The study of the neural networks involved in music processing has received less attention than work researching the brain's language networks. For the last two decades there has been a growing interest in discovering the functional mechanisms of the musical brain and understanding those disorders in which brain regions linked with perception and production of music are damaged.
DiscussionCongenital and acquired musical deficits in their various forms (perception, execution, music-memory) are grouped together under the generic term amusia. In this selective review we present the “cutting edge” studies on the cognitive and neural processes implicated in music and the various forms of amusia.
ConclusionsMusical processing requires a large cortico-subcortical network which is distributed throughout both cerebral hemispheres and the cerebellum. The analysis of healthy subjects using functional neuroimaging and examination of selective deficits (e.g., tone, rhythm, timbre, melodic contours) in patients will improve our knowledge of the mechanisms involved in musical processing and the latter's relationship with other cognitive processes.
El estudio de las redes neuronales implicadas en el procesamiento de la música ha recibido menos atención que la dispensada al lenguaje proposicional. Desde hace dos décadas existe un interés creciente en conocer los mecanismos funcionales del cerebro musical y los trastornos que surgen del daño de las estructuras implicadas en la percepción y producción de la música.
DesarrolloLos déficits congénitos y adquiridos del procesamiento musical en cualquiera de sus componentes (percepción, ejecución, memoria musical) se engloban dentro del término genérico amusia. En este trabajo se presenta una revisión selectiva del «estado-del-arte» de los procesos cognitivos y neurales implicados en la música y los diferentes tipos de amusias.
ConclusionesEl procesamiento musical depende de una amplia red neural córtico-subcortical distribuida en ambos hemisferios cerebrales y cerebelo. El análisis de sujetos sanos con neuroimagen funcional y de los déficits selectivos en los componentes musicales (p. ej., tono, ritmo, timbre, contorno melódico) en pacientes con amusia mejorarán nuestro conocimiento acerca de los mecanismos implicados en el procesamiento musical y su relación con otros procesos cognitivos.
From the depths of antiquity, music has occupied a privileged position in all cultures; this most universal form of language is a complex phenomenon which is difficult to describe. In Darwin's book The Descent of Man, and Selection in Relation to Sex (1871), the author addresses the enigmatic origin of music in human evolution without reaching any conclusions. He expressed his astonishment and perplexity with music's biological function within our species as follows: “[musical faculties] must be ranked among the most mysterious with which he is endowed”. Music arises as an innate ability that precedes spoken language. In fact, babies are sensitive to melodies and rhythms even before being born. As we grow, we learn that music is a fundamental element of our culture which fosters a particular style of communication and social interaction, as well as the ability to express emotion.1–3 As with verbal linguistic functions, the processing of music requires a structure of specific neuronal networks, since recent evidence indicates that musical processing is different from other cognitive processes. In fact, selective impairments in musical processing show that those neuronal networks involved in music are independent from other networks responsible for processing speech and ambient sounds.4–8 Over the last few years, there have been important advances in our understanding of brain processes involved in music. This progress has enabled researchers to design cognitive models based on the findings of the neurosciences.9
Music cognition modelWhen we listen to music, a series of basic processes are activated, such as recognition of the melody, musical memory, recognition of lyrics, emotional state, and many others. The integration of all of these processes at once is due to a complex brain processing mechanism in which multiple neural circuits participate simultaneously and/or successively. Theoretical models based on scientific evidence are needed if we are to identify the tasks involved in musical processing and understand potential interactions between them.
Peretz and Coltheart designed a functional architecture model for musical processing using studies of brain-damaged patients.10 According to this model, which was based on the perception of monophonic (single-voice) melodies, music reception is organised by 2 systems working independently at the same time. The one called the melodic system (MS) is responsible for melody processing, while the other, known as the temporal system (TS), is responsible for musical tempo processing, as its name suggests. The MS is responsible for processing all melody information, and it also discriminates between 2 crucial components: tones (each note in the melody) and intervals or difference between tones (distance between them and whether the change is ascending or descending). Processing the melody or melodic contour processing requires integrating all components within an overall perception mechanism. Recent research has shown that melodic processing occurs in the right superior temporal gyrus through its connections with ipsilateral frontal areas.11 In addition, the TS is responsible for temporally placing the melody defined by the MS by means of 2 processes: rhythm (note duration) and musical metre (number of primary and secondary beats per unit of time, or time signature [2/4 time; 6/8 time]). The TS is finally integrated in an overall perception mechanism.12 In addition, rhythm processing may be altered without the metre being affected and vice versa, as will be described in a later section.
Both systems work together, so a damaged brain may selectively lose its understanding of the ‘how’ (MS) or ‘when’ (TS) in its perception of music. Both networks (MS and TS) send information to the musical lexicon so as to create the musical repertoire (MR), or collection of known musical works. The musical lexicon includes the MR and contains the perceptual representation of all musical pieces and works to which we have been exposed in our lifetimes. The musical lexicon also includes musical memory, which stores all new musical pieces and works so that both familiar and non-familiar melodies can be recognised. Therefore, when the musical lexicon is damaged, the subject cannot recognise familiar melodies or record new ones. Output from the musical lexicon occurs either spontaneously or after receiving a stimulus and it is transmitted to a number of different components depending on what is required: (a) activation of the phonological lexicon (input and output) in order to retrieve lyrics; (b) phonological and articulation planning to prepare for the singing process; (c) activation of motor functions for music production, and (d) activation of multimodal associative memories to retrieve non-musical information (title of a musical piece, context of a concert, feelings evoked by a melody). Concerning the last requirement (d), perceptual modules are connected to emotional pathways alongside memory processes, but independently from them. This enables a listener to recognise a musical piece and experience emotions. Emotional processing is independent from the non-emotional analysis of music, and may therefore be damaged selectively.10 Recent functional neuroimaging studies with positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) are providing scientific validation for the theoretical model proposed by Peretz and Coltheart.13–15
In conclusion, impairment of any of these connections would explain difficulties integrating musical processes among patients with brain damage. Without these fundamental pillars, melody, harmony, scale, and other musical features cannot be perceived, just as texts without words, syllables, or letters cannot be understood in the context of language.
Definition and types of amusiaAmusia is a generic term for a syndrome also known as music agnosia caused by damage to one or more basic processes in music perception. The term ‘amusia’ was coined by Steinhals in 1871 as a general term for the inability to perceive music.16–18 Later on, following in the footsteps of Kussmaul, Lichtheim, and Wernicke, the German physician and anatomist Knoblauch proposed a diagrammatic model of music (1888/1890), which is now considered to be the first cognitive model for music processing.19 Amusia may be acquired or congenital.
Acquired amusia occurs due to brain injury. Depending on its location, injury may modify multiple musical functions (for example, expression, perception, execution, rhythm, reading, and writing), which is why researchers have described the numerous clinical types of amusia listed below. We do not know the incidence of acquired amusia. A review of the literature between 1965 and 2010 reveals several published cases of acquired amusia with widely different symptoms and anatomical locations (Tables 1 and 2).18,20–30
Main characteristics of acquired amusia (cases published in the literature between 1965 and 2010).
| Aetiology, n=53 |
| Infarct 33 |
| Haemorrhage 9 |
| Infectious disease 2 |
| Degenerative disease 5 |
| Tumour 3 |
| Arteriovenous malformation 1 |
| Age |
| ≤50 years: 18 |
| >50 years: 35 |
| Sex |
| Men: 17 |
| Women: 36 |
| Dominant hand |
| Right 48 |
| Left 1 |
| Ambidextrous 3 |
| Professional musician |
| Yes 25 |
| No 28 |
| Hemisphere of the lesion |
| Right 12 |
| Left 19 |
| Bilateral 22 |
| Aphasia |
| Yes |
| No 23 |
Types of deficits in acquired amusia (1965–2010).
| Type of deficit |
| Pitch discrimination 29Rhythm discrimination 22Melody recognition 20Timbre discrimination 15Singing 12Playing instruments 10Emotional connection to music 9Reading music 8Writing music 9Voice discrimination 3Environmental sound discrimination 4 |
Most cases present multiple deficits; only the most important is listed.
Congenital amusia appears at birth and it is also known as ‘tone deafness’ since almost all of these patients present pitch processing deficits. According to some studies, congenital amusia seems to be present in 5% of the population.31 Studies with twins and direct relatives of patients with congenital amusia have also shown deficits in pitch processing, suggesting that this disorder is hereditary and associated with structural variations in the frontal and temporal lobes.32–35
As with the classifications of aphasias, attempts to categorise amusias have generated controversy and given rise to a long list of diagnostic labels. Another factor that complicates the process of creating a reliable system for classifying amusia is that amusias are considered rare disorders and most clinical and pathological descriptions are based on isolated cases. In one attempt to classify the syndrome, Arthur L. Benton identified more than a dozen different types of amusias, including receptive and expressive amusia.36 According to current clinical classifications, motor amusia refers to the loss of ability to sing, whistle, or hum; sensory amusia (tone deafness), the loss of ability to discriminate pitches; musical amnesia, the loss of ability to recognise known pieces of music; musical apraxia or instrumental amusia, the loss of ability to play an instrument; musical agraphia, the inability to write music; and musical alexia, the inability to read music.
Recall that patients with amusia (congenital or acquired) experience difficulties in understanding music, although their auditory systems and other cognitive functions remain intact, no associated neurological disorders are present, and their environmental exposure to music is sufficient.11 However, evidence shows that the loss of musical abilities is not necessarily related to the loss of verbal functions, as can be observed in patients who preserve their musical abilities in spite of losing the ability to produce spoken language (aphasia). An example of such a phenomenon is the fascinating case of Russian composer Vissarion Y. Shebalin (1902–1963). This case was exhaustively studied by Alexander R. Luria et al.37 and published under the title ‘Aphasia in a composer’. Surprisingly, after suffering a stroke with associated complications of Wernicke's aphasia and jargon aphasia, he was able to compose his fifth symphony and thus demonstrate that his musical abilities were completely intact. However, other patients who suffered brain damage preserve their ability to recognise lyrics to known songs, poems, familiar voices, and environmental sounds, but are incapable of recognising the music accompanying them.38 Furthermore, other cases combine amusia with aphasia, as occurred with French composer Maurice Ravel (1875–1937). Ravel lost certain musical abilities in the context of developing progressive primary Wernicke's aphasia, ideomotor apraxia, alexia, and agraphia. Although the composer was rendered incapable of singing, playing the piano, writing, and reading music, he could recognise melodies and respond to them on an emotional level. Ravel was unable to express and write the music created by his brain, and it remained imprisoned in his mind. Upon attending a concert and hearing his own musical works, he exclaimed in frustration: “Et puis, j’avais encore tant de musique dans la tête” (And I had so much music left in my head!).30
Subjects with ‘tone deafness’ do not discriminate between tone directions (an essential step in the creation of a melodic contour). They cannot recognise familiar melodies or sing in tune, and they cannot identify musicians who are out of tune; all melodies sound similar to them. They are unable to see the point of music. As Sigmund Freud himself stated, “I do not understand music and its aesthetic effects”.16 Therefore, ‘tone deafness’ may be considered the opposite of absolute or perfect pitch (the ability to identify different tones without the benefit of an external reference as the result of an extraordinary auditory memory). Cases of rhythm deafness without tone deafness have also been described (‘rhythm agnosia’). However, this condition is uncommon, since rhythm involves many areas of the brain.39 In general, subjects with congenital amusia, and most cases of acquired amusia, tend to present musical tone processing deficits. However, rhythm impairment only affects a few of these cases.30,40,41 Patients with tone deafness or complete receptive amusia do not usually enjoy music, since they cannot perceive it as such. Music may even be unpleasant to these patients, who may develop strategies for avoiding musical events of all types (they do not attend concerts or listen to music in private). After hearing Rachmaninoff's Piano Concerto No. 2, a patient with congenital amusia and tone deafness stated, “It is furious and deafening”.42 Amusic patients report perceiving music as strident, grating, explosive, and similar to the sound of banging pots and pans together. It has been suggested that patients with severe amusia may present, in addition to tone deafness, impaired processing of timbre, or the ‘colour’ of a sound. This property is independent from the sound's pitch, and can be illustrated as the difference in sound when the same note is played on a piano and on a violin. For this reason, severe amusia is also called ‘dystimbria’.29,39,43
Anatomical correlates of musical processingFindings from recent research into the musical brain indicate that associating musical functions with the right hemisphere and linguistic functions with the left hemisphere is erroneous and simplistic.
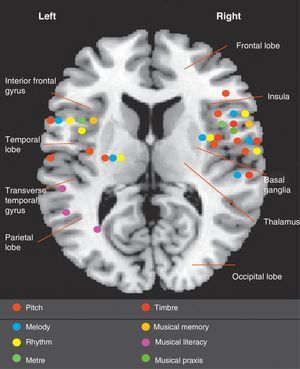
Documented cases of amusia show that not all musical deficits are located in the right hemisphere (Fig. 1). We also know that musical processing differs between subjects and that professional musicians and non-musicians process music in different ways, since they use different areas of the brain. While both hemispheres complement each other in the task of perceiving both melodic and temporal systems, the right hemisphere plays a more important role in overall perception, especially in right-handed non-musicians. The right primary auditory cortex (Brodmann area 41) and secondary auditory cortex (BA 42) are crucial to the perception of music. Therefore, a congenital or acquired anomaly in the right auditory cortex predicts significant musical impairment.4,44 A recent study of right-handed patients with right and left middle cerebral artery infarction showed that those with damage to the right hemisphere had more severe amusia than amusic patients with damage to the left hemisphere.45 Nevertheless, musical specialisation among right-handed professional musicians involves multiple areas of the left hemisphere, promoting interconnection between the two hemispheres and establishing more specific and concrete functions. Neuroimaging studies in professional musicians have shown the importance of the left hemisphere during musical tasks requiring more analytical processing.46,47
Recent knowledge about the structures involved in musical processing is mainly based on study of the correlations between affected areas and musical deficits identified in published cases (Fig. 1). With the help of new technologies and research, our knowledge is progressing significantly.48
In 1865, Bouillaud49 described the first series of patients with brain damage and loss of musical abilities. Henschen published the first monograph on amusia in 1920.50 In 1962, Milner researched musical functions in a group of patients with intractable epilepsy who underwent temporal lobectomy. Peretz51 studied patients who underwent left and right temporal corticectomy. They concluded that the right hemisphere is responsible for melody and melodic contour in general terms, while the left hemisphere has the more specific task of encoding each note and interval in that melodic contour. However, there is no clear association between rhythm and a specific hemisphere; rhythm is processed in multiple areas and rhythm impairment has been found in patients with either right-sided or left-sided brain damage.52
With regard to musical memory, some studies have revealed that the process of learning and retaining unfamiliar melodies involves the right hemisphere, while the process of recognising familiar melodies seems to depend more on the left hemisphere.53,54
According to MRI volumetric studies, patients with congenital amusia present less white matter than healthy subjects, especially in BA 47/44 of the right inferior frontal gyrus (rIFG). The reduction in white matter volume has been related to abnormal results on tests for detecting off-pitch notes in a musical phrase and melodic pitch memory in the Montreal Battery of Evaluation of Amusia (MBEA).55 Recent fMRI studies have shown lack of activation of the RIFG in amusic patients.56 Other PET studies57–59 have also revealed activation of the RIFG in healthy subjects during tasks involving musical memory. These areas probably participate in pitch processing by means of frontotemporal connections with the right auditory cortex of the temporal lobe. These connections are underdeveloped in amusic subjects. In contrast, these studies found that amusic patients had more grey matter in the same region of the rIFG (BA 47) than healthy subjects. The increase in grey matter in the rIFG may be due to an impairment in neuronal migration similar to those described in epilepsy and developmental dyslexia. However, it is more reasonable to assume that impaired musical perception is due to a reduced volume of white matter rather than to an increase in grey matter thickness. As mentioned previously, the abnormal rIFG found in congenitally amusic patients is probably related to genetic conditions60 involved in the development of early neuronal migration through frontotemporal connections,21–24 since both frontal and temporal lobes are necessary for music processing. Therefore, amusia may appear when one or both lobes, or the frontotemporal connections, have suffered unilateral or bilateral damage. On the other hand, although researchers have not yet found morphological anomalies in the white and grey matter of the right auditory temporal cortex in congenital amusic subjects, we cannot rule out this possibility.55–61
Cognitive evaluation and rehabilitation for amusiaContrary to what Knoblauch thought, amusia does not only affect professional musicians.16 In our experience, amusic subjects with no musical knowledge are unlikely to report their condition since they lack sufficient musical knowledge to perceive their own deficits. On the other hand, professional musicians and music lovers are quick to identify any musical deficits. If amusia were to be studied as systematically as language and cognitive functions, researchers would probably confirm that amusia is much more frequent than the literature suggests. Therefore, standardised evaluations need to be employed if we are to further our knowledge of musical abilities.
The MBEA62 was designed in 1987 as a tool for assessing amusic patients. Musical perception and musical memory are the most commonly studied musical functions. The MBEA includes 6 tests which allow researchers to assess perception of melodic contour, intervals, musical scales, rhythm, musical metre, and musical memory. Each test includes 30 unfamiliar musical phrases. Less well-known evaluation tools include the one developed by Wertheim and Botez in 1959, which involved adapting the test to the patient's pre-symptomatic musical level after first classifying subjects’ musical abilities;63 the Gordon musical aptitude profile;64 and the Bentley measures of musical abilities.65
In the last few years, researchers have been showing increasing interest in understanding the potential links between amusia and cognitive impairment in areas such as memory, visuospatial capacity, attention, etc. A recent study on acquired amusia in patients with right middle cerebral artery infarction showed that amusic patients score lower on tests of memory, attention, and cognitive flexibility than non-amusic subjects do.66
On the other hand, it is reasonable to think that perception of melodic contour in amusic patients may also affect the intonation (prosody) of speech.67 However, some studies show that 2 perceptual processes are at work: one for singing intonation and another for speaking intonation. Patel argues that ‘melodic contour deafness’ is not exclusive to music, as it also affects spoken language.68 Other studies have shown that musical perception depends on the same cognitive processes needed for spatial processing. Researchers have described cases of amusic patients with spatial impairment, which were believed to be related to poor mental representation of pitch intervals.69
We often hear tone-deaf subjects say, “I can’t play music since my ear is not good, but I love it”. These people are unable to sing in tune and they cannot tell when others are off-pitch, but they do enjoy music. Our opinion is that they should not be considered amusic in the strict sense of the term. Such individuals are probably lacking in musical education or exposure to music. Although their musical abilities remain intact, they are inactive. Alternatively, these individuals may be considered to have a ‘tolerant’ form of amusia, which may improve with the help of directed music training. Music teachers often improve their students’ musical abilities, since the ear for music can become more fine-tuned70 by the repetition of specific musical tasks which discriminate pitches, chords, intervals, rhythms, tonalities, and melodies. These tasks are activated when we play an instrument or attend a concert, for example. In addition, neuroimaging studies have found neuroplasticity in the musical pathways. This phenomenon is related to more ample musical experience, acquired by either playing or listening to music constantly (experience-based neuroplasticity).71–73 This is why some authors suggest that neuronal organisation in subjects with congenital amusia is not only endogenous, but also related to limited exposure to music. Amusic subjects are less likely to listen to music since it brings no pleasure. Therefore, avoiding exposure to music over the long term may lead to a decrease in the plasticity of frontotemporal connections (learning non-use). It is fundamental that we employ neuroimaging to study the neuroplasticity of the musical networks during directed musical tasks. This may lead to the discovery of rehabilitation techniques for amusic patients. In 2008, Weill-Chounlamountry et al.74 developed the first rehabilitation therapy for amusia. Their patient had suffered a cerebral infarction which caused tone deafness by impairing his pitch discrimination and therefore melody discrimination, but which did not affect his sense of rhythm. Researchers employed a computer programme that administered selective rehabilitation methods focusing solely on melody discrimination. This technique improved the patient's results on post-therapy MBEA tests. In addition, music plays an important role in correcting other cognitive deficits (melodic intonation therapy in patients with aphasia).75,76
ConclusionsAlthough several different areas of the brain are involved in music processing, more studies are needed in order to gain a better understanding of anatomical correlates. Although we do possess a large body of knowledge about speech and its corresponding areas of the brain, hemispheric specificity in music and brain regions involved in each of the components of music (pitch, rhythm, timbre, melody, musical memory) remain largely enigmatic. Neuroimaging tests (fMRI, PET, MEG) in amusic patients and in subjects with deficits in specific music processes will add to our knowledge of musical networks and refine models of anatomical and functional correlates. Congenital and acquired amusia may be more frequent than the literature suggests. The scarcity of tools for neuropsychological diagnosis and patients’ lack of awareness of their amusia may presumably explain the low rates of detection of these disorders.
FundingThis study received no public or private financial support.
Conflict of interestThe authors have no conflict of interest to declare.