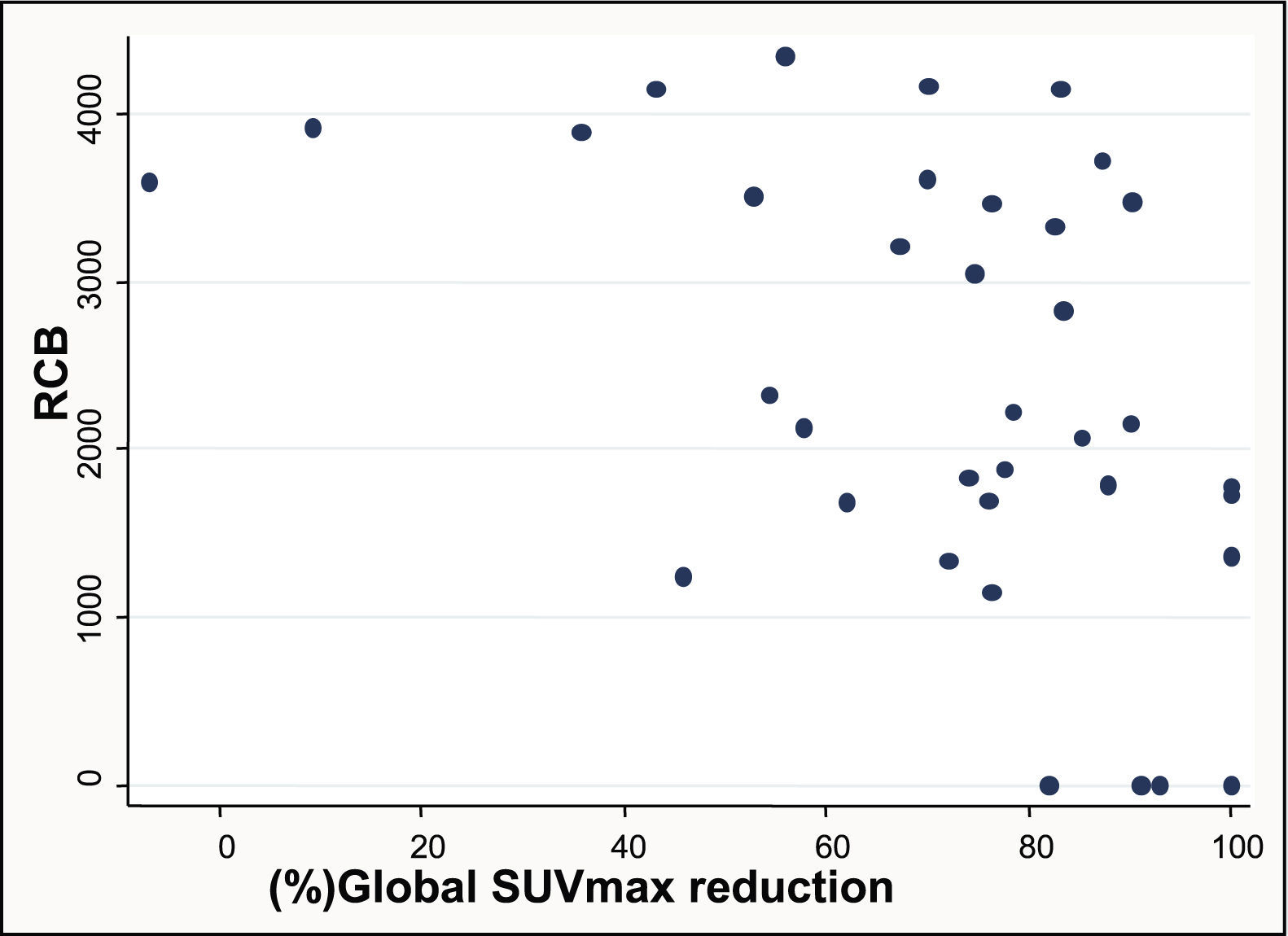
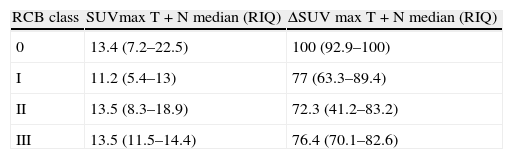
Material and methodsA written informed consent and approval were obtained from the Ethics Committee. PET-CT accuracy in the prediction of pathologic complete response (pCR) after NAC was studied in primary tumors and lymph node metastasis in 43 women (mean age: 50 years: range: 27–71 years) with histologically proven breast cancer between December 2009 and January 2011. PET-CT was performed at baseline and after NAC. SUVmax percentage changes (ΔSUVmax) were compared with pathology findings at surgery. Receiver-operator characteristic (ROC) analysis was used to discriminate between locoregional pCR and non-pCR. In patients not achieving pCR, it was investigated if ΔSUVmax could accurately identify the residual cancer burden (RCB) classes: RCB-I (minimal residual disease (MRD)), RCB-II (moderate RD), and RCB-III (extensive RD).
ResultspCR was obtained in 11 patients (25.6%). Residual disease was found in 32 patients (74.4%): 16 (37.2%) RCB-I, 15 (35.6%) RCB-II and 2 (4.7%) RCB-III. Sensitivity, specificity, and accuracy to predict pCR were 90.9%, 90.6%, and 90.7%, respectively. Specificity was 94.1% in the identification of a subset of patients who had either pCR or MRD.
ConclusionAccuracy of ΔSUVmax in the locoregional disease of stages II and III breast cancer patients after NAC is high for the identification of pCR cases. Its specificity is potentially sufficient to identify a subgroup of patients who could be managed with conservative surgery.
Estudiar de forma prospectiva el valor de la PET-TC con fluor-18-desoxiglucosa (FDG) para predecir la respuesta a la quimioterapia neoadyuvante (NAC) de la enfermedad locoregional en pacientes con cáncer de mama en estadios II y III.
Material y métodosSe obtuvo un consentimiento informado por escrito y la aprobación del Comité Ético. Se estudió la precisión de la PET-TC para predecir la respuesta completa patológica (pCR) tras la NAC en los tumores y en los ganglios de 43 mujeres (edad media: 50 años; rango: 27–71 años) que presentaban cáncer de mama diagnosticado por histología entre diciembre del 2009 y Enero del 2011. Los estudios PET-TC se realizaron al diagnóstico y tras la NAC. Los cambios en el porcentaje del SUVmax (delta-SUVmax) se compararon con los hallazgos de la anatomía patológica de la pieza quirúrgica. Se realizaron análisis de Característica Operativa del Receptor (ROC) para discriminar entre pCR y no-pCR en la enfermedad locoregional. En las pacientes que no alcanzaron la pCR, se investigó si el delta-SUVmax podía identificar de forma precisa las siguientes categorías de carga tumoral residual: RCB-I (enfermedad mínima residual (MRD)), RCB-II (moderada RD), y RCB-III (extensa RD).
ResultadosSe obtuvo pCR en 11 pacientes (25,6%). Se encontró enfermedad residual en 32 pacientes (74,4%): 16 (37,2%) RCB-I, 15 (35,6%) RCB-II y 2 (4,7%) RCB-III. La sensibilidad, especificidad y precisión para predecir la pCR fueron 90,9%, 90,6%, y 90,7%, respectivamente. En la identificación del subgrupo de pacientes con pCR o MRD la especificidad fue del 94,1%.
ConclusiónEl delta-SUVmax identifica con elevada precisión la pCR en la enfermedad locoregional de las pacientes con cáncer de mama en estadios II y III tras la NAC. La especificidad es potencialmente suficiente para identificar un subgrupo de pacientes que podrían ser candidatas a cirugía conservadora.
Article
If you experience access problems, you can contact the SEMNIM Technical Secretariat by email at secretaria.tecnica@semnim.es or by phone at +34 619 594 780.

Revista Española de Medicina Nuclear e Imagen Molecular (English Edition)