COVID-19 is characterized by various clinical manifestations, mainly respiratory involvement. Disease-related malnutrition is associated with impaired respiratory function and increased all-cause morbidity and mortality. Patients with COVID-19 infection carry a high nutritional risk. After designing a specific nutritional support protocol for this disease, we carried out a retrospective study on malnutrition and on the use of nutritional support in patients with COVID-19.
MethodsWe performed a retrospective study to determine whether nutritional support positively affected hospital stay, clinical complications, and mortality in patients with COVID-19. We compared the results with those of standard nutritional management. Our secondary objectives were to determine the prevalence of malnutrition in patients with COVID-19 and the value of nutritional support in the hospital where the study was performed.
ResultsAt least 60% of patients with COVID-19 experience malnutrition (up to 78.66% presented at least 1 of the parameters studied). The specialized nutritional support protocol was indicated in only 21 patients (28%) and was started early in only 12 patients (16%). Hospital stay was significantly shorter in patients managed with the early protocol (5.09 days, 95% CI, 1.338–8.853, p<0.01). Similarly, in this group, respiratory distress was less severe and less frequent (41% vs 82.5%, p<0.007), and statistically significantly fewer complications were recorded (9/12 vs 91/63; p<0.001).
ConclusionsCOVID-19 is associated with high rates of disease-related malnutrition. Early implementation of a specialized nutritional support plan can improve the prognosis of these patients by reducing hospital stay, the possibility of more severe respiratory distress, and complications in general.
La COVID-19 se caracteriza por diversas manifestaciones clínicas, principalmente afectación respiratoria. La desnutrición relacionada con la enfermedad se asocia con deterioro de la función respiratoria y aumento de la morbilidad y la mortalidad globales. Los pacientes con COVID-19 tienen un riesgo nutricional alto. Tras diseñar un protocolo de apoyo nutricional específico para esta enfermedad, se emprendió un estudio retrospectivo sobre desnutrición y uso de soporte nutricional en pacientes con COVID-19.
MétodosSe realizó un estudio retrospectivo para determinar si el apoyo nutricional tenía efectos positivos en la estancia hospitalaria, las complicaciones clínicas y la mortalidad de los pacientes con COVID-19. Se compararon los resultados con los del manejo nutricional habitual. Los objetivos secundarios eran determinar la prevalencia de desnutrición en los pacientes con COVID-19 y la utilidad del apoyo nutricional en el hospital en el que se realizó el estudio.
ResultadosAl menos el 60% de los pacientes con COVID-19 sufre desnutrición (hasta el 78,66% presentaba al menos uno de los parámetros estudiados). El protocolo de apoyo nutricional especializado estaba indicado en sólo 21 pacientes (28%) y se inició de forma temprana en sólo 12 pacientes (16%). La estancia hospitalaria fue significativamente menor en los pacientes sometidos al protocolo temprano (5,09 días, IC 95%, 1.338-8.853, p<0,01). Este grupo mostró asimismo un sufrimiento respiratorio menos intenso y frecuente (41 y 82,5%, respectivamente, p<0,007), y un número significativamente menor de complicaciones (9/12 frente a 91/63; p<0,001).
ConclusionesLa COVID-19 se asocia con tasas elevadas de desnutrición relacionada con la enfermedad. La implantación precoz de un plan de apoyo nutricional especializado puede mejorar el pronóstico de estos pacientes al reducir la estancia hospitalaria, la posibilidad de sufrimiento respiratorio más intenso y las complicaciones en general.
COVID-19 disease is characterized by a wide range of clinical manifestations, mainly respiratory involvement. The effects of malnutrition on respiratory function have been known for some time now and include reduced vital capacity and increased respiratory muscle resistance and residual volume, which in turn have been associated with reduced tolerance to exercise and longer time on mechanical ventilation. Patients also have reduced cough strength and alveolar macrophage count, thus delaying recovery from respiratory infections. Similarly, pulmonary surfactant is reduced, with the consequent increased respiratory effort.1 Disease-related malnutrition (DRM) is not unusual in our hospital system, affecting 1 in 4 patients and more commonly older people (37% of persons aged more than 70 years).2 Adequate early nutritional support has been shown to reduce mortality (by up to 35% according to the EFFORT study3) and poor clinical outcome (admission to intensive care units, readmission to hospital, major complications, impaired functional status, and mortality) (EFFORT). Patients with COVID-19 have a higher nutritional risk owing to the increased requirements resulting from severe acute inflammation and the difficulty in meeting these requirements owing to hyporexia and dyspnea. Jin et al.4 recommended nutritional support as part of the approach to patients hospitalized with COVID-19, whose nutritional requirements are estimated at 25–30kcal/kg of body weight and 1.5g of protein/kg/d. Based on these premises and in light of the fact that care of patients with these diseases is provided by nonspecialized staff, we drew up an institutional protocol on nutrition in patients with COVID-19. Nutritional screening was based on two questions: loss of weight or hiporexia. Positive answer for both of them defined undernutrition in patients younger than 70. All patients older than 70 were classified as high risk for malnutrition. Knowing the difficulties for obtaining anthropometric data in these patients, adjusted weights for 75Pc of different aged-groups of heights were used in order to calculate nutritional requirements. Modified basal diet (in order to provide higher protein content) was normally recommended. Additional nutritional oral supplement (400kcal–20g protein) was prescribed depending on sex and basal diet compliance. Frequent reevaluation (every 3–5 days) was recommended, and based on requirements fulfillment, different procedures of artificial nutrition (enteral nutrition/parenteral nutrition) were indicated. The protocol was distributed among the medical teams responsible for treating this infection and is available on line.5 Once the disease peak had been overcome, we decided to retrospectively analyze the usefulness of the protocol.
Material and methodsStudy hypothesisWe defined nutritional support as a clinical act comprising the following phases: nutritional screening, evaluation of nutritional status, calculation of nutritional requirements, implementation of nutritional treatment (diet therapy, oral supplementation with single nutrients or complete formulas, exclusive or complementary enteral or parenteral formulas), monitoring of treatment, and modification of nutritional treatment depending on the patients’ progress. We performed a study to determine whether nutritional support, as defined above and prescribed on an individual basis or according to a protocol, positively affects hospital stay, clinical complications, and mortality in patients with COVID-19. We compared the results with those of standard nutritional management.
Study objectivesMain objective: To determine whether there are clinical benefits (defined as a reduction in hospital stay) associated with a nutritional support plan that is protocol-based or prescribed and monitored according to the different points covered in a nutritional support plan, as set out in detail in the study hypothesis.
Secondary objectives: To determine whether there are any clinical benefits (defined as a reduction in morbidity and/or mortality) associated with a nutritional support plan that is protocol-based or prescribed and monitored according to the different points covered in a nutritional support plan, as set out in detail in the study hypothesis. The plan had to be introduced early (first 5 days).
To determine the prevalence of malnutrition in patients with COVID-19.
To determine the value of nutritional support in the hospital where the present study was performed. This was defined as adherence to the nutritional support protocol in patients with COVID-19. To compare adherence to this protocol with adherence to other clinical management protocols for the same disease.
MethodsWe performed a retrospective review of the clinical history of patients with COVID-19.
Inclusion criteria: admission to hospital during the period 4/4/2020 to 4/5/2020.
Exclusion criteria:
Admission to the intensive care unit.
Admission for palliative sedation.
We evaluated the following variables:
Sex, age, severe disease risk group as defined by the Ministry of Health,6 duration of admission, clinical presentation (respiratory disease, nonrespiratory disease, or asymptomatic disease), degree of respiratory involvement based on the SaO2/FiO2 ratio (lowest value during admission) and the CURB-65 scale7 (value at admission), adherence to treatment protocols (antibiotic therapy, anticoagulation therapy, investigational medication, corticosteroids) and nutritional support measures, type of nutritional support prescribed, modifications to nutritional support depending on the patient's clinical progress and monitoring, evaluation of nutritional support at admission and during admission, and complications (infections, organ failure, death, pressure ulcers).
Variables were coded in line with current Spanish legislation on protection of personal data after approval of the center was obtained.
Statistical analysisThe statistical analysis was performed using SPSS v.20 for Windows.
The Kolmogorov–Smirnov test was used to determine which variables were normally distributed and which were not.
Quantitative variables were expressed as the mean and standard deviation if they were normally distributed and as the median and range if they were not. Qualitative variables are shown as percentages.
We use a repeated-measures t test and repeated-measures analysis of variance in order to compare normally distributed variables. Nonparametric variables were analyzed using the Wilcoxon test (non-normally distributed quantitative variables), the McNemar test (nominal variables), and the Fisher exact test and chi-square test (qualitative variables and percentages). Statistical significance was set at p<0.05.
ResultsAfter reviewing the clinical histories of all patients admitted, the principal investigator ruled out those who did not fulfill the inclusion criteria. Of those admitted, 75 patients (50 men [66.7%] and 25 women [33.3%]) fulfilled the inclusion criteria. Mean age was 76.39±15.39 years. Forty patients (53.3%) belonged to one of the groups at risk of developing severe disease according to the Ministry of Health, and 65.3% (49 patients) were receiving medication for 3 or more diseases. Of these, 21 (28%) previously had moderate or severe cognitive impairment.
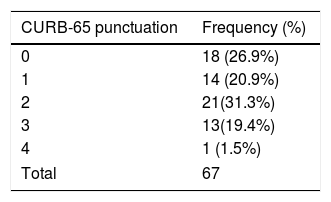
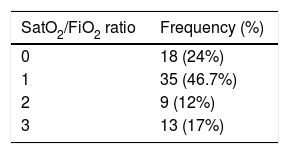
With respect to the clinical picture on admission, 62 patients (82.7%) had mainly respiratory symptoms, 9 (12%) had nonrespiratory symptoms, and 4 (5.3%) had no symptoms compatible with COVID-19. Onset of symptoms was 7.33±6.45 days before admission to hospital. The severity of pneumonia in COVID-19 according to the CURB-65 scale and the maximum degree of respiratory distress according to the SaO2/FiO2 ratio are shown in Tables 1 and 2.
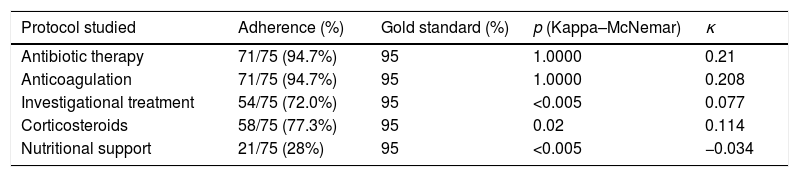
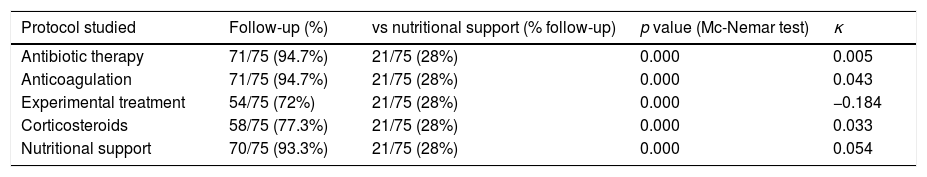
Hospital protocols were established for the use of empirical antibiotic therapy, anticoagulation therapy, investigational pharmacological therapy (azithromycin, lopinavir–ritonavir, hydroxychloroquine), and corticosteroids. Our department provided a nutritional support protocol for COVID-19 adapted to the specific characteristics of our center. We compared the rate of adherence to each protocol with respect to a gold standard of 95% (Table 3) and the rate of adherence to the nutritional support protocol (Table 4). The nutritional support protocol proved to be the least adhered to of all those designed (21 cases [28%]). The protocol was started early (first 5 days) in only 12 cases (16%) (Table 3) and was the least adhered to compared with the remaining established protocols. The difference was statistically significant (Table 4).
Comparison of the use of treatment protocols with respect to a 95% gold standard.
| Protocol studied | Adherence (%) | Gold standard (%) | p (Kappa–McNemar) | κ |
|---|---|---|---|---|
| Antibiotic therapy | 71/75 (94.7%) | 95 | 1.0000 | 0.21 |
| Anticoagulation | 71/75 (94.7%) | 95 | 1.0000 | 0.208 |
| Investigational treatment | 54/75 (72.0%) | 95 | <0.005 | 0.077 |
| Corticosteroids | 58/75 (77.3%) | 95 | 0.02 | 0.114 |
| Nutritional support | 21/75 (28%) | 95 | <0.005 | −0.034 |
Comparison of the use of treatment protocols with respect to the use of a nutritional support protocol.
| Protocol studied | Follow-up (%) | vs nutritional support (% follow-up) | p value (Mc-Nemar test) | κ |
|---|---|---|---|---|
| Antibiotic therapy | 71/75 (94.7%) | 21/75 (28%) | 0.000 | 0.005 |
| Anticoagulation | 71/75 (94.7%) | 21/75 (28%) | 0.000 | 0.043 |
| Experimental treatment | 54/75 (72%) | 21/75 (28%) | 0.000 | −0.184 |
| Corticosteroids | 58/75 (77.3%) | 21/75 (28%) | 0.000 | 0.033 |
| Nutritional support | 70/75 (93.3%) | 21/75 (28%) | 0.000 | 0.054 |
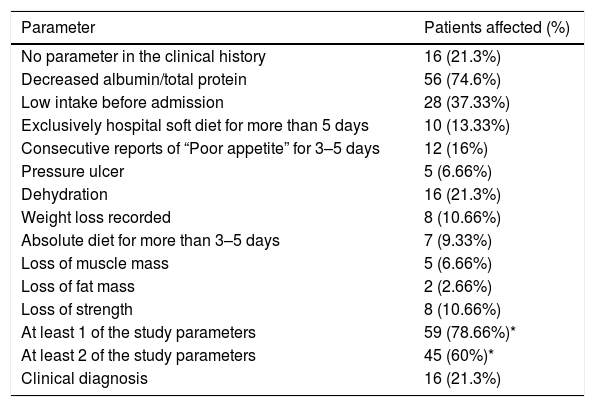
Nutritional screening during the first 48h after admission was only applied in 6 patients (8%). At least 26 patients (34.7%) presented ≥1 of the malnutrition parameters studied (data missing for 49 patients [55.4%]). During admission, 59 patients (78.66%) presented a malnutrition parameter (Table 5).
Parameters indicating malnutrition associated with the disease studied.
| Parameter | Patients affected (%) |
|---|---|
| No parameter in the clinical history | 16 (21.3%) |
| Decreased albumin/total protein | 56 (74.6%) |
| Low intake before admission | 28 (37.33%) |
| Exclusively hospital soft diet for more than 5 days | 10 (13.33%) |
| Consecutive reports of “Poor appetite” for 3–5 days | 12 (16%) |
| Pressure ulcer | 5 (6.66%) |
| Dehydration | 16 (21.3%) |
| Weight loss recorded | 8 (10.66%) |
| Absolute diet for more than 3–5 days | 7 (9.33%) |
| Loss of muscle mass | 5 (6.66%) |
| Loss of fat mass | 2 (2.66%) |
| Loss of strength | 8 (10.66%) |
| At least 1 of the study parameters | 59 (78.66%)* |
| At least 2 of the study parameters | 45 (60%)* |
| Clinical diagnosis | 16 (21.3%) |
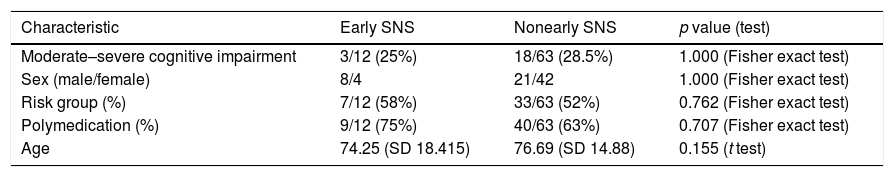
We compared 2 groups: patients who received protocol-based specialized nutritional support during the first 5 days after admission (early nutritional support) and patients who did not. Both groups were similar in terms of age, sex, distribution, risk group, polymedication, duration of symptoms before admission, and presence of moderate or severe cognitive impairment (Table 6).
Characteristics of the study populations.
| Characteristic | Early SNS | Nonearly SNS | p value (test) |
|---|---|---|---|
| Moderate–severe cognitive impairment | 3/12 (25%) | 18/63 (28.5%) | 1.000 (Fisher exact test) |
| Sex (male/female) | 8/4 | 21/42 | 1.000 (Fisher exact test) |
| Risk group (%) | 7/12 (58%) | 33/63 (52%) | 0.762 (Fisher exact test) |
| Polymedication (%) | 9/12 (75%) | 40/63 (63%) | 0.707 (Fisher exact test) |
| Age | 74.25 (SD 18.415) | 76.69 (SD 14.88) | 0.155 (t test) |
SNS: specialized nutritional support.
In the early SNS group, protein requirements were met at 5 days, and, at least 75% of calorie requirements were met in 11 of 12 patients. All of the patients had a nutritional risk/malnutrition according to the criteria of the protocol.
Hospital stay was 9.83±4.84 days in the group receiving early specialized nutritional support compared with 14.43±9.41 days in the nonearly group (mean difference of 5.09 days). In 95% of cases, the reduction in mean stay was between 1.338 and 8.853 days (p<0.01).
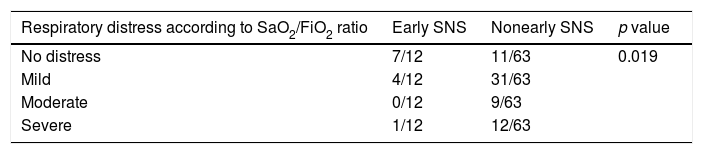
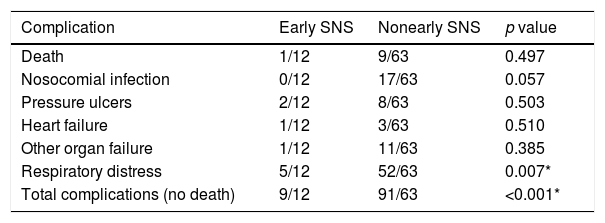
Severe respiratory distress was significantly less frequent in the early specialized nutritional support group (p<0.007), and, although 12 patients presented with respiratory symptoms at admission, a high percentage did not develop moderate-to-severe distress (Table 7). This difference was statistically significant (p<0.019). Furthermore, we observed a trend toward presenting a lower number of nosocomial infectious complications (0/12 vs 17/63), although the difference was not statistically significant (p<0.057).
Similarly, we observed a lower percentage of deaths (8.3% vs 14.28%) and pressure ulcers (8.3% vs 12.69%), although the difference was not statistically significant. Lastly, the early SNS group generally had fewer complications (75.0% versus 144.44%). This difference was statistically significant (p<0.001) (Table 8).
Complications in the group studied according to the type of nutritional support received.
| Complication | Early SNS | Nonearly SNS | p value |
|---|---|---|---|
| Death | 1/12 | 9/63 | 0.497 |
| Nosocomial infection | 0/12 | 17/63 | 0.057 |
| Pressure ulcers | 2/12 | 8/63 | 0.503 |
| Heart failure | 1/12 | 3/63 | 0.510 |
| Other organ failure | 1/12 | 11/63 | 0.385 |
| Respiratory distress | 5/12 | 52/63 | 0.007* |
| Total complications (no death) | 9/12 | 91/63 | <0.001* |
SNS: specialized nutritional support.
The first studies on DRM in hospitals were published in the 1970s, with the classic article by Butterworth, “The skeleton in the hospital closet”.8 DRM continues to be a considerable problem 40 years later, and it has been confirmed that between 20% and 50% of patients admitted to hospital experience DRM.9
Disease-related malnutrition in Spanish hospitals is more prevalent in older patients (≥70 years), that is, >50% at both admission and discharge.10 Therefore, it is not surprising that at least 60% of patients in the present series fulfilled the criteria for DRM during admission. Different clinical series describing prevalence of DRM in COVID-19 disease have been publicated (42.1% in 114 patients by Bedock et al.,11 52.7% in 182 patients by Li et al.12). Our data are concordant with this in therms of high prevalence of DRM in SARS-Cov-2 infection. Variation depends on the criteria for defining DMR and characteristics of study population. We must remember that the causes of DRM include not only those directly associated with the disease itself, but also those that arise from hospitalization, the care team, and the health care provided.13 The causes associated with health care include poor awareness or lack of knowledge on the part of the care teams about the importance of identifying malnutrition and aggravating factors, thus depriving patients of the beneficial effects of nutritional support.14–16
In the present series, this observation is reflected in the following points: notable underuse of the nutritional support protocol (28% of all patients admitted during the study and, sensu stricto, early and controlled implementation in only 16% of patients, especially when compared with the other established protocols), scarce screening on admission (8%), and a low rate of coding for malnutrition at discharge (present in 21.3% of hospital reports, even though at least 60% of patients were affected).
Independently of the criteria used for defining DRE or nutritional risk in COVID-19, this has been associated with higher morbimortality,17 but up to now, there are no data describing effects of early and effective nutritional support on COVID-19 disease. Although the sample size was small, the participants were comparable in terms of sex, age, polymedication, and risk of COVID-19. We found that effective and early implementation of specialized nutritional support (high percentage of needs met during the first few days) generated the expected effects according to previous publications on nutritional interventions in other diseases, with a major and significant reduction in hospital stay (admission with a mean stay of 5.095 days lower than in the early specialized nutritional support group, that is, 31.88% shorter). This reduction in hospital stay has been reported in other hospitals in our region when early specialized nutritional support was implemented.18 The findings are compatible with those reported in the international literature.19,20
Furthermore, it is noteworthy that patients who received early nutritional support had a lower degree of respiratory distress, measured as the SaO2/FiO2 ratio, during the pulmonary and inflammatory phases of the disease (since the mean duration of symptoms admission was more than 7 days, the viral phase had finished). In other viral infections such as influenza, malnutrition has proven to be a prognostic factor for the development of severe disease.21 A retrospective analysis of data available on the 1918 influenza pandemic revealed that disease severity depended on viral and host factors. In the latter, a key role is played by cellular and humoral immune responses and nutritional status.22 Malnutrition is one of the most common causes of immunosuppression, and infection is a major cause of morbidity and mortality in severely malnourished patients. Involvement is based mainly on the cellular immune response, with abnormality of cutaneous-mucous membrane permeability, reduced immunoglobulin A titers, reduced peripheral blood lymphocyte count, and alteration of the CD4/CD8 ratio. With respect to the humoral response, there have been reports of reduced B-lymphocyte production, decreased complement C3 fraction, and diminished synthesis of antibodies in response to vaccination.23 If we take these effects together with the effect of malnutrition on lung function described at the beginning of this article, it is no surprise that nutritional support played a favorable role in the preservation of lung function in this scenario.
The limited sample size prevented us from making a detailed analysis of the remaining complications evaluated. However, we did observe a tendency toward a reduction in the frequency of fatal and nonfatal complications. Taking the nonfatal complications together, we see that early nutritional support can help to reduce the frequency of complications in patients hospitalized with COVID-19. This difference is statistically significant, thus confirming the beneficial effects of nutritional support on the progress of patients admitted to hospital for any reason.24–30
ConclusionCOVID-19 is associated with higher rates of malnutrition in patients admitted to hospital. Early implementation of a specialized nutritional support plan can improve the prognosis of patients by reducing their stay, the likelihood of more severe respiratory distress, and the frequency of complications in general.
The small study sample and the fact that our data are from a single center somewhat limit the statistical power. Consequently, research based on a larger sample and data from various centers are necessary in order to confirm our results. However, the study may still prove useful for determining the viability of implementing a nutritional support program as a key component of treatment of patients hospitalized with COVID-19.
Clinical relevancy statementAs far as we know, malnutrition is associated with higher mortality and complications. On the other hand, adequate early nutritional support has been shown to reduce mortality and poor clinical outcome (admission to intensive care units, readmission to hospital, major complications, impaired functional status, and mortality). Patients with COVID-19 have a higher nutritional risk owing to the increased requirements resulting from severe acute inflammation and the difficulty in meeting these requirements owing to hyporexia and dyspnea, but very few data has been publicated reporting potential benefits of early nutritional support in this new pathology. This short study shows how frequent malnutrition is in COVID-19 disease, but also, how important early nutritional support can be in therms of reducing hospital stay, complications and in particular possible protective effect on developing respiratory distress. Concerning that, adequate early nutritional support shows to be even more necessary in this pathology.
FundingNone declared.
Conflict of interestNone declared.