Given that surgical treatment of refractory mesial temporal lobe epilepsy may cause memory impairment, determining which patients are eligible for surgery is essential. However, there is little agreement on which presurgical memory assessment methods are best able to predict memory outcome after surgery and identify those patients with a greater risk of surgery-induced memory decline.
ObjectiveWe conducted a systematic literature review to determine which presurgical memory assessment methods best predict memory outcome.
Material and methodsThe literature search of PubMed gathered articles published between January 2005 and December 2015 addressing pre- and postsurgical memory assessment in mesial temporal lobe epilepsy patients by means of neuropsychological testing, functional MRI, and other neuroimaging techniques. We obtained 178 articles, 31 of which were included in our review.
ResultsMost of the studies used neuropsychological tests and fMRI; these methods are considered to have the greatest predictive ability for memory impairment. Other less frequently used techniques included the Wada test and FDG-PET.
ConclusionsCurrent evidence supports performing a presurgical assessment of memory function using both neuropsychological tests and functional MRI to predict memory outcome after surgery.
El tratamiento quirúrgico de los pacientes con epilepsia mesial del lóbulo temporal refractaria al tratamiento farmacológico conlleva el riesgo de deterioro de la memoria, siendo así importante una correcta selección de los pacientes. Sin embargo, no existe un consenso claro en cuanto a qué métodos de la evaluación prequirúrgica de la memoria permitirían predecir mejor sus cambios tras la cirugía para identificar a aquellos pacientes con mayor riesgo de deterioro.
ObjetivosIdentificar qué métodos de evaluación de la memoria podrían ser más útiles en la predicción de sus posibles cambios tras la cirugía, a través de una revisión sistemática de la literatura.
Material y métodosSe realizó una búsqueda de los estudios publicados entre los años 2005 y 2015 en la base de datos Pubmed, incorporando aquellos en los que se evaluaba la memoria, mediante test neuropsicológicos, neuroimagen funcional, y otras, antes y después de la intervención neuroquirúrgica en pacientes con epilepsia mesial del lóbulo temporal, con objeto de predecir sus cambios. Se identificaron 178 artículos, de los cuales 31 fueron finalmente incluidos en la revisión.
ResultadosLa mayoría de los estudios utilizan test neuropsicológicos y RM funcional, con una amplia variedad de técnicas diferentes, que son los métodos con mayor utilidad predictiva. Otras técnicas, como el test de Wada o el FDG-PET, son menos utilizadas.
ConclusionesLa evidencia actual apoya que una evaluación prequirúrgica adecuada de la memoria mediante test neuropsicológicos y RM funcional constituye el mejor modo de predicción de sus cambios tras la cirugía.
Mesial temporal lobe epilepsy (mTLE) is the most frequent form of focal epilepsy in adults. As it is frequently refractory to antiepileptic treatment, it constitutes one of the main indications for epilepsy surgery, which achieves seizure freedom in 60-80% of cases.1–3 However, this surgery may negatively affect memory. The procedure has a considerable impact on verbal and non-verbal memory in patients with left and right mTLE, respectively; verbal memory is more vulnerable.3–7 A thorough evaluation, including a detailed clinical history plus, at least, EEG, neuroimaging (MRI), and proper cognitive assessment, should therefore be conducted before surgery to determine the exact location of the epileptic focus, detect any related lesions, and evaluate the risk of cognitive impairment after surgery. The data gathered enable physicians to evaluate whether surgery is indicated for each specific case and to inform patients about the risks involved.
Several risk factors for postoperative memory impairment have been identified3,8; these include older age at the time of the intervention, type and extent of the underlying disease (hippocampal sclerosis is associated with poorer prognosis), whether surgery is performed on the dominant hemisphere (this is associated with higher risk of verbal memory impairment), and other factors related to epilepsy, such as age at onset and disease progression time.
Preoperative cognitive reserve has also been identified as a major prognostic factor3,8: patients with normal baseline memory are at greater risk of memory impairment. Preoperative memory may be assessed with different tools, including neuropsychological tests, functional imaging techniques (particularly functional MRI [fMRI]), and such invasive methods as the Wada test, which is becoming less frequently used worldwide.6,8 There is no consensus as to which specific methods of memory assessment are most effective for predicting memory impairment, and the studies conducted to date are heterogeneous in terms of methodology and results.
We conducted a systematic review of studies, aiming to establish a preoperative memory assessment method able to predict postoperative memory changes in patients with mTLE.
Material and methodsDatabases and keywords usedWe searched PubMed for articles published between 1 January 2005 and 31 December 2015 using the following MeSH terms: (mesial temporal lobe epilepsy OR sclerosis OR hippocampal sclerosis) AND surgery AND memory. Results were filtered to include only studies on humans. Our literature search yielded a total of 178 studies.
Study selection criteriaWe reviewed the studies identified in the literature search and applied the following selection criteria:
Study typeTo be included in the review, studies had to provide data on memory assessment and compare baseline and postoperative data from a single sample of patients; studies could be prospective or retrospective.
We excluded case reports, editorials, reviews, letters to the editor, and any other type of article not providing original information, as well as studies not conducted on humans and those published in languages other than English or Spanish.
ParticipantsWe included studies on adults (>14 years) and excluded those including only children. Patients had to have been diagnosed with mTLE according to their symptoms, EEG results, neuroimaging results, or histopathology findings.
We only considered studies including patients undergoing memory assessment and surgery for the treatment of mTLE, regardless of the technique used.
Types of assessmentThe studies selected included thorough assessment of either verbal or non-verbal memory both before and after surgery, regardless of the patient’s follow-up time. The evaluation method used for each case (neuropsychological test battery, neuroimaging, and/or Wada test) had to be specified. We also accepted studies including assessment of such other cognitive functions as language, as long as the objective was to analyse the association between these functions and memory in order to predict memory impairment after surgery.
We excluded studies merely describing memory changes, those whose main aim was to describe the short- or long-term outcomes of a surgical technique, and those aiming to compare 2 different surgical techniques or approaches but not analysing their association with preoperative memory assessment results or identifying the patients at greater risk of postoperative memory impairment.
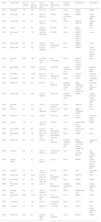
ResultsOf the 178 studies found on PubMed, we finally selected 31.9–39Table 1 lists the year of publication and first author of each study, the number of patients and total percentage of patients diagnosed with hippocampal sclerosis exclusively, the type of participants in the control group (when applicable), memory tests used, and other tests aiming to find an association with memory function and predict memory impairment, according to each study’s objectives.
Summary of the studies included in this literature review (references 9 to 39; listed in that order).
| Year | Lead author | No. of patients | % of patients with HS | No. of patients per side | Left dominancen (%) | Control group(n) | Memory test | Other tests |
|---|---|---|---|---|---|---|---|---|
| 2015 | Rathore C | 116 | 100 | Left: 116 | 94 (81) | None | WMS, ROCFT | Wada |
| 2015 | Sidhu MK | 50 | NA | Left: 23; Right: 27 | 46 (92) | Healthy individuals (26) | BMIPB | Verbal memory fMRI |
| 2015 | Doucet GE | 32 | 56 | Left: 16; Right: 16 | 32 (100) | None | WMS-III, CVLT-II | Resting-state fMRI |
| 2014 | Jutila L | 98 | 58 | Left: 44; Right: 54 | 98 (100) | None | WMS, ROCFT | None |
| 2014 | St-Laurent M | 57 | 100 | Left: 28; Right: 28 | 56 (100) | None | WWRT, RAVLT, WFRT, RVDLT, CALa | None |
| 2013 | Baxendale S | 68 | 100 | Left: 34; Right: 34 | Not established | None | AMIPB, BMIPB | None |
| 2013 | Bonelli SB | 46 | 89.1 | Left: 26; Right: 20 | 33 (71) | None | AMIPB | Verbal and non-verbal memory fMRI |
| 2013 | Gargaro AC | 426 | 100 | Left: 225; Right: 201 | Not established | None | WMS-R, RAVLT, RVDLT, ROCFT | None |
| 2013 | McCormick C | 38 | 68.42 | Left: 18; Right: 20 | 34 (89.47) | Healthy individuals (19) | WWRT, WFRT | Resting-state fMRI |
| 2012 | Vulliemoz S | 45 | 64.44 | Left: 25; Right: 20 | Not established | None | RAVLT, RVDLT | Postictal memory |
| 2010 | Labudda K | 16 | 100 | Left: 16 | 16 (100) | None | VLMT | Language fMRI |
| 2010 | Binder JR | 30 | NA | Left: 30 | Not specified | Patients with right mTLE (37) | WMS-R/WMS-III, SRT, 7/24 Spatial Recall Test | Language and memory fMRI |
| 2010 | Bonelli SB | 72 | 94.4 | Left: 41; Right: 31 | 39 (54.16) | Healthy individuals (20) | AMIPB | Verbal and non-verbal memory fMRI |
| 2009 | Leeman BA | 14 | 57.1 | Left: 14 | 8 (57), rest unknown | None | WMS-III | FDG PET |
| 2009 | Potter JL | 41 | 56.1 | Not specified | 25 (61), rest unknown | None | WMS-R/WMS-III, WFRT | None |
| 2009 | Elshorst N | 59 | 88.1 | Left: 59 | 45 (76.27) | None | CVLT, RAVLT | Tests and MRI vs Wada |
| 2009 | Ujil SG | 183 | 47 | Left: 104; Right: 79 | 159 (87) | None | Fifteen-Word Test, ROCFT | Wada |
| 2009 | Grammaldo LG | 82 | 63 | Left: 34; Right: 47 | Not established | None | RAVLT, SR, ROCFT | None |
| 2008 | Baxendale S | 237 | 100 | Left: 132; Right: 105 | 96 (40.5)7 atypicalRest unknown | None | AMIBP | None |
| 2008 | Binder JR | 60 | NA | Left: 60 | Not specified | Patients with right mTLE (62) | WMS-R/WMS-III, SRT, 7/24 Spatial Recall Test | Language fMRI |
| 2008 | Frings L | 22 | 60 | Left: 12; Right: 10 | 10 (45), rest unknown | None | VLMT, geometric figures | Non-verbal memory fMRI |
| 2008 | Powell HWR | 15 | 80 | Left: 7; Right: 8 | 14 (93) | None | AMIPB | Verbal and non-verbal memory fMRI |
| 2008 | Köylü B | 26 | 73 | Left: 14; Right: 12 | 26 (100) | None | MGT | Language fMRI |
| 2008 | Harvey DJ | 161 | 72.67 | Left: 80; Right: 81 | Not established | None | WMS-III | None |
| 2007 | Baxendale S | 91 | NA | Left: 43; Right: 48 | 91 (100) | None | AMIPB | Wada |
| 2006 | Richardson MP | 30 | 100 | Left: 30 | Not established | Healthy individuals (13) | AMIPB | Verbal memory fMRI |
| 2006 | Alpherts WCJ | 85 | 55 | Left: 34; Right: 51 | 85 (100) | None | Fifteen-Word Test | None |
| 2006 | Baxendale S | 288 | 76.38 | Left: 163; Right: 125 | Not established | None | AMIPB | None |
| 2005 | LoGalbo A | 17 | 100 | Left: 17 | 10 (59), rest unknown | None | CVLT | None |
| 2005 | Janszky J | 16 | 75 | Right: 16 | 15 (93.75) | None | RVDLT | Non-verbal memory fMRI |
| 2005 | Kirsch HE | 204 | 50 | Left: 96; Right: 108 | Not specified | None | RAVLT | Wada |
AMIPB: Adult Memory and Information Processing Battery; BMIPB: BIRT Memory and Information Processing Battery; CAL: Spatial Conditional Associative Learning Task; CVLT: California Verbal Learning Test; fMRI: functional magnetic resonance imaging; HS: hippocampal sclerosis; MGT: Münchner Gedächtnistest (German-language version of the CVLT); mTLE: mesial temporal lobe epilepsy; NA: not accessible; RAVLT: Rey Auditory Verbal Learning Test; ROCFT: Rey-Osterrieth Complex Figure Test; RVDLT: Rey Visual Design Learning Test; SR: Story Recall Test; SRT: 6-Trial Selective Reminding Test; VLMT: Verbaler Lern- und Merkfähigkeitstest (German-language version of the RAVLT); WFRT: Warrington’s Face Recognition Test; WMS: Wechsler Memory Scale; WWRT: Warrington’s Word Recognition Test.
A series of demographic, clinical, and surgical variables have been found to have an impact on memory assessment results, acting as confounding factors; these include age at epilepsy onset, disease progression time, seizure frequency, whether surgery successfully controlled seizures, years of schooling, and intelligence quotient (IQ). Most studies provide sufficient data on these variables, often in tables. Significant differences between the groups being compared are rarely reported, and when differences are found, the variable is included in the statistical analysis (for example, in studies identifying higher IQ as a protective factor against postoperative memory impairment). The type of epilepsy, lesion location, and whether the affected hemisphere is the dominant one are also important factors to consider in patient assessment. Hippocampal sclerosis, the most common cause of mTLE, has been identified as a potential factor of poor prognosis, causing greater memory impairment than other causes both before and after surgery. Table 1 shows the percentage of patients diagnosed with hippocampal sclerosis and no other lesions in all the studies that provided this information.
Involvement of the dominant hemisphere is also associated with poorer prognosis3,8; data obtained from these patients may not be comparable to those from patients with lesions to the non-dominant hemisphere, especially in terms of verbal memory. As a result, patients with left and right mTLE are analysed separately. Despite this subclassification, however, controlling for cerebral dominance for language is still interesting due to the presence of atypical representations (right-sided or bilateral). In general terms, the more patients with left dominance that are included in a study, the more homogeneous the study groups will be in terms of lesions to the dominant and non-dominant hemispheres (left and right). Table 1 summarises the main patient characteristics, indicating lesion location (right or left hemisphere), and the number and percentage of patients with left dominance. As we can see, most studies include patients with unilateral lesions, whether right-sided or left-sided. No study included patients with bilateral lesions.
In 4 studies,15,19,23,32 not all patients underwent surgery. Surgery patients constitute a separate group in the analysis of postoperative memory impairment, as data on these patients help to establish the predictive value of preoperative memory assessment results.
In one of the included studies,29 the epileptic focus was located in the lateral temporal region (extramesial) in one patient. However, this study was not excluded from our literature review as this single patient was not found to have a significant impact on the results.
Neuropsychological test batteryAll the studies included in this review used neuropsychological tests to assess memory both before and after surgery (Table 1). They differ greatly, however, in terms of test batteries used, follow-up time, and the time point when the postoperative assessment is performed (ranging between 3 months and 6 years). Tests may focus on different areas, and some focus only on specific areas (immediate recall, recent memory after a distraction, or both). One study used a non-standardised test; consequently, the results of this study may not be extrapolated to other centres. This heterogeneity makes it difficult to draw conclusions on memory assessment in these patients.
Verbal memory is assessed in 30 of the 31 studies, whereas non-verbal memory is assessed in 20. Eleven studies10,19,22,24,31,33–37,39 evaluate verbal memory exclusively, perhaps because the most frequent deficits are associated with this type of memory, which is also the most relevant from a clinical viewpoint. Only one study38 evaluated non-verbal memory exclusively; this study used a memory-fMRI paradigm for assessing non-verbal episodic memory. The remaining studies evaluate both types of memory.
The heterogeneity of the tests used may be explained by the availability of normalised tests depending on the study population. The Adult Memory and Information Processing Battery (AMIPB) and a more recent version, the BIRT Memory and Information Processing Battery (BMIPB), have a similar structure to that of the California Verbal Learning Test (CVLT) but they are not normalised for the population of the United Kingdom. Other tests used are German-language adaptations of the CVLT and the Rey Auditory Verbal Learning Test (RAVLT). The Fifteen-Word Test is a Dutch-language adaptation of the RAVLT.
The tests most frequently used for verbal memory assessment are the AMIPB/BMIPB, the CVLT, the RAVLT (and its adaptations to other populations), and the Wechsler Memory Scale (WMS). The tests most frequently used to assess non-verbal memory were the visual memory component of the WMS and the Rey-Osterrieth Complex Figure Test.
Postoperative memory changes measured by these tests are regarded as statistically significant if so indicated in the corresponding study. To assess whether changes were significant, 13 studies used reliable change index scores, which enable correction for practice effects and errors typical of test-retest situations. The remaining studies measure changes in a variety of ways, mainly by subtracting postoperative scores from presurgery scores.
Studies using neuropsychological tests exclusivelyMemory assessment with neuropsychological tests is especially important when the study does not evaluate memory by other means, as occurs in 12 studies. Five studies build predictive models using tests and demographic variables exclusively, 2 analyse test validity as a predictor in different situations, 2 include patients with intact preoperative memory, and 3 use long-term follow-up data to identify predictors of memory function during that follow-up period.
Are neuropsychological tests valid for predicting postoperative memory outcomes?The tests used were found to be sensitive to memory changes in patients with mTLE. One study analysed the validity of WMS-III scores for predicting postoperative memory impairment, and found a significant correlation between preoperative function and postoperative verbal and non-verbal memory in patients with left mTLE, and a weaker correlation between preoperative visual memory function and postoperative changes in patients with right mTLE.32
Are neuropsychological tests valid for predicting postoperative memory changes?The study by LoGalbo et al.,37 which included a sample of patients with normal preoperative memory function, underscores the need for functional assessment of patients with hippocampal dysfunction (performed using neuropsychological tests in their study), since it has been found to be more useful for predicting postoperative memory impairment than simply evaluating the presence of such structural diseases as hippocampal sclerosis.
Potter et al.23 and Baxendale et al.36 built logistic regression models incorporating numerous variables (mainly surgery-related variables, age at the time of intervention, presence and type of structural lesions, and test results) to predict postoperative memory impairment; most patients with postoperative memory impairment were classified correctly. Test results explain most of the variance. In both studies, better preoperative memory function was associated with more severe postoperative memory impairment.
The logistic regression model built in another study by Baxendale et al.27 aimed to identify patients likely to show improvements in memory function after surgery. Patients generally showed more marked functional improvements contralateral to the hippocampal lesion; this was associated with lower preoperative test scores, and with higher IQs in patients with left mTLE. IQ was also found to be an important predictive factor in a subsequent study including a sample of patients with normal preoperative memory function, 69% of whom showed postoperative memory impairment.14 The patients maintaining a stable level of memory function had significantly higher IQs, which suggests that these patients may be able to develop more effective compensatory intellectual strategies.
Gargaro et al.16 used a different approach, classifying patients according to whether they showed a typical (unilateral memory deficits) or atypical memory profile. They concluded that these profiles are predictive of postoperative memory impairment: patients undergoing resection of left mesial temporal structures and a normal (no deficit) or contralateral (deficit contralateral to the affected side) memory profile showed considerable verbal memory impairment compared to the remaining profiles, especially those with a normal memory profile, who also achieved the best scores on presurgery memory tests. Surprisingly, patients undergoing resection of right mesial temporal structures showed improved verbal memory function.
St-Laurent et al.13 performed a principal component analysis, identifying components reflecting verbal and visuospatial memory with greater predictive power than test scores in themselves, and concluded that their approach provides valuable data for increasing the predictive capacity of these tests.
Another study explored whether postictal memory testing is more predictive than interictal testing.18 Although the purpose of these tests was to locate the epileptic focus, they added no predictive value when the presence/absence of hippocampal sclerosis and focus lateralisation were also considered; they may nonetheless be useful for assessing functional reserve, given that the function of the hemisphere where seizures originate would be partially suppressed in these situations.
Are neuropsychological tests useful for long-term follow-up?
Three studies followed up patients for long periods (2, 3, and 6 years).13,26,35 In 2 of these,13,35 preoperative test results were found to be predictive of postoperative memory changes throughout the follow-up period, whereas no significant association was observed in the remaining study.26 The latter study, which followed up patients for 2 years, reports long-term memory improvements, whereas the other 2 show memory impairment. However, memory changes are dynamic: memory function may improve or stabilise over the first 2 years after surgery, especially in cases of right temporal lobe surgery, and progressively deteriorate after that time.35
Studies on the Wada testFive studies discuss the usefulness of the Wada test for predicting postoperative memory impairment. The Wada test seems to have little or no predictive capacity,33,39 and does not add any predictive value to a model considering preoperative test results and age at the time of surgery,33 or neuropsychological test results and structural MRI findings.24 In fact, in a recent study, patients who failed the Wada test (according to different failure cut-off points) showed no differences in postoperative memory impairment compared to patients who passed the test.9 A bilateral Wada test (with the drug also injected into the side contralateral to the lesion) was found to predict memory impairment but the results had no impact on treatment decision-making.25 All studies advise against using the Wada test for routine assessment of patients with mTLE who are eligible for surgery; surgery should never be ruled out based on Wada test results exclusively.
Studies using functional magnetic resonance imagingA total of 13 studies used fMRI as the main procedure for predicting postoperative memory impairment. Table 1 lists the memory tests and paradigms used in each study. A detailed analysis of other methodological aspects, such as the regions of interest (ROI) defined in each study, is beyond the scope of this review; we will therefore comment on these aspects only briefly.
Two studies used resting-state fMRI. Four used language paradigms, with semantic categorisation tasks (3) and verbal fluency tasks (1). The remaining studies used memory paradigms: verbal (2), non-verbal (2), or both (3). In all of these studies, fMRI predicted postoperative memory changes, constituting the main predictor for memory impairment or adding a significant predictive value to the model compared to other predictors, mainly neuropsychological test results and clinical variables.
Resting-state functional magnetic resonance imagingThe 2 studies of brain connectivity included in the review concur that resting-state fMRI reflects episodic memory capacity and predicts postoperative changes. McCormick et al.17 studied the connections between the posterior cingulate cortex and the hippocampus; connectivity was predictive of verbal and non-verbal memory function in patients with right and left mTLE. Doucet et al.11 performed a different analysis, and found that certain connectivity measures in the pars orbitalis of the left frontal lobe (in left mTLE) and the right mesial temporal region (in right mTLE) predict verbal memory function, whereas measures for the precuneus predict non-verbal memory function in patients with left but not right mTLE. The latter study, unlike the one by McCormick et al.,17 shows that measures for the healthy contralateral hippocampus did not constitute a good predictor of episodic memory.
Verbal memory paradigmsFive studies focused on predicting verbal memory changes using a memory paradigm.10,15,21,30,34 All of these presented words visually, required participants to perform a deep encoding task, and used an event-related analysis, asking participants to recognise those words in a subsequent task outside the MRI scanner. Cerebral activation for each stimulus was classified according to whether the stimulus was later remembered or forgotten. The possibility of indicating that words were “familiar”10,34 increased the statistical power of the study, as it increased the contrast between stimuli clearly remembered and forgotten. The study by Sidhu et al.10 was methodologically different in that it also required patients to memorise words during the scan.
In this study, the index of frontotemporal activation asymmetry (that is, greater activation in the left than in the right frontotemporal region) was predictive of verbal memory changes in patients with left mTLE. This index was also predictive in the case of one right-dominant patient with right mTLE, showing postoperative verbal memory impairment. In the remaining patients with right mTLE, none of the factors studied (memory lateralisation, language lateralisation, or clinical variables) were able to predict memory impairment.
The remaining studies analyse the surgically treated temporomesial areas, especially the anterior hippocampus. The asymmetry index of hippocampal ROIs was predictive of visual and verbal memory changes21,30: the left/dominant anterior hippocampus for verbal memory21,30,34 and the right anterior hippocampus for non-verbal memory21; the latter association was less consistently found in the literature. The 2 studies conducted by Bonelli et al.15,21 analyse posterior hippocampal activation, which seems to be associated with reduced memory impairment: greater preoperative activation is correlated with better verbal memory outcomes in patients with left mTLE not showing significant deterioration following surgery.15
It may be possible to create predictive models incorporating memory lateralisation indices in addition to such other variables as language lateralisation or neuropsychological test results, which are capable of predicting verbal memory impairment.10,21
Non-verbal memory paradigmsAs mentioned previously, activation of the hippocampus21,30 and right amygdala (which is larger than the left)30 was predictive of non-verbal memory changes after surgery performed on the right or non-dominant hemisphere. Using the Roland Hometown Walking task, Janszky et al.38 found an association between non-verbal memory changes and mesial temporal activation; reduced activation ipsilateral to the epileptic focus was correlated with favourable postoperative outcomes in patients with right mTLE. In that study, all patients with greater ipsilateral than contralateral mesial temporal activation showed postoperative non-verbal memory impairment.
Despite using a task more suitable for assessing visuospatial than verbal memory, the study by Frings et al.29 shows that lateralisation of hippocampal activation is significantly correlated with postoperative verbal memory impairment, with greater ipsilateral activation predicting more severe impairment. No significant correlation was found with non-verbal memory.
Language paradigmsLanguage lateralisation evaluated with fMRI using a semantic decision task can predict postoperative verbal memory impairment and adds predictive value to models including such other factors as age at epilepsy onset and neuropsychological test scores,28 or even lateralisation of hippocampal activation as measured with a visual scene encoding task, which has been found not to be predictive.20 In another study,31 mesial temporal activation as measured with the same task was also found to be correlated with pre- and postoperative verbal episodic memory and may help predict postoperative memory function in patients with left mTLE. However, these results should be interpreted with caution since the study does not correlate fMRI results with changes in test scores but rather with absolute values of test scores before and after surgery.
Finally, a verbal fluency paradigm may also be used to predict verbal memory impairment in patients with left mTLE: significant postoperative impairment has been found to be associated with greater preoperative posterior temporal activation ipsilateral to the epileptic focus.19 Activation in this and such other regions as the middle and superior temporal gyri was found to be negatively correlated with postoperative changes in verbal memory.
Other methods of memory assessmentWe only found one study using FDG PET to predict postoperative memory impairment.22 The results of this study suggest that hippocampal FDG PET asymmetry does not predict verbal memory changes following surgery on the left hemisphere.
DiscussionThe main difficulty in performing the present review was the great heterogeneity of the studies in terms of methodology, analysis, and results. We may conclude that functional assessment of the hippocampus and adjacent areas is essential for indicating and planning surgery in patients with mTLE; in most cases, it is possible to identify which patients are at greater risk of postoperative memory impairment without using invasive techniques.
Two models have been proposed to explain why some patients experience memory impairment whereas others remain stable.40 According to the functional adequacy model, it is the capacity of the ipsilateral hippocampus to support memory after surgery that determines whether memory changes will occur. The hippocampal reserve model, in contrast, posits that it is the reserve or capacity of the contralateral hippocampus that maintains memory function and therefore determines postoperative impairment. Although we reviewed studies supporting either one of the models, most agree with the functional adequacy model.
Neuropsychological tests constitute a widely used tool for measuring memory function; better performance before surgery is associated with greater memory impairment. This may be partially explained by the idea that “the more you have, the more you have to lose”: patients with poorer baseline performance would show less marked postoperative memory impairment due to the floor effect. The data also suggest that, in these cases, surgery disrupts memory function; tests would therefore reflect the functional integrity of the hippocampus to be resected, according to the functional adequacy model.7,14 This is especially relevant with regard to verbal memory in patients with left mTLE, which has consistently been reported to be impaired; data on visuospatial memory are more variable, however, as our review shows. If the right hippocampus were able to fully assume the left hippocampus’ function in terms of verbal memory, patients with good preoperative function would not be affected by surgery on the left hippocampus. Worse results in verbal memory tests are associated with more severe left hippocampal dysfunction; these patients show less marked impairment after surgery,7,14,28 which may be attributed to the functional reserve of the right hippocampus. Thus, a functional shift to the right hemisphere, though protective, would also be associated with poorer preoperative performance.28
The introduction of fMRI in this context has expanded our understanding of the function of the hippocampus and adjacent structures in patients with mTLE.6,8 This neuroimaging technique can also predict memory changes; as occurs with neuropsychological tests, fMRI results are more constant for verbal memory in patients with left mTLE, and more variable for non-verbal memory and in patients with right mTLE. Measures range from absolute mesial temporal lobe activation to asymmetry indices; the latter cannot determine whether the main risk factor for memory impairment is sustained function of the ipsilateral hippocampus or lack of activation of the contralateral hippocampus (that is, whether they support the functional adequacy or the hippocampal reserve model).30
However, most studies using fMRI seem to support the functional adequacy model, reporting that absolute hippocampal activation predicts specific ipsilateral memory function using word and face encoding paradigms or the Roland Hometown Walking task.21,30,34,38 The most important studies on the topic conducted to date are those by Bonelli et al.15,21 These studies found an association between activation of the ipsilateral anterior hippocampus and postoperative memory impairment, and between activation of the ipsilateral posterior hippocampus and a lack of impairment. Furthermore, posterior hippocampal activation was still observed 4 months after surgery; differences in preoperative function were observed between patients with and without postoperative memory impairment. Patients with no postoperative memory impairment showed greater preoperative hippocampal activation, suggesting that early, preoperative memory reorganisation in the ipsilateral posterior hippocampus is efficient and protects against memory impairment, whereas early postoperative reorganisation in the absence of preoperative reorganisation is unable to preserve memory function.15 The authors also observe, however, that the follow-up time may be too short to reflect the role of functional reserve, given that memory changes are dynamic.12,35 This may explain the differences observed between these and other studies including long follow-up periods with fMRI, where postoperative memory function was associated with activation of the mesial temporal region contralateral to surgery.41
According to the material specificity hypothesis, supported by most studies into memory prediction in mTLE, each temporal lobe is specialised either in verbal or non-verbal memory, depending on the material stored; the left hippocampus is associated with verbal memory and the right with non-verbal memory. However, this model seems very restrictive and has therefore been questioned by some authors.42 Visual scene-encoding tasks constitute a good example of this as they seem to activate verbal and non-verbal memory circuits bilaterally, as shown in studies published before the timeframe set for our literature review,43 as well as in some studies included in the review.20,29 This means that patients may use verbal strategies to process visual information: an apparently visuospatial task may therefore be better able to predict verbal than non-verbal memory impairment.29 The activation evoked by these tasks would reflect the asymmetry of the verbal+non-verbal memory system, constituting a good indicator of hippocampal damage rather than an indicator of lateralisation of any one type of memory.20 We should also comment on the heterogeneity of studies measuring visuospatial memory with neuropsychological tests, which may not be sufficiently sensitive to detect right mesial temporal damage, calling attention to the need for more reliable markers.42
Language lateralisation appears to be a good predictor of postoperative verbal memory changes and adds predictive power to models including other factors in several studies.10,21,28 Language tasks enable the identification of ROIs including the activation of mesial temporal structures, but which are larger than those limited to the hippocampus, which may constitute a more stable and reliable measure.20,28 Binder et al.20,28 show that language lateralisation is correlated with verbal episodic memory lateralisation and postulate that the type of material each hippocampus works with depends on the type of information it receives from the ipsilateral cortex; when language is lateralised to the left hemisphere, verbal memory is also represented in the left hemisphere and the patient has a greater risk of verbal memory impairment after surgery on the left hemisphere. Only when the right hippocampus receives verbal information from the right hemisphere does it contribute to maintaining verbal memory.28
In summary, further research into this topic is necessary before fMRI can be applied in clinical practice. Furthermore, due to the differences between individual patients, fMRI protocols for predicting memory impairment are still highly variable, although the existing results are promising. There is clear evidence that the technique adds predictive value to the model compared with classical predictors such as neuropsychological test results, both for memory paradigms and language tasks.
We also analysed other methods for predicting postoperative memory impairment in patients with mTLE. The Wada test is no longer recommended in clinical practice except for particular cases, in line with the results of other more specific review articles.44 Good results have been achieved with connectivity studies using correlation matrices to analyse functional networks through resting-state fMRI. Other techniques, such as FDG PET, require further development and must be shown to be superior to such other tools as neuropsychological tests or fMRI.
ConclusionsMost patients with mTLE at risk of memory impairment may be identified before surgery with a non-invasive structural and functional assessment; functional assessment is an essential part of this process. Neuropsychological tests are currently the most important and most widely used tools for predicting postoperative memory impairment, although fMRI is becoming increasingly common. A predictive model incorporating both neuropsychological test results and findings from fMRI with memory and language paradigms may constitute a powerful tool for identifying patients at greater risk of postoperative memory impairment.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Parra-Díaz P, García-Casares N. Evaluación de la memoria en la epilepsia del lóbulo temporal para predecir sus cambios tras la cirugía. Una revisión sistemática. Neurología. 2019;34:596–606.