This article reviews the scientific evidence on the relationship between periodontitis and neurological disease, and particularly cerebrovascular disease and dementia. We also issue a series of recommendations regarding the prevention and management of periodontitis and these neurological diseases at dental clinics and neurology units.
DevelopmentIn response to a series of questions proposed by the SEPA-SEN working group, a literature search was performed, with no restrictions on study design, to identify the most relevant articles on the association between periodontitis and cerebrovascular disease and dementia from the perspectives of epidemiology, treatment, and the biological mechanisms involved in these associations.
ConclusionsPeriodontitis increases the risk of ischaemic stroke and Alzheimer dementia. Recurrent bacterial infections and increased low-grade systemic inflammation seem to be possible biological mechanisms underlying this association. Limited evidence suggests that various oral health interventions can reduce the future risk of cerebrovascular disease and dementia.
Revisar la evidencia científica disponible sobre la relación entre la periodontitis y las enfermedades neurológicas, en particular la enfermedad cerebrovascular y la demencia. Además, se facilitan una serie de recomendaciones en relación a la prevención y manejo de la periodontitis y estas enfermedades neurológicas desde las consultas dentales y las unidades de neurología.
DesarrolloSe realizó una búsqueda bibliográfica sin restricción en cuanto al diseño de estudio para identificar aquellos artículos más relevantes sobre la asociación entre periodontitis, enfermedad cerebrovascular y demencia desde un punto de vista epidemiológico, de intervención, así como de mecanismos biológicos involucrados en estas relaciones para responder a diferentes preguntas planteadas por los miembros del grupo de trabajo SEPA-SEN.
ConclusionesLa periodontitis aumenta el riesgo de ictus isquémico y demencia de tipo Alzheimer. Bacteriemias recurrentes con aumento de un estado inflamatorio sistémico de bajo grado parecen ser posibles mecanismos biológicos que explicarían esta asociación. Evidencia limitada apunta a que diferentes intervenciones de salud oral pueden reducir el riesgo futuro de padecer enfermedad cerebrovascular y demencia.
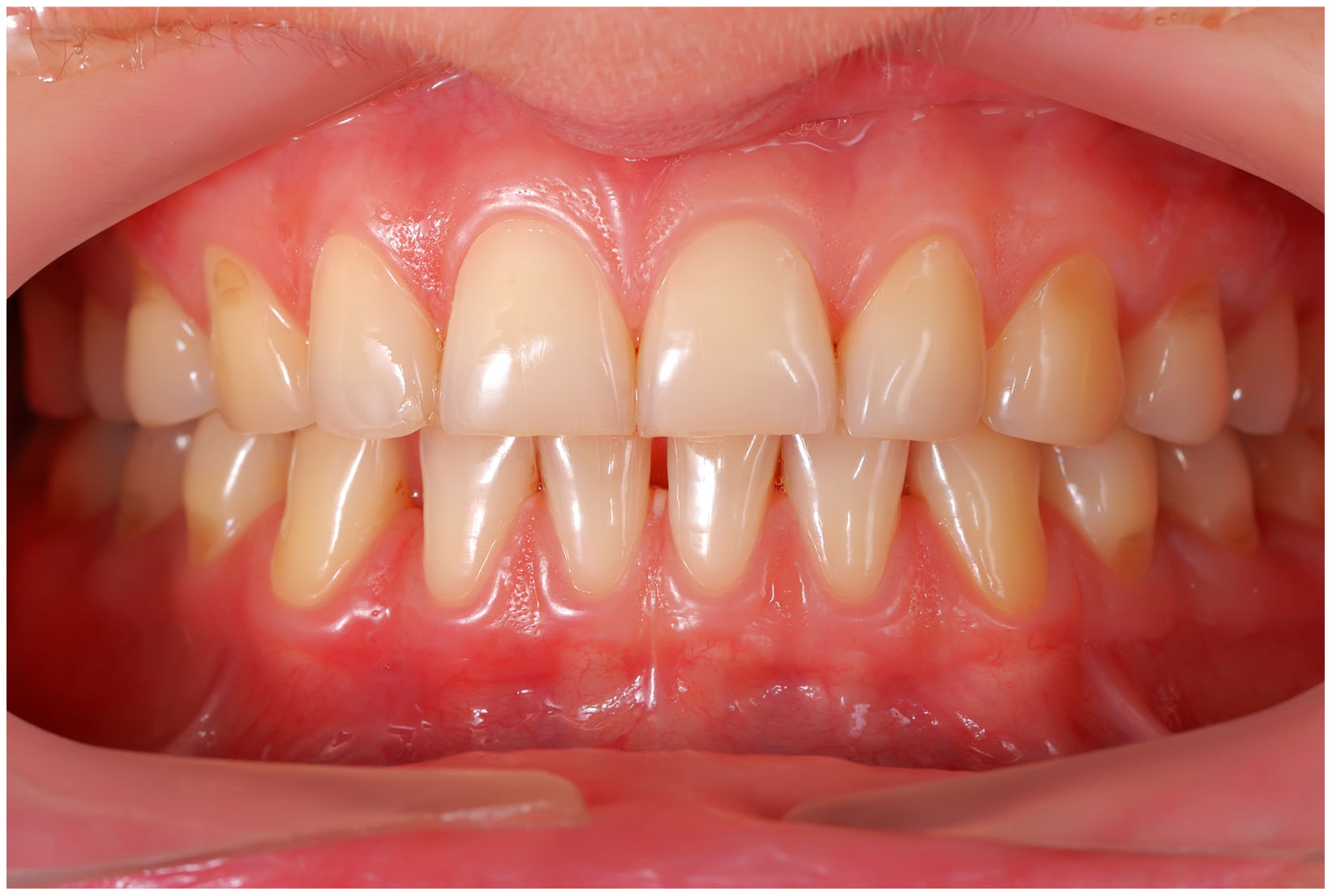
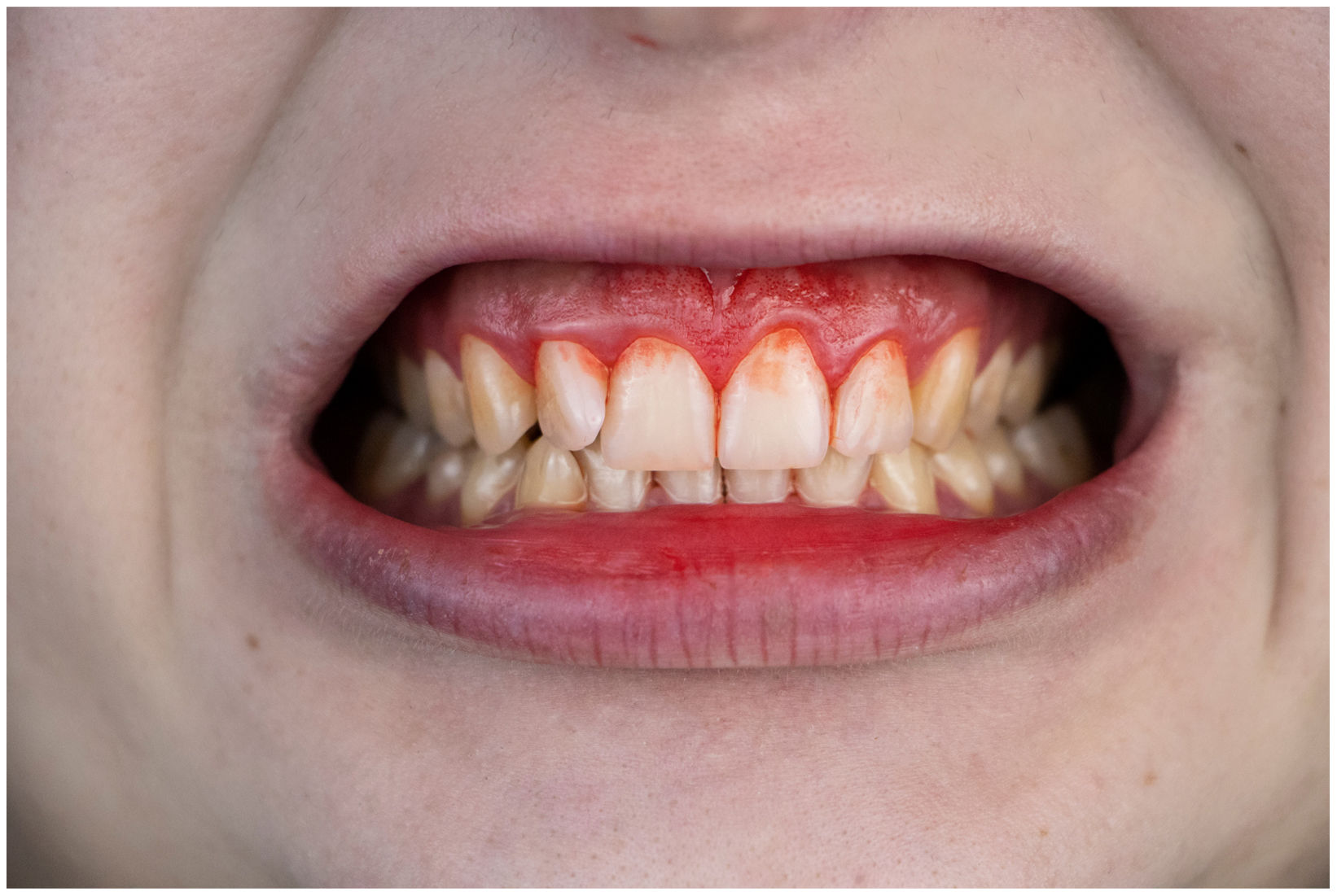
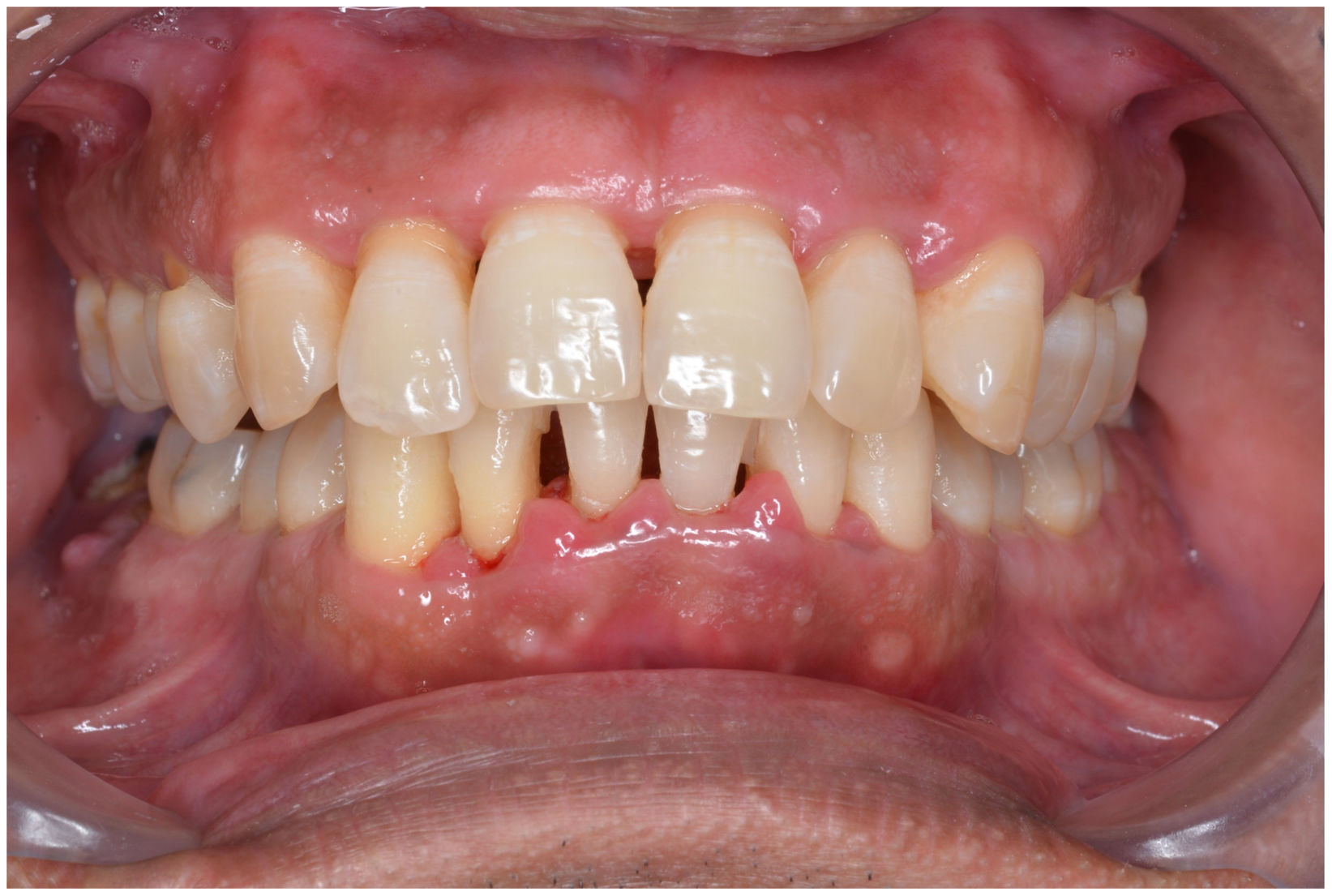
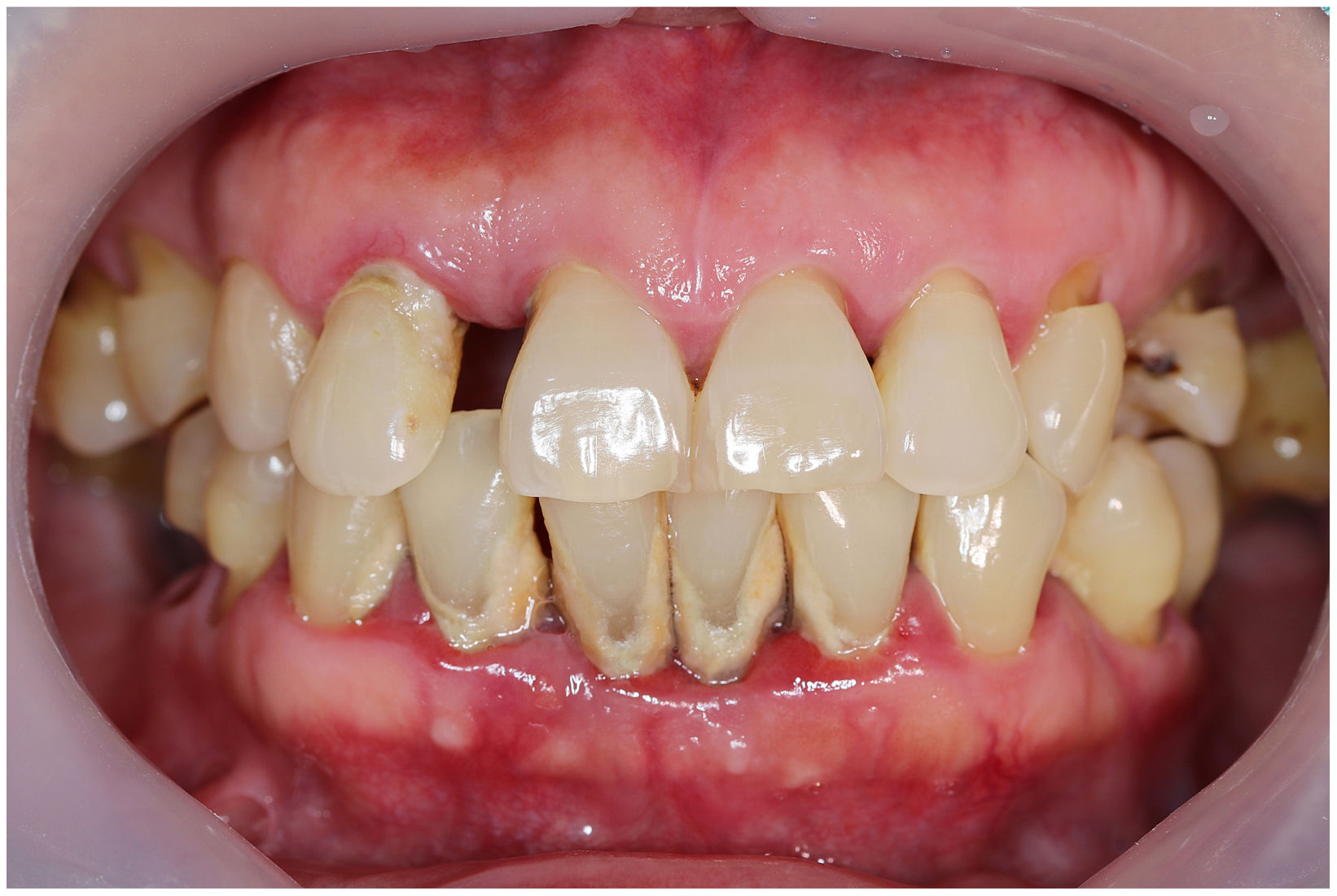
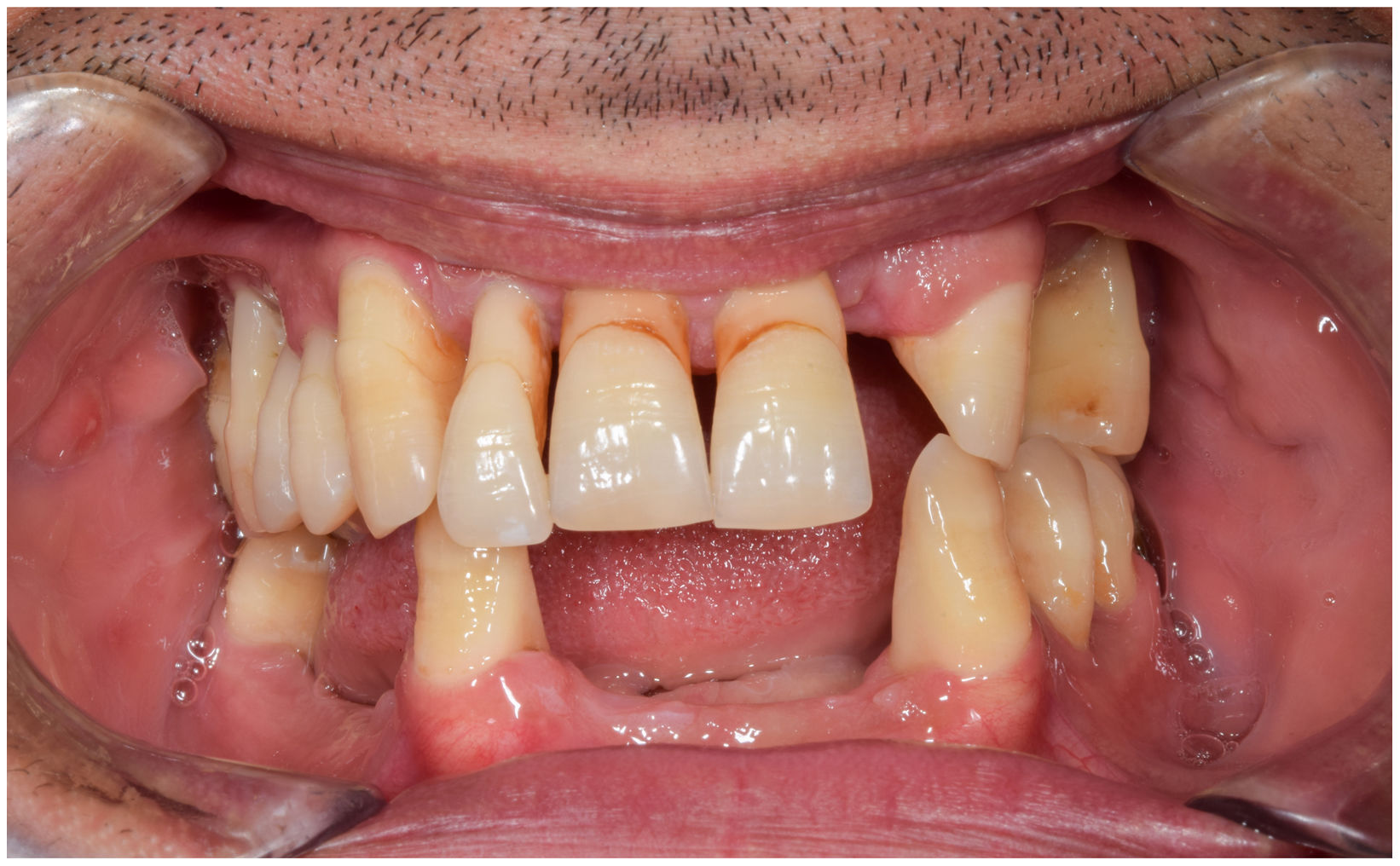
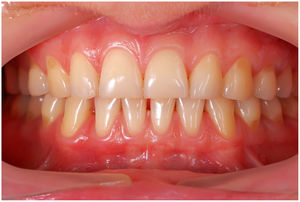
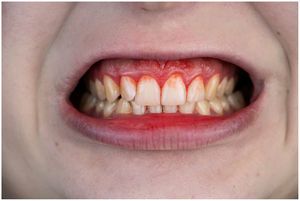
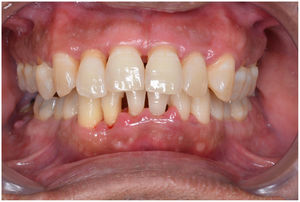
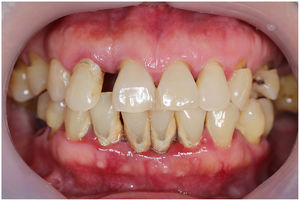
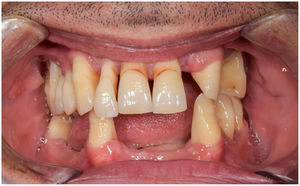
In healthy conditions, periodontal tissues are aesthetically and functionally structured around the teeth (Fig. 1). Tooth enamel is a structure that is not periodically replaced, and therefore the accumulation and formation of biofilms is favoured at its junction with the gums. If these are not removed using oral hygiene techniques, whether at home or professionally, biofilms enter the gingival sulcus, eventually resulting in gingivitis, which affects 50%-90% of the adult population. Gingivitis is characterised by the reversible inflammation of the gums, with the tooth preserving its support, as other structures of the periodontium (cementum, periodontal ligament, and alveolar bone) are spared (Fig. 2). The loss of balance between the bacterial attack (mainly by anaerobic species) and the host defences, together with the presence of several risk factors (genetics, smoking, specific systemic conditions such as diabetes, etc) and inadequate oral hygiene and/or lack of periodic, professional dental check-ups, leads to the development of periodontitis, characterised by the progressive destruction of the dental support, together with the inflammatory component of gingivitis (Fig. 3). If it is not promptly diagnosed and treated, the damage increases and destroys the bone support, which leads to separation or loss of gums, tooth mobility, dental displacement, and significant functional and aesthetic alterations (Fig. 4). Progression of periodontitis leads to tooth loss, which causes severe local, masticatory, and psychological complications, and impacts quality of life (Fig. 5).1
Periodontitis is the most frequent noncommunicable inflammatory disease worldwide, with its most severe form alone affecting 8% of the adult population.2 A recently published epidemiological study concluded that approximately 38% of Spanish adults present periodontitis, reaching more than 60% among those older than 55 years.3 Periodontitis may go unnoticed by patients for several years, with such warning signs as gingival bleeding (always pathological), inflammation, and changes in gum size, shape, and position, followed by the eventual appearance of tooth mobility and displacement, gum loss with exposed tooth roots, or periodontal abscesses, among others. All these signs are indicative of advanced disease. Treatment of periodontitis consists in the removal of biofilms and calculus by a professional to stop disease progression. In more advanced stages, surgical periodontal treatment may be needed to access deep areas showing loss of the supporting alveolar bone. In any case, and considering that this is a chronic disease, daily oral hygiene is essential, as well as periodic visits to the dentist for periodontal maintenance therapy.
A relevant aspect is that periodontitis does not only affect the mouth. Over the last decades, studies have analysed the interaction between periodontal inflammation and certain chronic noncommunicable diseases, some of which are highly prevalent and have a considerable impact in terms of morbidity and mortality, such as diabetes mellitus, atherosclerotic cardiovascular disease, rheumatoid arthritis, and infectious lung disease.4–8 However, the association with other systemic conditions seems to be exaggerated, with a limited scientific basis.9 Remote effects of periodontitis are explained by the fact that ulceration of the epithelium of deep periodontal pockets causes an open wound between the gums and teeth,10 enabling entry of bacteria and their toxic products into the microcirculation, leading to bacteraemia, even during everyday activities such as chewing or brushing or flossing the teeth.11 This causes chronic, systemic, low-grade inflammation involved in the aetiopathogenesis of the above mentioned diseases. The available evidence is based on studies using animal models, clinical trials with surrogate variables, and epidemiological studies, as randomised clinical trials with clinical comorbidity assessment criteria require long-term follow-up of thousands of patients, and involve such ethical issues as leaving periodontitis untreated in control groups.12,13
At the neurological level, periodontitis has been suggested as a possible added risk factor/indicator in 2 very important groups of diseases: cerebrovascular events, mainly ischaemic strokes, and such neurodegenerative processes as dementia, with Alzheimer disease being the most relevant.14,15
Literature searchTo gather the most relevant evidence on the association between periodontitis and cerebrovascular disease and dementia, we performed a literature search on the Ovid MEDLINE database, from 1946 to 10 February 2021. We used the Endnote X9 reference management tool to manage our results. We only included studies published in English or Spanish. No restriction was applied regarding study design. We selected the most relevant original and review articles to address the questions posed by the working group of the Spanish Society of Periodontology (SEPA) and the Spanish Society of Neurology (SEN), made up of 3 dentists (YL, MC, and PD) and 3 neurologists (JV, ÁM, and AF).
Association between periodontitis and cerebrovascular diseaseIs periodontitis associated with increased risk of cerebrovascular disease?The findings of a meta-analysis of longitudinal epidemiological studies show that patients with periodontitis present 2.8 times greater risk of presenting an ischaemic stroke than patients without periodontitis.16 Furthermore, a prospective cohort study concluded that periodontitis significantly increased the risk of death due to ischaemic stroke.17 Data regarding its association with haemorrhagic stroke are inconsistent.17–19 However, a recent study performed in Finland has shown that severe periodontitis may increase the risk of presenting an intracranial aneurysm.20
Does the presence of periodontitis impact the progression and outcomes of cerebrovascular disease?Although the available evidence is limited, some observational studies have revealed that patients who had an ischaemic stroke and confirmed diagnosis of periodontitis present a higher risk of a recurrent vascular event,21 poorer functional prognosis,22 greater neurological impairment,23 and higher likelihood of post-stroke depression24 than those without periodontitis.
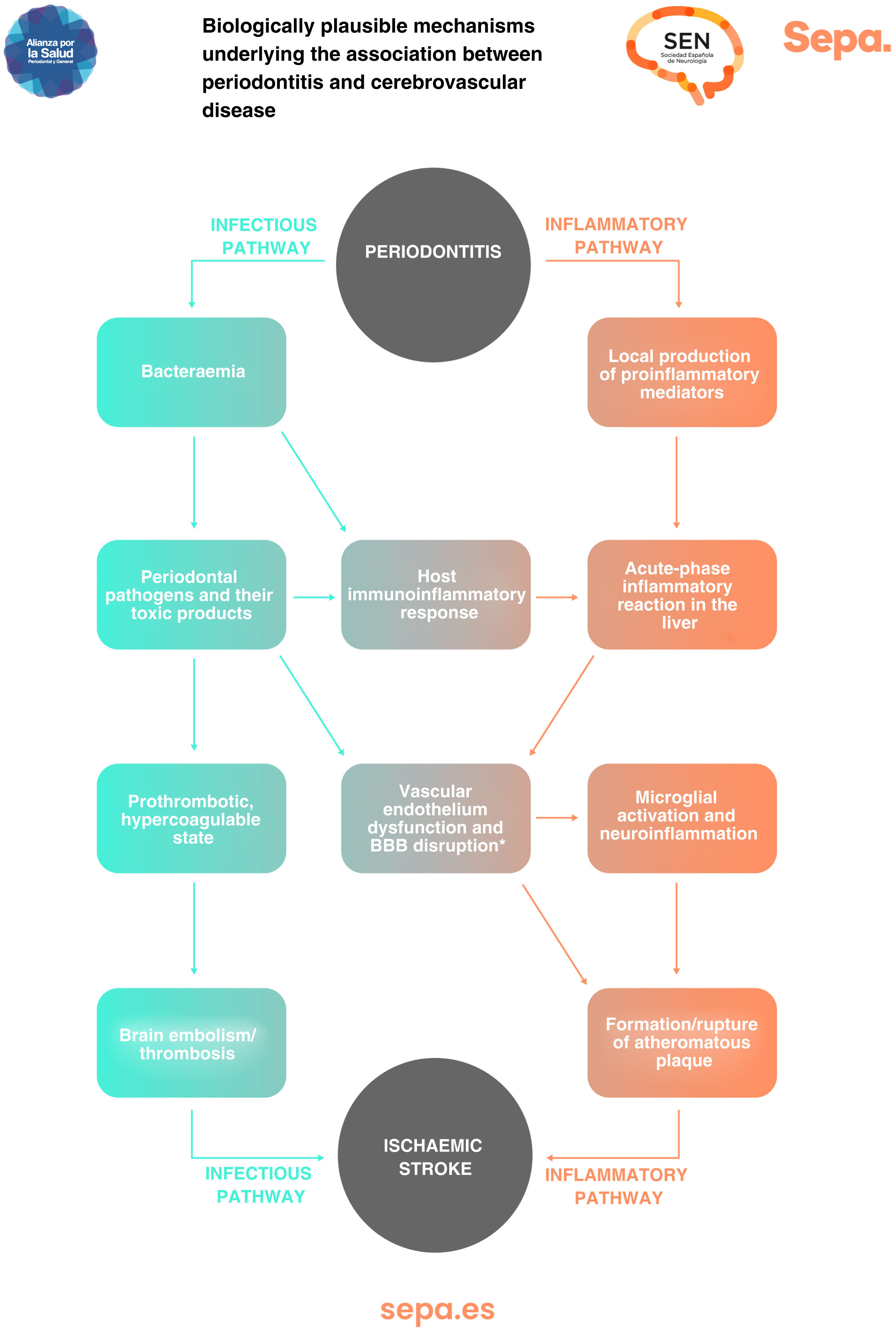
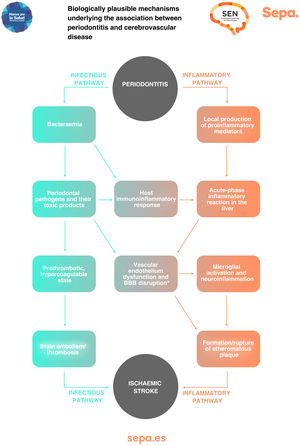
Can any biologically plausible mechanism explain the association between periodontitis and cerebrovascular disease?In patients with periodontitis, both periodontal bacteria and their toxic products, as well as proinflammatory mediators locally produced in periodontal tissues, may access the bloodstream through the ulcerated periodontal epithelium. This causes an immunoinflammatory response that, together with a possible acute-phase systemic inflammatory reaction in the liver, would trigger a prothrombotic hypercoagulable state and dysfunction of the vascular endothelium, increasing the risk of cerebral embolism/thrombosis and formation/rupture of atheromatous plaques, which would manifest clinically as the development of ischaemic stroke (Fig. 6).25
Can periodontal treatment decrease the risk of cerebrovascular disease and/or modify its progression?To date, no interventional clinical trial has been published on the effect of periodontal treatment in the primary or secondary prevention of cerebrovascular disease. However, numerous population studies have observed a significant decrease in the risk of ischaemic and haemorrhagic stroke in association with different oral hygiene interventions such as regular teeth brushing (≥ 3 times daily), periodic professional dental cleaning, or periodontal treatment.26–31 Furthermore, regular visits to the dentist (at least annually) have been shown to be a protective factor against stroke in the future.32
Conclusions- •
Patients with periodontitis present 2.8 times greater risk of developing an ischaemic stroke than those without periodontitis.
- •
Data regarding the association with haemorrhagic stroke are inconsistent.
- •
There is limited evidence on the higher incidence of recurrent vascular events in patients with periodontitis.
- •
Chronic immunoinflammatory response to periodontitis would trigger a prothrombotic hypercoagulable state and dysfunction of the vascular endothelium, which may increase the risk of cerebral embolism/thrombosis.
- •
No interventional clinical trial has been published on the effect of periodontal treatment in the primary or secondary prevention of cerebrovascular disease.
- •
Several observational studies have reported a significantly decreased risk of ischaemic and haemorrhagic cerebrovascular events in association with different oral health interventions, including regular visits to the dentist.
The findings of a meta-analysis of epidemiological studies show that patients with periodontitis present 1.7 times greater risk of developing Alzheimer dementia than patients without periodontal disease.33 This risk is significantly increased (up to 3 times greater) in patients presenting more severe forms of periodontitis. The available evidence on the association between periodontitis and vascular dementia is more limited, although it seems to follow the same trend.34
Does presence of periodontitis impact performance in cognitive tests?Numerous epidemiological studies have observed that patients with periodontitis perform worse in different neuropsychological assessments of cognitive function (Mini–Mental State Examination, Digit Symbol Substitution Test, Block Design Test score, and Serial Digit Learning test) than patients without periodontal disease.35–38
Is there a bidirectional association between periodontitis and cognitive impairment/dementia?Several population studies have suggested that patients with dementia or cognitive impairment present greater risk of developing periodontitis than cognitively healthy individuals.39–41 These findings are supported by those from a population-based cross-sectional study that clearly showed that good cognitive function is a protective factor against periodontitis.42
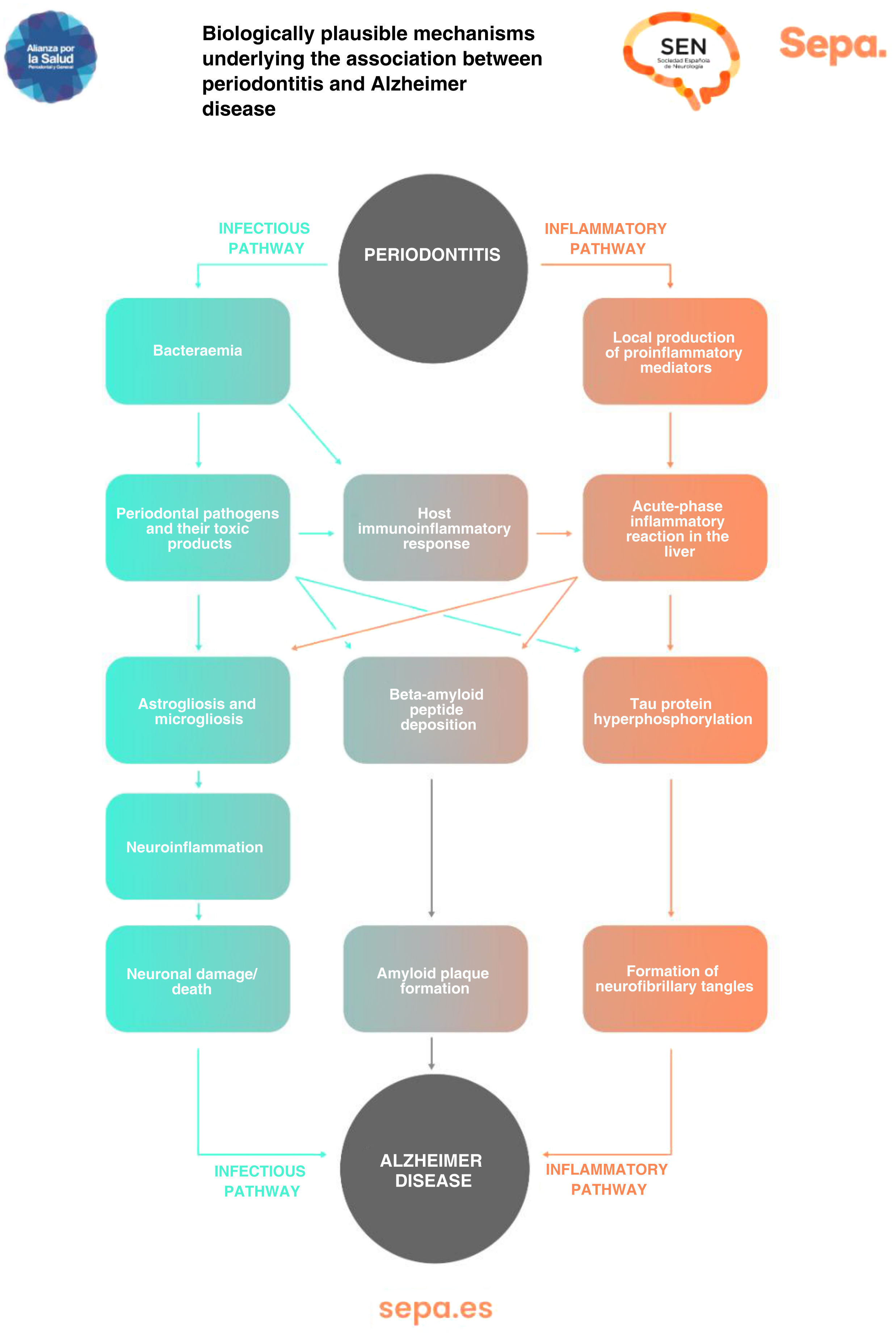
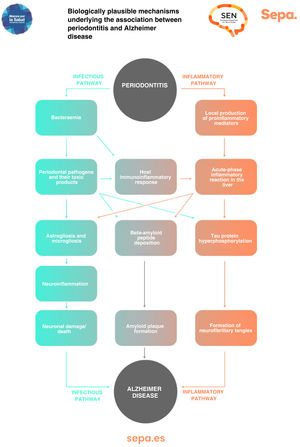
Can any biologically plausible mechanism explain the association between periodontitis and cognitive impairment?As previously mentioned, periodontitis causes recurrent episodes of bacteraemia and endotoxaemia, as well as a state of low-grade chronic inflammation. All this would significantly contribute to the development of neurodegenerative processes involved in cognitive dysfunction, particularly those typical of Alzheimer disease, such as astrogliosis, microgliosis, neuroinflammation, and neuronal damage/death, as well as the formation of beta-amyloid senile plaques and neurofibrillary tangles, which contain hyperphosphorylated tau protein (Fig. 7).43
Can periodontal treatment impact the progression of cognitive impairment and/or its markers?To date, no interventional clinical trial has been published on the effect of periodontal treatment in the primary prevention of dementia. However, numerous population studies have identified a significant decrease in the risk of dementia after several oral health interventions, such as professional dental prophylaxis or periodontal treatment.44,45 Furthermore, regular visits to the dentist (at least annually) have been shown to be a protective factor against dementia in the future.46 A recent quasi-experimental study combining data from patients with and without periodontitis from 2 different epidemiological databases confirmed a beneficial effect of periodontal treatment on the brain atrophy typical of Alzheimer disease.47 Regarding research into secondary prevention, limited evidence is available on the possible improvement caused by dental treatment or certain oral hygiene measures in the cognitive function of patients with cognitive impairment.48,49
Conclusions- •
Patients with periodontitis present 1.7 times greater risk of developing Alzheimer dementia than patients without periodontal disease. The available evidence on the association between periodontitis and vascular dementia is more limited.
- •
Individuals with periodontitis performed worse than those without periodontal disease in different neuropsychological assessments of cognitive function.
- •
Periodontitis causes recurrent episodes of bacteraemia and endotoxaemia, leading to a state of low-grade chronic inflammation that significantly contributes to the development of neurodegenerative processes involved in cognitive dysfunction.
- •
No interventional clinical trial has been published on the effect of periodontal treatment in the primary prevention of dementia. Regarding secondary prevention, very limited evidence is available.
- •
Observational studies have confirmed a significant decrease in the risk of dementia in association with different oral health interventions, including periodic visits to the dentist, as well as a beneficial effect of periodontal treatment on brain atrophy.
- •
All patients
- o
Avoid toxic habits in general, especially alcohol abuse.
- o
Avoid tobacco use.
- o
Promote healthy habits.
- o
Promote regular physical exercise and cognitive activities.
- o
Promote adequate control of vascular diseases and risk factors (arterial hypertension, diabetes mellitus, dyslipidaemia, obesity…).
- o
- •
Patients with stroke
- o
Avoid the discontinuation of antiplatelet/anticoagulant therapy, except in those procedures in which this is considered strictly necessary and for the minimum time possible. When indicated, clinicians should assess the need for a replacement bridging antithrombotic therapy, and inform the patient about the risk of discontinuation.
- o
Inform patients about warning symptoms and advise immediate contact with emergency services.
- o
- •
Patients with post-stroke seizures
- o
Avoid the use of antibiotics that decrease the seizure threshold (quinolones).
- o
Inform patients about the general measures to be considered in the event of epileptic seizure: lateral safety position, protection from the environment, avoiding introduction of objects into the patient’s mouth….
- o
- •
Patients with cognitive impairment
- o
Inform the patient and their caregiver about the relevance of proper oral hygiene and monitoring signs of dental infection or disease.
- o
Treat pain in all patients with cognitive impairment and dental disease, particularly in those showing agitation, even when the patient does not explicitly complain of pain. Avoid the use of opiates to treat pain.
- o
Avoid sedation as far as possible; where necessary, the minimum dose should be used.
- o
- •
All patients
- o
Encourage all patients (or their caregivers) to incorporate teeth brushing into their daily hygiene habits (at least twice a day, with fluoride toothpaste, for 2minutes).
- o
Stress to all patients the importance of periodic dental check-ups (at least once a year).
- o
- •
Suspicion of periodontitis
- o
Encourage all patients with risk factors or signs/symptoms of suspicion of periodontitis to attend a dental check-up as soon as possible.
- o
Assess risk factors:
- o
Smoking
- o
Diabetes mellitus
- o
Neutropenia
- o
Immunodeficiencies (congenital/acquired)
- o
Immunosuppressive therapy.
- o
- o
Ask for and examine warning signs/symptoms:
- o
Gingival bleeding (spontaneous or with brushing)
- o
Whitish, reddish, or bluish gums
- o
Bad breath (halitosis)
- o
Tooth mobility
- o
Progressive displacement of teeth
- o
Exposed tooth root.
- o
- o
- •
Confirmed diagnosis of periodontitis
- o
Encourage all patients diagnosed with periodontitis to seek specific periodontal treatment as soon as possible.
- o
Stress the need to follow the instructions provided by the dentist and attend periodic check-ups.
- o
None.
Conflicts of interestNone.
Part of this report has been disseminated by both scientific societies. The SEPA-SEN working group would like to thank the SEPA executive committee for its invaluable help in the development of this project, and in particular Javier García, Jaume Pros, and Eugenia Huerta; and is grateful for the institutional support of doctors José Miguel Láinez (President of the SEN), Antonio Bujaldón (former president of the SEPA), José Nart (president of the SEPA), Paula Matesanz (vice-president of the SEPA), and Olalla Argibay (board member of the SEPA). Lastly, we would like to thank doctor Debora Marletta (Library services, University College London) for her help in the literature search. Yago Leira has been awarded a Sara Borrell fellowship, funded by the Instituto de Salud Carlos III (CD22/00051).